Abstract
Methylmalonic acidemia and propionic acidemia are organic acidemias biochemically characterized by predominant tissue accumulation of methylmalonic acid (MMA) and propionic acid (PA), respectively. Affected patients present predominantly neurological symptoms, whose pathogenesis is not yet fully established. In the present study we investigated the in vitro effects of MMA and PA on important parameters of lipid and protein oxidative damage and on the production of reactive species in synaptosomes from cerebrum of developing rats. Synaptosomes correspond to nerve terminals that have been used to investigate toxic properties of compounds on neuronal cells. The in vivo effects of intrastriatal injection of MMA and PA on the same parameters and on enzymatic antioxidant defenses, were also studied. MMA-induced in vitro and in vivo lipid peroxidation and protein oxidative damage. Furthermore, the lipid oxidative damage was attenuated or prevented, pending on the doses utilized, by the free radical scavengers α-tocopherol, melatonin and by the NMDA glutamate receptor antagonist MK-801, implying the involvement of reactive species and glutamate receptor activation in these effects. In addition, 2′,7′-dichlorofluorescein diacetate oxidation was significantly increased in synaptosomes by MMA, reinforcing that reactive species generation is elicited by this organic acid. We also verified that glutathione peroxidase activity was inhibited by intrastriatal MMA injection. In contrast, PA did not induce any significant effect on all parameters examined in vitro and in vivo, implying a selective action for MMA. The present data demonstrate that oxidative stress is induced by MMA in vitro in nerve terminals and in vivo in striatum, suggesting the participation of neuronal cells in MMA-elicited oxidative damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methylmalonic acidemia and propionic acidemia are frequent organic acidurias caused by a severe deficiency of methylmalonyl-CoA mutase (EC 5.4.99.2) and propionyl-CoA carboxylase activities (EC 6.4.1.3), respectively. They are biochemically characterized by predominant blood accumulation of methylmalonic acid (MMA) (1–2.5 mM) and propionic acid (PA) (5 mM), respectively, although the concentrations of 3-hydroxypropionic acid, methylcitric acid, propionylglycine, and tiglylglycine are also increased. Other laboratory findings include ketoacidosis, hypoglycemia, hyperglycinemia, hyperammonemia, neutropenia, and trombocitopenia (Fenton et al. 2001; Hörster et al. 2009). Affected patients usually present early in life lethargy, vomiting, dehydration, hepatomegaly, hypotonia, and encephalopathy that may lead to coma and death (Deodato et al. 2006; Manoli and Venditti 2010). Other neurological abnormalities are psychomotor delay/mental retardation, focal and generalized convulsions, EEG alterations, delayed myelination (progressive cortical atrophy), and hypodensity of the basal ganglia (Brismar and Ozand 1994; Chemelli et al. 2000; Harting et al. 2008).
Clinical presentation of propionic acidemia usually occurs in the newborn period with severe metabolic acidosis, vomiting, hypotonia, lethargy, seizures, and hepatomegaly, although in some patients symptoms may occur later, with acute encephalopathy, episodic ketoacidosis, and developmental retardation (Wolf et al. 1981; Surtees et al. 1992; Manoli and Venditti 2010). Treatment of these patients is difficult usually leading to neurological sequelae, including dystonia, chorea, pyramidal signs, developmental delay, focal and general convulsions, cerebral atrophy, and EEG abnormalities (Surtees et al. 1992).
Regarding methylmalonic acidemia, clinical presentation usually occurs in the first week of life and the most common signs and symptoms are lethargy, failure to thrive, recurrent vomiting, respiratory distress and hypotonia, although hepatomegaly and coma is relatively frequent (Shevell et al. 1993; Fenton et al. 2001). Neurological sequelae are similar to that of propionic acidemic patients.
On the other hand, although patients present severe neurological symptoms and cerebral abnormalities, the pathophysiology of brain damage in these disorders is only partly understood. In this context, it has been suggested that brain damage in methylmalonic and propionic acidemias is mainly driven by the accumulating metabolites since it appears that these endogenous compounds are produced and trapped in neural cells (Kölker et al. 2006; Stellmer et al. 2007; Sauer et al. 2006, 2010).
MMA has been demonstrated to provoke behavioral alterations, seizures and striatal lesions in rats after intrastriatal administration through activation of glutamate receptors, energy depletion and oxidative damage (de Mello et al. 1996; Wyse et al. 2000; Fighera et al. 2003; Malfatti et al. 2003; Ribeiro et al. 2005; Royes et al. 2003, 2005, 2006; Furian et al. 2007). Other experimental studies confirmed that impairment of brain mitochondrial energy metabolism, alterations of the redox status and glutamatergic neurotransmission may represent important pathomechanisms of MMA neurotoxicity (Wajner and Coelho 1997; McLaughlin et al. 1998; Fontella et al. 2000; Kölker et al. 2000; Brusque et al. 2001, 2002; Okun et al. 2002; Malfatti et al. 2003; Pettenuzzo et al. 2006).
Furthermore, there is solid evidence showing that oxidative stress and mitochondrial dysfunction play important roles as pathogenic mechanisms in patients and mice models with methylmalonic acidemia (Treacy et al. 1996; Richard et al. 2005, 2007, 2009; Chandler et al. 2009; de Keyzer et al. 2009; Ribas et al. 2010a, b).
Neurotoxic effects of PA have been also reported but to a lesser extent (Wyse et al. 1998; Brusque et al. 1999; de Mattos-Dutra et al. 2000; Fontella et al. 2000; Pettenuzzo et al. 2002; Trindade et al. 2002; Rigo et al. 2006, Ribas et al. 2010a, b). Thus, it has been observed that PA induces lipid peroxidation and decreases non-enzymatic antioxidant defenses in vitro (Fontella et al. 2000) and reduces tissue antioxidant defenses in the hippocampus in vivo (Pettenuzzo et al. 2002). In addition, Rigo et al. (2006) demonstrated that PA causes seizures and induces carbonyl formation in rat striatum after intrastriatal administration and that these effects are prevented by MK-801, a glutamatergic receptor antagonist, suggesting the involvement of glutamate receptors in these effects. Leukocyte DNA damage and induction of lipid and protein oxidative damage were also observed in plasma of patients affected by propionic acidemia and methylmalonic acidemia (Ribas et al. 2010a, b).
However, although oxidative stress was shown to occur in animal models and in humans with methylmalonic and propionic acidemia (Fontella et al. 2000; Furian et al. 2007; Richard et al. 2007, 2009; Mc Guire et al. 2009; Ribas et al. 2010a, b), the reported studies did not distinguish whether neurons or other neural cells are vulnerable to MMA and PA effects. Since synaptosomes are nerve terminals that have been used to investigate the functional consequences of neurotoxins on neurons (Nicholls 2003), our main goal here was to determine whether these neural cells are involved in MMA- and PA-elicited oxidative stress. Therefore, we employed synaptosomal preparations from cerebrum of young rats to study the in vitro effects of MMA and PA on important parameters of lipid and protein oxidative damage and on the production of reactive species. We also investigated the in vivo effects of intrastriatal administration of MMA and PA to young rats on the same parameters and also on the enzymatic antioxidant defenses in the hope to better characterize the deleterious influence of these organic acids on the striatum that is particularly affected in methylmalonic and propionic acidemias.
Methods
Animals and Reagents
Thirty-day-old Wistar rats obtained from the Central Animal House of the Department of Biochemistry, ICBS, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil, were used. The animals were housed six per cage with food (protein commercial chow; SUPRA, Porto Alegre, RS, Brazil) and water available ad libitum and were maintained on a normal 12 h light/dark cycle (lights on 7:00–19:00 h) in air conditioned constant temperature (22 ± 1°C) colony room. This study was performed in accordance with the “Principles of Laboratory Animal Care” (NIH publication no. 80-23, revised 1996) and with the approval of Ethics Committee for Animal Research of the Universidade Federal do Rio Grande do Sul. All efforts were made to minimize the number of animals used and their suffering.
All chemicals were purchased from Sigma (St. Louis, MO, USA). MMA, PA and NaCl solutions were prepared on the day of the experiments in the incubation medium used for each technique and the pH was adjusted to 7.4.
Synaptosomal Preparation from Brain
For the in vitro studies, synaptosomal preparations were used. The animals were killed by decapitation without anesthesia and the brain without the medulla, pons, olfactory bulb, and cerebellum was cut into small pieces using surgical scissors, extensively washed and then manually homogenized in 15 ml of buffer containing 0.32 M sucrose, 2 mM EDTA, 2 mM EGTA, and 20 mM HEPES, pH 7.2. The homogenate was centrifuged at 450×g for 10 min at 4°C and the supernatant transferred to a new tube. Then, the supernatant was centrifuged at 20,000×g for 10 min at 4°C, and the resulting crude synaptosomal pellet was resuspended in 2 ml of Locke’s buffer (154 mM NaCl, 5.6 mM KCl, 2.3 mM CaCl2, 1 mM MgCl2, 3.6 mM NaHCO3, 5 mM glucose, and 5 mM HEPES, at pH 7.2) (Springer et al. 1998). Synaptosomal preparations were incubated for 60 min at 37°C with MMA or PA at concentrations ranging from 0.2 to 10 mM. Controls did not contain these organic acids in the incubation medium. In some experiments, MK-801 (glutamatergic receptor antagonist) and antioxidants were added to the medium at the beginning of incubation together with MMA, at the following final concentrations: 500 or 1000 μM MK-801, 10 or 20 μM trolox (soluble α-tocopherol; TRO), 1000 or 2000 μM melatonin (MEL), combination of superoxide dismutase (SOD) plus catalase (CAT) (100 or 250 mU/ml each), 100 μM l-carnitine (CAR) or 750 μM Nω-nitro-l-arginine methyl ester (l-NAME). Thereafter, aliquots were taken for the measurement of thiobarbituric acid-reactive substances (TBA-RS) (lipid peroxidation), sulfhydryl oxidation and carbonyl formation (protein oxidation) and 2′,7′-dichlorofluorescein diacetate (DCF-DA) (reactive species production). The doses of the antioxidants employed in this investigation was those reported by other investigators as efficient for their scavenging properties (Guajardo et al. 2006; Halliwell and Gutteridge 2007; Gavazza and Catalá 2009; Stasiak et al. 2010; Stuss et al. 2010; Sadowska-Woda et al. 2010).
Administration of Methylmalonic Acid or Propionic Acid and Striatum Preparation
For these studies MMA or PA was injected bilaterally into the striatum. Male Wistar rats of 30 days of life were anesthetized with ketamine and xylasine (75 and 10 mg/kg ip, respectively). Two small holes were drilled in the skull for microinjection and 1 μl of 8 M MMA, 8 M PA or 8 M NaCl (each solution prepared in water and pH was adjusted to 7.4 with NaOH) was slowly injected over 3 min into each striatum via needles connected by a polyethylene tube to a 10 μl Hamilton syringe. The needle was left in place for another 1 min before being softly removed, so that the total procedure lasted 4 min. The coordinates for injection were as follows: 0.6 mm posterior to the bregma, 2.6 mm lateral to the midline and 4.5 mm ventral from dura (Paxinos and Watson 1986). The correct position of the needle was tested by previous injection of 0.5 μl of a methylene blue solution (4% in saline) and further histological analysis.
Thirty minutes after injection the rats were killed by decapitation, the brain was removed and the striatum isolated. The striatum was homogenized in 1:10 (w/v) in 20 mM sodium phosphate buffer with 140 mM KCl, pH 7.4. The homogenate was then centrifuged at 750×g for 10 min at 4°C and the supernatant collected. Thereafter, aliquots were separated and used to measure TBA-RS levels (lipid peroxidation), sulfhydryl oxidation (protein oxidation), and the activities of the antioxidant enzymes glutathione peroxidase (GPx), CAT, and SOD.
Thiobarbituric Acid-Reactive Substances
TBA-RS levels were measured according to the method described by Yagi (1998) with slight modifications. Briefly, 200 μl of 10% trichloroacetic acid and 300 μl of 0.67% TBA in 7.1% sodium sulfate were added to 100 μl of tissue supernatant (0.3 mg of protein) and incubated for 2 h in a boiling water bath. The mixture was allowed to cool on running tap water for 5 min. The resulting pink-stained complex was extracted with 400 μl of butanol. Fluorescence of the organic phase was read at 515 and 553 nm as excitation and emission wavelengths, respectively. Calibration curve was performed using 1,1,3,3-tetramethoxypropane and subjected to the same treatment as supernatants. TBA-RS levels were calculated as nmol TBA-RS/mg protein.
Sulfhydryl Content
Total sulfhydryl group content was assessed according to the method of LoPachin et al. (2004), which is based on the reduction of 5,5′-ditio-bis-2-nitrobenzoic acid (DTNB) by thiol groups present in the sample, forming a yellow derivate (TNB). Sixty hundred and fifty microliters of PBS containing 1 mM EDTA, pH 7.4, and 100 μl of sodium dodecyl sulfate 1% were added to 250 μl of sample (0.3 mg of protein) and rested for 5 min. Soon after, 30 μl of DTNB were added and after an incubation of 5 min the absorption was read at 412 nm. The absorbance due to the amount of TNB is proportional to the amount of reduced thiol groups present in the sample. The results were expressed as nmol TNB/mg protein.
Protein Carbonyl Content
Protein carbonyl formation was measured spectrophotometrically according to Levine et al. (1994). Two hundred microliters of sample (0.3 mg of protein) were treated with 100 μl of 50 mM TRIS buffer and 200 μl of a solution of 10 mM 2,4-dinitrophenylhydrazine (DNPH) prepared in 2.5 N HCl or 2.5 N HCl (blank) and left in the dark at 37°C for 1 h. Samples were then precipitated with 325 μl of 20% trichloroacetic acid and centrifuged for 10 min at 3,000×g. The pellet was then washed with a mixture of ethanol:ethyl acetate (1:1, V/V) and suspended in 700 μl of 6 M guanidine prepared in 2.5 N HCl at 37°C for 5 min. The difference between the DNPH- and HCl-treated samples (blank) was used to calculate the carbonyl content determined at 365 nm. The results were calculated as nmol of carbonyl groups/mg of protein.
2′,7′-Dichlorofluorescein Diacetate Oxidation
The production of reactive species was determined according to the method of LeBel et al. (1992) using DCF-DA. DCF-DA prepared in 20 mM sodium phosphate buffer, pH 7.4, containing 140 mM KCl, was incubated with 80 μl of synaptosomal preparation (1 mg of protein) in the presence of MMA or PA during 1 h at 37°C. Intracellular esterases cleave the acetate group of DCF-DA, generating the reduced form DCFH, which is then rapidly oxidized to form the highly fluorescent product DCF in the presence of reactive species. Fluorescence was measured using wavelengths of 480 nm (excitation) and 535 nm (emission). The calibration curve was performed with standard DCF (0-10 mM) and the concentration of reactive species was expressed as pmol DCF/mg protein.
Glutathione Peroxidase Activity
GPx activity was measured according to Wendel (1981) using tert-butylhydroperoxide as substrate. The enzyme activity was determined by monitoring the NADPH disappearance at 340 nm in a medium containing 600 μl of buffer (100 mM potassium phosphate containing 1 mM EDTA, pH 7.0), 10 μl of 40 mM sodium azide, 15 μl of 100 mM glutathione, 15 μl of 10 U/ml glutathione reductase, 10 μl of 10 mM NADPH, and 10 μl of sample (3 μg of protein). One GPx unit (U) is defined as 1 μmol of NADPH consumed per minute. The specific activity was calculated as U/mg protein.
Catalase Activity
CAT activity was assayed according to Aebi (1984) by measuring the absorbance decrease at 240 nm at room temperature (22 ± 2°C) in a reaction medium containing 20 mM H2O2, 0.1% Triton X-100, 10 mM potassium phosphate buffer, pH 7.0, and the supernatants containing approximately 1 μg of protein. CAT activity was calculated as U/mg protein, using the extinction coefficient of 43.6 M/cm for H2O2. One unit (U) of the enzyme is defined as 1 μmol of H2O2 consumed per minute.
Superoxide Dismutase Activity
SOD activity was assayed according to Marklund (1985) and is based on the capacity of pyrogallol to autoxidize, a process highly dependent on superoxide anion, which is the substrate for SOD. The inhibition of the autoxidation of pyrogallol occurs in the presence of SOD and, therefore, is proportional to the activity of the SOD present in homogenates. The reaction medium contained 50 mM Tris buffer/1 mM ethylenediaminetetraacetic acid, pH 8.2, 80 U/ml CAT, 0.38 mM pyrogallol and approximately 1 μg of protein and the absorbance was read at 420 nm. A calibration curve was performed with purified SOD as standard, in order to calculate the activity of SOD present in the samples. The results were reported as U/mg protein.
Protein Content
The protein content was determined by the method of Lowry et al. (1951) or Bradford (1976) using bovine albumin as standard.
Statistical Analysis
Data were expressed as means ± SD for absolute values. Assays were performed in duplicate or triplicate and the mean was used for statistical analysis. Data were analyzed using one-way analysis of variance (ANOVA) followed by the post-hoc Duncan multiple range test when F was significant (in vitro) or Student t test for unpaired samples (in vivo). Only significant F and t values are displayed in the text. Differences between groups were rated significant at P < 0.05. All analyses were carried out in an IBM-compatible PC computer using the Statistical Package for the Social Sciences (SPSS) software.
Results
MMA Induces Lipid Peroxidation in Brain Synaptosomes and Striatum of Young Rats
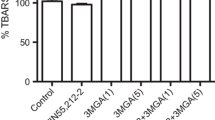
We first observed that TBA-RS levels were significantly increased by MMA in synaptosomal preparations (up to 60%) [F (4,25) = 59.2; P < 0.001] in a concentration-dependent manner (β = 0.921; P < 0.001) (Fig. 1a), whereas PA did not alter this parameter (results not shown). We also evaluated the effect of 3-nitropropionic acid (3NPA), a known irreversible inhibitor of complex II activity of the respiratory chain on TBA-RS levels and found that, similarly to PA, this acid had no effect on this parameter (results not shown). We also found that the NMDA receptor antagonist MK-801 (500 μM) [F (5,30) = 25.6; P < 0.001] and the free radical scavengers trolox (soluble α-tocopherol, 10 μM TRO) [F (3,20) = 44.8; P < 0.001] and melatonin (MEL, 1000 μM) [F (3,20) = 93.7; P < 0.001] attenuated the MMA-induced lipid peroxidation (Fig. 1b–d), whereas the combination of SOD plus CAT (100 mU/ml each), l-carnitine (100 μM) and l-NAME (750 μM) did not change this effect (data not shown). However, when increasing the amounts of these antioxidants in the medium, we observed that 2000 μM MEL and 1000 μM MK-801 totally prevented and 20 μM TRO only attenuated MMA-induced increase of TBA-RS levels [F (5,18) = 10.2; P < 0.001], suggesting that the hydroxyl radical was mainly responsible for this effect. In addition, the combination of SOD plus CAT at 250 mU/ml each had no effect on the increased TBA-RS values (Fig. 1e). Similarly, MMA intrastriatal administration provoked a significant increase in TBA-RS levels in striatum of young rats (43%) [t (8) = 3.88; P < 0.01] (Fig. 2). Taken together, these data indicate that lipid oxidative damage is markedly induced by MMA in vitro in rat brain synaptosomes and in vivo in striatum probably via reactive species generation.
In vitro effect of methylmalonic acid (MMA) on thiobarbituric acid-reactive substances (TBA-RS) in brain synaptosomes. Synaptosomes were incubated during 1 h in the presence of MMA (a). In some experiments synaptosomes were co-incubated for 1 h with 10 mM MMA and either MK-801 (500 μM, b), ∝-tocopherol (TRO, 10 μM, c), or melatonin (MEL, 1000 μM, d). Higher concentrations of MK-801 (1000 μM), MEL (2000 μM), TRO (20 μM), superoxide dismutase plus catalase (SOD + CAT, 250 mU/ml each) were also tested (e). Controls did not contain MMA in the incubation medium, but rather the buffer used in the technique (20 mM sodium phosphate buffer with 140 mM KCl, pH 7.4). Values are means ± standard deviation of six independent experiments (animals) performed in triplicate. **P < 0.01, ***P < 0.001, compared to control; # P < 0.05, ## P < 0.01, ### P < 0.001 compared to MMA (Duncan multiple range test)
In vivo effect of intrastriatal administration of methylmalonic acid (MMA) (8 μmol) on thiobarbituric acid-reactive substances (TBA-RS) in rat striatum. Controls received intrastriatal administration of NaCl (8 μmol). Values are means ± standard deviation of five independent experiments (animals) performed in triplicate. **P < 0.01, compared to control (Student t test)
MMA Induces Protein Oxidative Damage in Brain Synaptosomes and Striatum of Young Rats
The next step of the present study was to evaluate the effect of MMA and PA on protein oxidative damage by measuring sulfhydryl oxidation and carbonyl formation in the brain. Our results show that MMA caused an increase of carbonyl formation (up to 99%) in synaptosomal preparations when supplemented to the incubation medium [F (4,15) = 4.58; P < 0.05] (Fig. 3a). Furthermore, MMA moderately but significantly induced sulfhydryl oxidation (10%) in striatum after intrastriatal administration [t (8) = 3.82; P < 0.01] (Fig. 3b). In contrast, sulfhydryl oxidation was not altered by the exposition of synaptosomal preparations to MMA, whereas PA did not alter these parameters in vivo or in vitro (data not shown). These results indicate that MMA provokes protein oxidative damage in brain synaptosomes and striatum from young rats.
Effects of methylmalonic acid (MMA) on carbonyl formation (a) and sulfhydryl content (b). Carbonyl formation was measured after exposing synaptosomal preparations to various concentrations of MMA (a), whereas sulfhydryl content was measured after intrastriatal administration of MMA (8 μmol) (b). In the in vitro experiments controls did not contain MMA in the incubation medium, but rather the buffer used in the technique (20 mM sodium phosphate buffer with 140 mM KCl, pH 7.4), whereas the controls in vivo received intrastriatal administration of NaCl (8 μmol). Values are means ± standard deviation of five to six independent experiments (animals) performed in triplicate. **P < 0.01, compared to control (Duncan multiple range test and Student t test)
MMA Induces Reactive Species in Brain Synaptosomes from Young Rats
The in vitro effect of MMA and PA on DCF-DA oxidation was also investigated in synaptosomal preparations from brain of young rats. We verified that MMA strongly induced DCF-DA oxidation (up to 177%) [F (4,25) = 65.3; P < 0.001] (Fig. 4), indicating that this organic acid induces an increase of reactive species formation. In contrast, PA had no effect on this parameter (results not shown).
In vitro effect of methylmalonic acid (MMA) on 2′,7′-dichlorofluorescein diacetate (DCF-DA) oxidation in brain synaptosomes. Controls did not contain MMA in the incubation medium, but rather the buffer used in the technique (20 mM sodium phosphate buffer with 140 mM KCl, pH 7.4). Values are means ± standard deviation of six independent experiments (animals) performed in triplicate. ***P<0.001, compared to control (Duncan multiple range test)
MMA Intrastriatal Administration Decreases GPx Activity in Striatum of Young Rats
Finally, we investigated the effect of MMA and PA intrastriatal administration on the activities of the antioxidant enzymes GPx, CAT, and SOD (Table 1). MMA significantly decreased the activity of GPx [t (7) = 9.00; P < 0.001] (37%), but did not affect SOD and CAT activities. In addition, PA did not alter any of these activities (data not shown).
Discussion
In the present work we evaluated the effects of MMA and PA on lipid and protein oxidative damage and on the production of reactive species using synaptosomal preparations from brain of young rats to assess whether oxidative damage is elicited by these metabolites in neuronal cells. We also tested the effect of in vivo intrastriatal administration of MMA and PA on lipid and protein oxidative damage and on the activities of antioxidant enzymes because the striatum is mainly affected in methylmalonic and propionic acidemias, especially during metabolic crises when the concentrations of the accumulating organic acids dramatically increase. We killed the animals 30 min after NaCl, MMA, or PA injection to investigate short-lived effects. It is emphasized that, to our mind, this is the first work that employed synaptosomes to investigate neurotoxic properties of MMA and PA and, besides, determine whether these organic acids are able to alter the enzymatic antioxidant defenses in the striatum.
We first verified that MMA, but not PA and 3NPA, another inhibitor of complex II of the respiratory chain, significantly increased TBA-RS levels in vitro and in vivo in rat brain. These results indicate a selective oxidative effect of MMA, rather than a nonspecific action of acidic compounds. Considering that TBA-RS measurement reflects the amount of malondialdehyde formation, an end product of membrane fatty acid peroxidation (Halliwell and Gutteridge 2007), the increased values of this parameter elicited by MMA strongly indicate that this metabolite causes lipid peroxidation. We also observed that the lipid oxidative damage induced by MMA in brain synaptosomal preparations (in vitro) was partially prevented by MEL (1000 μM), TRO (10 μM), and MK-801 (500 μM), but not by l-carnitine (100 μM), l-NAME (750 μM) or the combination of SOD and CAT (100 mU/ml each). Furthermore, higher doses of MEL (2000 μM) and MK-801 (1000 μM) fully prevented, whereas TRO (20 μM) attenuated and the combination of SOD plus CAT (250 mU/ml each) did not change MMA-elicited effects. Since TRO and MEL scavenge preferentially peroxyl and hydroxyl radicals, it may be suggested that these reactive oxygen species and especially the hydroxyl radical that is efficiently scavenged by MEL, were at least partly involved in MMA-induced lipid oxidation. It should be emphasized that the antioxidants were added simultaneously with MMA to the incubation medium so that they were able to scavenge reactive species before they could react with cell constituents. On the other hand, it is unlikely that reactive nitrogen species were responsible for the induction of lipid peroxidation in synaptosomes used in the present investigation since the classical inhibitor of nitric oxide synthase l-NAME did not reduce the increase of TBA-RS levels provoked by MMA. Furthermore, the protective effects of the NMDA antagonist MK-801 support the involvement of these glutamate receptors in MMA effects, as previously observed (de Mello et al. 1996; Kölker et al. 2000; Brusque et al. 2001; Malfatti et al. 2007). MMA also provoked a moderate protein oxidative damage, as observed by the increase of carbonyl formation in synaptosomes elicited at a high concentration and by the mild enhancement of sulfhydryl oxidation after intrastriatal administration. In contrast, PA did not affect these parameters. Carbonyl group generation is currently used as a marker of free radical-mediated protein oxidation (Levine et al. 1994), being the amino acid residues Pro, Arg, Lys, and Thr of the side chain of proteins the most vulnerable to oxidative attack (Dalle-Donne et al. 2003). On the other hand, since approximately two-thirds of sulfhydryl groups are bound to proteins, whereas one-third is a component of small molecules such as glutathione (Requejo et al. 2010), oxidation of cellular protein-bound sulfhydryl groups from specific cysteine residues also reflects oxidative damage that may potentially lead to protein inactivation (Aksenov and Markesbery 2001; Davies 2003).
We also found that MMA markedly increased DCF-DA oxidation, which is converted to DCF, mainly by hydroxyl radicals, hydrogen peroxide, and peroxynitrite (LeBel et al. 1992; Ischiropoulos et al. 1999; Ohashi et al. 2002; Myhre et al. 2003; Bonini et al. 2006). These data, allied to the findings showing that peroxyl and hydroxyl scavengers attenuated MMA-elicited lipid peroxidation, reinforce the presumption that these reactive species were involved in MMA-induced oxidative effects. Interestingly, it was recently demonstrated increased levels of reactive species in fibroblasts from patients with methylmalonic acidemia (Richard et al. 2005, 2007, 2009), supporting our present in vitro and in vivo findings.
With regard to the antioxidant defense system, MMA in vivo administration provoked a selective decrease of GPx activity, without altering the activities of CAT and SOD. The reduced activity of GPx caused by MMA in striatum may result from decreased de novo synthesis or from inactivation of the enzyme protein due to a direct binding of MMA to vulnerable groups of the enzyme or indirectly through increased reactive species that attack essential sulfhydryl or other vulnerable groups of the enzyme (Singh et al. 2004; Jafari 2007). On the other hand, reduction of GPx activity may lead to a diminished capacity of the striatum to scavenge hydrogen peroxide and fatty acid hydroperoxides that can generate other forms of carbon-, nitrogen-, and oxygen-centered radicals, such as hydroxyl radicals via the Fenton reaction (Halliwell and Gutteridge 2007). In this context, it should be emphasized that hydroxyl radicals readily initiate the process of lipid peroxidation, which may be related to the lipid oxidative damage induced by MMA.
Since oxidative stress results from an imbalance between the total antioxidant defenses and the reactive species generated in a tissue (Halliwell and Gutteridge 2007), our present data strongly indicate that MMA induces oxidative stress in brain of young rats. At this point, it should be emphasized that the brain has low cerebral antioxidant defenses compared with other tissues. Besides, the high oxygen consumption and high iron and lipid contents, especially polyunsaturated fatty acids (Halliwell and Gutteridge 2007), contribute to make the brain more vulnerable to increased reactive species generation.
Taken together the various observations that oxidative damage occurs in patients affected by methylmalonic acidemia (Treacy et al. 1996; Richard et al. 2005, 2007, 2009; Ribas et al. 2010a, b) and the animal experimental studies demonstrating that MMA induces oxidative stress in the brain (McLaughlin et al. 1998; Brusque et al. 2001; Okun et al. 2002; Fighera et al. 2003; Royes et al. 2005, 2006, 2007; Furian et al. 2007; Richard et al. 2005, 2007, 2009; Ribeiro et al. 2005, 2009), it is presumed that alterations of the biological oxidations in the brain may possibly represent one of the mechanisms by which MMA is neurotoxic. However, to our knowledge, no previous study investigated which neural cells were involved in MMA oxidative effects. Our present work was the first to demonstrate that MMA action inducing oxidative damage occurs in nerve terminals (synaptosomal preparations) used as a model to study the effects of neurotoxins in neuronal cells (Nicholls 2003). A great advantage of the utilization of synaptosomes, that correspond to mini-cell in which mitochondria exist in a physiological milieu and supply ATP to the cytoplasm and plasma membrane, is that these preparations can be made from animals of any age, in contrast to the neonatal requirement for virtually all primary neuronal cell cultures. Therefore, we assume that MMA affects biological oxidations in the central nervous system by acting on neuronal cells.
In contrast, PA had no effect on all parameters examined in vitro and in vivo, strongly indicating that this organic acid does not elicit oxidative stress in neuronal cells and in the striatum. However, a previous report showing that PA provokes lipid peroxidation and reduces the nonenzymatic antioxidant defenses in rat cerebral cortex homogenates, implying that oxidative damage may occur in this cerebral region (Fontella et al. 2000). Furthermore, oxidative stress markers were found to be increased in plasma of patients affected by propionic acidemia, although it was not investigated which accumulating organic acids were related to these findings (Ribas et al. 2010a, b). Therefore, it is possible that different approaches and tissue specific effects may possibly explain these apparently conflicting results.
It is difficult to determine the pathophysiological relevance of our present data since to our knowledge MMA brain concentrations are not yet established in methylmalonic acidemia. However, it should be noted significant effects on some parameters of oxidative stress occurred at concentrations as low as 0.2 and 1 mM, concentrations that are within the levels found in plasma and cerebrospinal fluid (CSF) (3.0 mM) (Fenton et al. 2001) of patients affected by this disorder. Furthermore, it is feasible that even higher brain MMA concentrations may take place in stress situations, such as occurs during episodes of metabolic decompensation characterized by intense catabolism and proteolysis. Under these circumstances, the levels of the accumulating metabolites within neural cells may predominate over those found in plasma and CSF (Hoffmann et al. 1993). We should also consider that MMA may be directly produced in the brain, being thereafter trapped in neural cells (Kölker et al. 2006; Sauer et al. 2006, 2010). This is in line with the data showing that patients suffering liver transplantation do not present a decrease in CSF MMA levels and remain with neurological manifestations after this procedure, which is in accordance with the trapping hypothesis (van’t Hoff et al. 1999; Chakrapani et al. 2002; Nyhan et al. 2002; Kölker and Okunm 2005; Nagarajan et al. 2005; Kaplan et al. 2006; Kölker et al. 2006; Sauer et al. 2006, 2010). However, we should also emphasize that the protein oxidative damage induced by MMA was only moderate and occurred at high concentrations of MMA, which may not be physiologically important.
In conclusion, we report for the first time that MMA induces lipid and protein oxidative damage by indirectly increasing the generation of free radicals and other reactive species in brain synaptosomes. Similar findings and a reduction of the enzymatic antioxidant defenses were found in rat striatum after MMA intrastriatal injection, a brain structure that is severely compromised in methylmalonic acidemia (Brismar and Ozand 1994; Chemelli et al. 2000; Harting et al. 2008). In case the present findings are confirmed in human postmortem brain from methylmalonic acidemic patients, the stimulation of highly reactive radical production by MMA in the CNS will potentially lead to deleterious consequences to the brain. Based on the present findings and on previous evidence suggesting that oxidative stress plays a role in this disease and that antioxidants prevent the oxidative damage induced by MMA (Fontella et al. 2000; Malfatti et al. 2003; Royes et al. 2006, 2007; Furian et al. 2007), it is presumed that this pathomechanism may be relevant to explain at least in part the brain dysfunction and abnormalities observed in this disorder, particularly during crises of metabolic decompensation in which the concentrations of MMA dramatically increase. Finally, it is conceivable that administration of antioxidants as adjuvant agents to the usual therapy of methylmalonic acidemia may be useful, especially during these episodes.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Aksenov MY, Markesbery WR (2001) Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci Lett 302:141–145
Bonini MG, Rota C, Tomasi A, Mason RP (2006) The oxidation of 2′-7′-dichlorofluorescin to reactive oxygen species: a self-fulfilling prophesy? Free Radic Biol Med 40:968–975
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brismar J, Ozand PT (1994) CT and MR of the brain in disorders of the propionate and methylmalonate metabolism. AJNR Am J Neuroradiol 15:1459–1473
Brusque AM, Mello CF, Buchanan DN, Terracciano ST, Rocha MP, Vargas CR, Wannmacher CM, Wajner M (1999) Effect of chemically induced propionic academia on neurobehavioral development rats. Pharmacol Biochem Behav 64:529–534
Brusque AM, Rotta LN, Tavares RG, Emanuelli T, Schwarzbold CV, Dutra-Filho CS, de Souza Wyse AT, Duval Wannmacher CM, Gomes de Souza DO, Wajner M (2001) Effects of methylmalonic and propionic acids on glutamate uptake by synaptosomes and synaptic vesicles and on glutamate release by synaptosomes from cerebral cortex of rats. Brain Res 920:194–201
Brusque AM, Borba Rosa R, Schuck PF, Dalcin KB, Ribeiro CA, Silva CG, Wannmacher CM, Dutra-Filho CS, Wyse AT, Briones P, Wajner M (2002) Inhibition of the mitochondrial respiratory chain complex activities in rat cerebral cortex by methylmalonic acid. Neurochem Int 40:593–601
Chakrapani A, Sivakumar P, McKiernan PJ, Leonard JV (2002) Metabolic stroke in methylmalonic acidemia five years after liver transplantation. J Pediatr 140:261–263
Chandler RJ, Zerfas PM, Shanske S, Sloan J, Hoffmann V, DiMauro S, Venditti CP (2009) Mitochondrial dysfunction in mut methylmalonic acidemia. FASEB J 23:1252–1261
Chemelli AP, Schocke M, Sperl W, Trieb T, Aichner F, Felber S (2000) Magnetic resonance spectroscopy (MRS) in five patients with treated propionic acidemia. J Magn Reson Imaging 11:596–600
Dalle-Donne I, Giustarini D, Colombo R, Rossi R, Milzani A (2003) Protein carbonylation in human diseases. Trends Mol Med 9:169–176
Davies MJ (2003) Singlet oxygen-mediated damage to proteins and its consequences. Biochem Biophys Res Commun 305:761–770
de Keyzer Y, Valayannopoulos V, Benoist JF, Batteux F, Lacaille F, Hubert L, Chrétien D, Chadefeaux-Vekemans B, Niaudet P, Touati G, Munnich A, de Lonlay P (2009) Multiple OXPHOS deficiency in the liver, kidney, heart, and skeletal muscle of patients with methylmalonic aciduria and propionic aciduria. Pediatr Res 66:91–95
de Mattos-Dutra A, Meirelles R, Bevilaqua da Rocha B, Kommers T, Wofchuk ST, Wajner M, Pessoa-Pureur R (2000) Methylmalonic and propionic acids increase the in vitro incorporation of 32P into cytoskeletal proteins from cerebral cortex of young rats through NMDA glutamate receptors. Brain Res 856:111–118
de Mello CF, Begnini J, Jimenez-Bernal RE, Rubin MA, de Bastiani J, da Costa E Jr, Wajner M (1996) Intrastriatal methylmalonic acid administration induces rotational behavior and convulsions through glutamatergic mechanisms. Brain Res 721:120–125
Deodato F, Boenzi S, Santorelli FM, Dionisi-Vici C (2006) Methylmalonic and propionic aciduria. Am J Med Genet C Semin Med Genet 142:104–112
Fenton WA, Gravel RA, Rosenblatt DS (2001) In: Scriver CR, Beaudet AL, Valle AD, Sky WS (eds) The metabolic and molecular bases of inherited disease. McGraw-Hill Inc, New York, pp 2165–2193
Fighera MR, Bonini JS, de Oliveira TG, Frussa-Filho R, Dutra-Filho CS, Rubin MA, Mello CF (2003) GM1 ganglioside attenuates convulsions and thiobarbituric acid reactive substances production induced by the intrastriatal injection of methylmalonic acid. Int J Biochem Cell Biol 35:465–473
Fontella FU, Pulrolnik V, Gassen E, Wannmacher CM, Klein AB, Wajner M, Dutra-Filho CS (2000) Propionic and L-methylmalonic acids induce oxidative stress in brain of young rats. Neuroreport 11:541–544
Furian AF, Fighera MR, Oliveira MS, Ferreira AP, Fiorenza NG, de Carvalho Myskiw J, Petry JC, Coelho RC, Mello CF, Royes LF (2007) Methylene blue prevents methylmalonate-induced seizures and oxidative damage in rat striatum. Neurochem Int 50:164–171
Gavazza M, Catalá A (2009) Relative efficacies of alpha-tocopherol, N-acetyl-serotonin, and melatonin in reducing non-enzymatic lipid peroxidation of rat testicular microsomes and mitochondria. Mol Cell Biochem 321(1–2):37–43
Guajardo MH, Terrasa AM, Catalá A (2006) Lipid-protein modifications during ascorbate-Fe2+ peroxidation of photoreceptor membranes: protective effect of melatonin. J Pineal Res 41(3):201–210
Halliwell B, Gutteridge JMC (2007) Measurement of reactive species. In: Halliwell B, Gutteridge JMC (eds) Free radicals in biology and medicine. Oxford University Press, Oxford, pp 268–340
Harting I, Seitz A, Geb S, Zwickler T, Porto L, Lindner M, Kölker S, Höster F (2008) Looking beyond the basal ganglia: the spectrum of MRI changes in methylmalonic acidaemia. J Inherit Metab Dis 31:368–378
Hoffmann GF, Meler-Augenstein W, Stockler S, Surtees R, Rating D, Nyhan WL (1993) Physiology and pathophysiology of organic acids on cerebrospinal fluid. J Inherit Metab Dis 16:648–669
Hörster F, Garbade SF, Zwickler T, Aydin HI, Bodamer OA, Burlina AB, Das AM, de Klerk JBC, Dionisi-Vici C, Geb S, Gökcay G, Guffon N, Maier EM, Morava E, Walter JH, Schwahn B, Wijburg FA, Lindner M, Grünewald S, Baumgartner MR, Kölker S (2009) Prediction of outcome in isolated methylmalonic acidurias: combined use of clinical and biochemical parameters. J Inherit Metab Dis 32:630–639
Ischiropoulos H, Gow A, Thom SR, Kooy NW, Royall JA, Crow JP (1999) Detection of reactive nitrogen species using 2,7-dichlorodihydrofluorescein and dihydrorhodamine 123. Methods Enzymol 301:367–373
Jafari M (2007) Dose- and time-dependent effects of sulfur mustard on antioxidant system in liver and brain of rat. Toxicology 231:30–39
Kaplan P, Ficicioglu C, Mazur AT, Palmieri MJ, Berry GT (2006) Liver transplantation is not curative for methylmalonic acidopathy caused by methylmalonyl-CoA mutase deficiency. Mol Genet Metab 88:322–326
Kölker S, Okunm JG (2005) Methylmalonic acid-an endogenous toxin? Cell Mol Life Sci 62:621–624
Kölker S, Ahlemeyer B, Krieglstein J, Hoffmann GF (2000) Methylmalonic acid induces excitotoxic neuronal damage in vitro. J Inherit Metab Dis 23:355–358
Kölker S, Sauer SW, Surtees RA, Leonard JV (2006) The aetiology of neurological of organic acidaemias—a role for the blood-brain barrier. J Inherit Metab Dis 29:701–704
LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–231
Levine RL, Williams JA, Stadman ER, Shacter E (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 233:346–357
LoPachin RM, Schwarez AI, Gaughan CL, Mansukhani S, Das S (2004) In vivo and in vitro effects of acrylamide on synaptosomal neurotransmitter uptake and release. Neurotoxicology 25:349–363
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Malfatti CRM, Royes LFF, Francescato L, Sanabria ERG, Rubin MA, Cavalheiro EA, Mello CF (2003) Intrastriatal methylmalonic acid administration induces convulsions and TBARS production, and alters Na+, K+-ATPase activity in the rat striatum and cerebral cortex. Epilepsia 44:761–767
Malfatti CR, Perry ML, Schweigert ID, Muller AP, Paquetti L, Rigo FK, Fighera MR, Garrido-Sanabria ER, Mello CF (2007) Convulsions induced by methylmalonic acid are associated with glutamic acid decarboxylase inhibition in rats: a role for GABA in the seizures presented by methylmalonic academic patients? Neuroscience 146:1879–1887
Manoli I, Venditti CP (2010) Methylmalonic acidemia. In: Pagon RA, Bird TC, Dolan CR, Stephens K (eds) GeneReviews (internet). University of Washington, Seattle, WA, 1993–2005 (updated 2010 Sep 28)
Marklund SL (1985) Pyrogallol autoxidation. In: Handbook for oxygen radical research. CRC Press, Boca Raton, FL, pp 243–247
Mc Guire PJ, Parikh A, Diaz GA (2009) Profiling of oxidative stress in patients with inborn errors of metabolism. Mol Genet Metab 98:173–180
McLaughlin BA, Nelson D, Silver JA, Erecinska M, Chesselet MF (1998) Methylmalonate toxicity in primary neuronal cultures. Neuroscience 86:279–290
Myhre O, Andersen JM, Aarnes H, Fonnum F (2003) Evaluation of the probes 2′,7′-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem Pharmacol 65:1575–1582
Nagarajan S, Enns GM, Millan MT, Winter S, Sarwal MM (2005) Management of methylmalonic acidaemia by combined liver-kidney transplantation. J Inherit Metab Dis 28:517–524
Nicholls DG (2003) Bioenergetics and transmitter release in the isolated nerve terminal. Neurochem Res 28:1433–1441
Nyhan WL, Gargus JJ, Boyle K, Selby R, Koch R (2002) Progressive neurologic disability in methylmalonic acidemia despite transplantation of the liver. Eur J Pediatr 161:377–379
Ohashi T, Mizutani A, Murakami A, Kojo S, Ishii T, Taketani S (2002) Rapid oxidation of dichlorodihydrofluorescin with heme and hemoproteins: formation of the fluorescein is independent of the generation of reactive oxygen species. FEBS Lett 511:21–27
Okun JG, Hörster F, Farkas LM, Feyh P, Hinz A, Sauer S, Hoffmann GF, Unsicker K, Mayatepek E, Kölker S (2002) Neurodegeneration in methylmalonic aciduria involves inhibition of complex II and the tricarboxylic acid cycle, and synergistically acting excitotoxicity. J Biol Chem 277:14674–14680
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Academic Press, San Diego
Pettenuzzo LF, Schuck PF, Fontella F, Wannmacher CM, Wyse AT, Dutra-Filho CS, Netto CA, Wajner M (2002) Ascorbic acid prevents cognitive deficits caused by chronic administration of propionic acid to rats in the water maze. Pharmacol Biochem Behav 73:623–629
Pettenuzzo LF, Ferreira GC, Schmidt AL, Dutra-Filho CS, Wyse AT, Wajner M (2006) Differential inhibitory effects of methylmalonic acid on respiratory chain complex activities in rat tissues. Int J Dev Neurosci 24:45–52
Requejo R, Hurd TR, Costa NJ, Murphy MP (2010) Cysteine residues exposed on protein surfaces are the dominant intramitochondrial thiol and may protect against oxidative damage. FEBS J 277:1465–1480
Ribas GS, Manfredini V, de Mari JF, Wayhs CY, Vanzin CS, Biancini GB, Sitta A, Deon M, Wajner M, Vargas CR (2010a) Reduction of lipid and protein damage in patients with disorders of propionate metabolism under treatment: a possible protective role of l-carnitine supplementation. Int J Dev Neurosci 28:127–132
Ribas GS, Manfredini V, de Marco MG, Wayhs CY, Vanzin CS, Biancini GB, Wajner M, Vargas CR (2010b) Prevention by l-carnitine of DNA damage induced by propionic and l-methylmalonic acids in human peripheral leukocytes in vitro. Mutat Res 702:123–128
Ribeiro MC, de Avila DS, Schneider CY, Hermes FS, Furian AF, Oliveira MS, Rubin MA, Lehmann M, Krieglstein J, Mello CF (2005) Alpha-tocopherol protects against pentylenetetrazol- and methylmalonate-induced convulsions. Epilepsy Res 66:185–194
Ribeiro LR, Fighera MR, Oliveira MS, Furian AF, Rambo LM, Ferreira AP, Saraiva AL, Souza MA, Lima FD, Magni DV, Dezengrini R, Flores EF, Butterfield DA, Ferreira J, dos Santos AR, Mello CF, Royes LF (2009) Methylmalonate-induced seizures are attenuated in inducible nitric oxide synthase knockout mice. Int J Dev Neurosci 27:157–163
Richard E, Jorge-Finnigan A, Garcia-Villoria J, Merinero B, Desviat LR, Gort L, Briones P, Leal F, Pérez-Cerdá C, Ribes A, Ugarte M, Pérez B; the MMACHC Working Group (Aguirre A, Andrés M, Badía J, Baldellou A, Couce ML, García-Carzola A, García-Silva MT, Lama R, Lopez-Mendoza S, Martínez-Pardo M, Olivares JL, Parini R, Parraga D, Pedrón C, Peña L, Pineda M, Pintos G, Porta R, Roselló P, Ruiz A, Toro M, Urbón A, Vernet A, Vilaseca MA, Yoldi ME) (2009) Genetic and cellular studies of oxidative stress in methylmalonic aciduria (MMA) cobalamin deficiency type C (cblC) with homocystinuria (MMACHC). Hum Mutat 30:1558–1566
Richard E, Monteoliva L, Juarez S, Pérez B, Desviat LR, Ugarte M, Albar JP (2005) Quantitative analysis of mitochondrial protein expression in methylmalonic acidemia by two-dimensional difference gel electrophoresis. J Proteome Res 5:1602–1610
Richard E, Alvarez-Barrientos A, Pérez B, Desviat LR, Ugarte M (2007) Methylmalonic acidemia leads to increased production of reactive oxygen species and induction of apoptosis through the mitochondrial/caspase pathway. J Pathol 213:453–461
Rigo FK, Pasquetti L, Malfatti CR, Fighera MR, Coelho RC, Petri CZ, Mello CF (2006) Propionic acid induces convulsions and protein carbonylation in rats. Neurosci Lett 408:151–154
Royes LF, Fighera MR, Furian AF, Oliveira MS, da Silva LG, Malfatti CR, Schneider PH, Braga AL, Wajner M, Mello CF (2003) Creatine protects against the convulsive behavior and lactate production elicited by the intrastriatal injection of methylmalonate. Neuroscience 118:1079–1090
Royes LF, Fighera MR, Furian AF, Oliveira MS, de Carvalho Myskiw J, Fiorenza NG, Frussa-Filho R, Mello CF (2005) Involvement of NO in the convulsive behavior and oxidative damage induced by intrastriatal injection of methylmalonate. Neurosci Lett 376:116–120
Royes LF, Fighera MR, Furian AF, Oliveira MS, de Carvalho Myskiw J, Fiorenza NG, Petry JC, Coelho RC, Mello CF (2006) Effectiveness of creatine monohydrate on seizures and oxidative damage induced by methylmalonate. Pharmacol Biochem Behav 83:136–144
Royes LF, Fighera MR, Furian AF, Oliveira MS, Fiorenza NG, Petry JC, Coelho RC, Mello CF (2007) The role of nitric oxide on the convulsive behavior and oxidative stress induced by methylmalonate: an electroencephalographic and neurochemical study. Epilepsy Res 73:228–237
Sadowska-Woda I, Wójcik N, Karowicz-Bilińska A, Bieszczad-Bedrejczuk E (2010) Effect of selected antioxidants in beta-cyfluthrin-induced oxidative stress in human erythrocytes in vitro. Toxicol In Vitro 24(3):879–884
Sauer SW, Okun JG, Fricker G, Mahringer A, Müller I, Crnic LR, Mühlhausen C, Hoffmann GF, Höster F, Goodman SI, Harding CO, Koeller DM, Kölker S (2006) Intracerebral accumulation of glutaric and 3-hydroxyglutaric acids secondary to limited flux across the blood-brain barrier constitute a biochemical risk factor for neurodegeneration in glutaryl-CoA dehydrogenase deficiency. J Neurochem 97:899–910
Sauer SW, Opp S, Mahringher A, Kaminki MM, Thiel C, Okun JG, Fricker G, Morath MA, Kölker S (2010) Glutaric aciduria type I and methylmalonic aciduria: simulation of cerebral import models of the blood-brain barrier and the choroid plexus. Biochim Biophys Acta 1802:552–560
Shevell MI, Matiaszuk N, Ledley FD, Rosenblatt DS (1993) Varying neurological phenotypes among mut 0 and mut − patients with methylmalonilCoA mutase deficiency. Am J Med Genet 45:619–624
Singh P, Jain A, Kaur G (2004) Impact of hypoglycemia and diabetes on CNS: correlation of mitochondrial oxidative stress with DNA damage. Mol Cell Biochem 260(1–2):153–159
Springer JE, Azbill RD, Carlson SL (1998) A rapid and sensitive assay for measuring mitochondrial metabolic activity in isolated neural tissue. Brain Res Protoc 2:259–263
Stasiak M, Zasada K, Lewinski A, Karbownik-Lewinska M (2010) Melatonin restores the basal level of lipid peroxidation in rat tissues exposed to potassium bromate in vitro. Neuroendocrinol Lett 31(3):363–369
Stellmer F, Keyser B, Burckhardt BC, Koepsell H, Streichert T, Glatzel M, Jabs S, Thiem J, Herdering W, Koeller DM, Goodman SI, Lukacs Z, Ullrich K, Burckhardt G, Braulke T, Mühlhausen C (2007) 3-Hydroxyglutaric acid is transported via the sodium-dependent dicarboxylate transporter NaDC3. J Mol Med 85:763–770
Stuss M, Wiktorska JA, Sewerynek E (2010) N-acetylserotonin reduces lipopolysaccharide-induced lipid peroxidation in vitro more effectively than melatonin. Neuroendocrinol Lett 31(4):489–496
Surtees RAH, Matthews EE, Leonard JV (1992) Neurologic outcome of propionic acidemia. Pediatr Neurol 8:333–337
Treacy E, Arbour L, Chessex P, Graham G, Kasprzak L, Casey K, Bell L, Mamer O, Scriver CR (1996) Glutathione deficiency as a complication of methylmalonic acidemia: response to high doses of ascorbate. J Pediatr 129:445–448
Trindade VM, Brusque AM, Raasch JR, Pettenuzzo LF, Rocha HP, Wannmacher CM, Wajner M (2002) Ganglioside alterations in the central nervous system of rats chemically injected with methylmalonic and propionic acids. Metab Brain Dis 17:93–102
van’t Hoff W, McKiernan PJ, Surtees RA, Leonard JV (1999) Liver transplantation for methylmalonic acidemia. Eur J Pediatr 2:S70–S74
Wajner M, Coelho JC (1997) Neurological dysfunction in methylmalonic acidemia is probably related to the inhibitory effect of methylmalonate on brain energy production. J Inherit Metab Dis 20:761–768
Wendel A (1981) Glutathione peroxidase. Methods Enzymol 77:325–332
Wolf B, Hsia YE, Sweetman L, Gravel R, Harris DJ, Nyhan WL (1981) Propionic acidemia: a clinical update. J Pediatr 99:835–846
Wyse AT, Brusque AM, Silva CG, Streck EL, Wajner M, Wannmacher CM (1998) Inhibition of Na+, K+-ATPase from rat brain cortex by propionic acid. Neuroreport 9:1719–1721
Wyse AT, Streck EL, Barros SV, Brusque AM, Zugno AI, Wajner M (2000) Methylmalonate administration decreases Na+, K+-ATPase activity in cerebral cortex of rats. Neuroreport 11:2331–2334
Yagi K (1998) Simple procedure for specific assay of lipid hydroperoxides in serum or plasma. Methods Mol Biol 108:107–110
Acknowledgments
This work was supported by grants from CNPq, PRONEX II, FAPERGS, PROPESQ/UFRGS, FINEP research grant Rede Instituto Brasileiro de Neurociência (IBN-Net) # 01.06.0842-00 and Instituto Nacional de Ciência e Tecnologia em Excitotoxicidade e Neuroproteção (INCT-EN).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fernandes, C.G., Borges, C.G., Seminotti, B. et al. Experimental Evidence that Methylmalonic Acid Provokes Oxidative Damage and Compromises Antioxidant Defenses in Nerve Terminal and Striatum of Young Rats. Cell Mol Neurobiol 31, 775–785 (2011). https://doi.org/10.1007/s10571-011-9675-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-011-9675-4