Abstract
Degeneration of neural retina causes vision impairment and can lead to blindness. Neural stem and progenitor cells might be used as a tool directed to regenerative medicine of the retina. Here, we describe a novel platform for cell phenotype-specific drug discovery and screening of proneurogenic factors, able to boost differentiation of neural retinal progenitor cells. By using single cell calcium imaging (SCCI) and a rational-based stimulation protocol, a diversity of cells emerging from differentiated retinal neurosphere cultures were identified. Exposure of retinal progenitor cultures to KCl or to α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) stimulated Ca2+ transients in microtubule-associated protein 2 (MAP-2) positive neurons. Doublecortin (DCX) and polysialated neural cell adhesion molecule (PSA-NCAM) positive neuroblasts were distinguished from differentiated neurons on the basis of their response to muscimol. Ca2+ fluxes in glial fibrillary acidic protein (GFAP) or glutamine synthetase (GS) positive cells were induced by ATP. To validate the platform, neurospheres were treated with brain-derived neurotrophic factor (BDNF) (proneurogenic) or ciliary neurotrophic factor (CNTF) (gliogenic factor). BDNF increased the percentage of differentiated cells expressing Tuj-1 sensitive to KCl or AMPA and reduced the population of cells responding to muscimol. CNTF exposure resulted in a higher number of cells expressing GFAP responding to ATP. All together, our data may open new perspectives for cell type-specific discovery of drug targets and screening of novel proneurogenic factors to boost differentiation of neural retina cells to treat degenerative retinal diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
At early stages of eye development, a pool of multipotent precursors are able to generate all the six different types of neurons and the Müller glia cells present in the mammalian retina (Turner and Cepko 1987). Initially, retinal ganglion cells (RGC), horizontal cells and cones differentiate, while rods, bipolar, Müller and certain subtypes of amacrine cells are among the last to emerge (reviewed in Das et al. 2006a). Indeed, there are two different populations of progenitors in the retina, named early and late-born progenitors (Cayouette et al. 2006), that ultimately give rise to such populations. Understanding the mechanisms related to the differentiation of these progenitors might allow a cell-based therapy to particular retina disorders, such as retinitis pigmentosa or glaucoma that affects millions of people worldwide (Gamm et al. 2007).
Retinal neurons constituting the vertical axis, photoreceptors—bipolar cells—RGC, use glutamate or aspartate as the main excitatory neurotransmitter. Signals mediated by other transmitters are processed in local microcircuits with the participation of horizontal and amacrine cells. Müller glia differentiate at later developmental stages in the vertebrate retina (Prada et al. 1991). These cells span the whole length of the retinal tissue and locate their cell bodies in the inner nuclear layer, close to amacrine and bipolar cells.
In the last decade, Müller glia has been highlighted as a possible source of postnatal retinal progenitors. Retinal damage and exposure to exogenous growth factors lead Müller cells to re-enter the cell cycle, dedifferentiate and express different transcription factors, which are normally expressed by embryonic retinal progenitors (Fischer and Reh 2001, 2003). As a consequence of the activation of the dedifferentiation program, Müller glia can acquire neuronal markers (Kubrusly et al. 2005). Moreover, these cells are able to generate clonal neurospheres in culture and acquire the phenotype of site-specific neurons on transplantation (Das et al. 2006b).
It is known that transcription factors (Hatakeyama and Kageyama 2004) and extrinsic cues, such as trophic factors (Bhattacharya et al. 2008), neurotransmitters (Martins and Pearson 2008), and extracellular matrix components (Biehlmaier et al. 2007) direct the choice of cell fate in the retina, controlling the production of proper cell types, their differentiation and synaptic lamination. In addition to the major neurotransmitters in the retina, glutamate and GABA, other molecules, such as ATP, have also been related to neuron–glia signaling in the retina (Newman 2003) regulating several functions such as proliferation, survival, protection, and differentiation (Sarthy and Ripps 2001; de Melo Reis et al. 2008a; Bringmann et al. 2009). Among different factors present in the retinal niche, ciliary neurotrophic factor (CNTF) has been shown to regulate neuronal versus glial differentiation of rat retinal progenitors, leading to the generation of bipolar cells at a low concentration or Müller glial cells at a higher concentration (Bhattacharya et al. 2008).
In the present work, single cell calcium imaging (SCCI) was used to functionally identify neurons and glial cells differentiated from neurospheres prepared from postnatal progenitors of mice retina. This technological platform allows us to identify, under live imaging, a diversity of phenotypes based on functional response of single cells to selective agents in a heterogeneous populations of retinal cells. Moreover, this novel approach might be relevant to the functional identification of cell-specific molecular targets in differentiating retinal cells.
Materials and Methods
All experiments were performed in accordance with Brazilian (IBCCF-035) and European Union (86/609/EEC) guidelines for the care and use of laboratory animals.
Retinal Cell Cultures
Neurospheres
Retinal neurosphere cultures were prepared from postnatal (P0–P3) mice using the same principles described elsewhere for subventricular zone (Agasse et al. 2008) and for retinal cells (Bhattacharya et al. 2008). Briefly, retinae were carefully dissected in Hank’s balanced salt solution (Gibco, Rockville, MD) and digested in 0.025% trypsin and 0.265 mM EDTA (Gibco) prepared in the same solution. After dissociation by gentle trituration with a P1000 pipette, cells were washed twice in Dulbecco’s modified Eagle’s medium (DMEM/Ham’s F-12 medium, GlutaMAX-I, Gibco) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 1% B27 supplement, and 20 ng/ml epidermal growth factor (EGF). Dissociated retinal cell (Fig. 1a) were cultured in the same medium (1–3 × 105 cells per cm2), and incubated for 5 days to generate neurospheres (Fig. 1b). In order to investigate whether free-floating spheres were able to maintain self-replication, neurospheres were trypsinized, mechanically dissociated into single cells, grown for an extra 5 days period in the presence of EGF and the formation of secondary spheres was then evaluated (Fig. 1c). Differentiation was assessed by immunocytochemistry and SCCI. For this, neurospheres were differentiated in 10 μg/ml poly-l-lysine (PLL) plus 10 μg/ml human placental laminin (Sigma) coated coverslips. These conditions are permissive for the formation of a mixed retinal carpet around the sphere, containing glia and neurons, within 3–4 days. To search for the effect of brain-derived neurotrophic factor (BDNF) and CNTF on the differentiation of retinal precursors, neurospheres were incubated with 50 ng/ml BDNF or 50 ng/ml CNTF during this period (Fig. 1d).
Neurospheres derived from retinal stem cells. Retinal cell dissociates (a) were cultured (3 × 105 cells per cm2) in DMEM/F12 medium containing 20 ng/ml epidermal growth factor (EGF) for 5 days to generate neurospheres (b). In order to investigate if free-floating neurospheres were able to maintain self-renewal properties, neurospheres were trypsinized, mechanically dissociated and grown for an additional 5 days in the presence of EGF to obtain secondary neurospheres (c). The protocol for the differentiation of mice retinal neurospheres is shown in d. Cells in the pseudomonolayer emerging from the border of the neurospheres (“carpet”) (e) were then analyzed using single cell calcium imaging (SCCI), according to the protocol described in f
Müller Glial Cultures
Müller glial cultures were prepared from P0 to P3 mice retinal cells by modifying the protocol described elsewhere (de Melo Reis et al. 2008b). Briefly, retinae were carefully dissected and incubated in 0.05% trypsin for 10 min. Then, cells were mechanically dissociated in DMEM/Ham’s F-12 medium with GlutaMAX-I supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% fetal calf serum (FCS) (all from Gibco). The tissue was then centrifuged, washed twice and the dissociated retinal cells were plated (2 × 106) in 90-mm plastic dishes with 10 ml DMEM supplemented with 10% FCS. After 8 days, when the culture was confluent, 4 mM ascorbic acid was added for 2 h to eliminate all neurons. After extensive washing with DMEM without serum, neuronal-free enriched glial cultures were obtained. Cells were then trypsinized and plated (approximately 5 × 103 cells/dish) on coverslips for assays on SCCI or immunocytochemistry.
Retinal Neurons
Enriched neuronal cultures were prepared from P0 to P3 mice retinal progenitors after a modification of the protocol used in our laboratory to prepare chick retinal neuronal cultures (Loureiro dos Santos et al. 2001). Briefly, retina pieces were carefully dissected in DMEM supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin and trypsinized for 10 min as described above. Cells were then mechanically dissociated, centrifuged and, after washing, the retinal cells were plated in PLL-treated coverslips in the presence of Neurobasal medium supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 1% B27 supplement. For immunocytochemistry and SCCI assays, cultures were used after 4 days after plating.
Single Cell Calcium Imaging
Variations of free intracellular calcium levels ([Ca2+]i) were evaluated in single cells obtained from retinal neurospheres following an adaptation of the protocol from Agasse et al. (2008). Neurospheres were differentiated as described in coated coverslips to allow the formation of a mixed retinal carpet containing glia and neurons, in 3–4 days (Fig. 1d). As controls, we used cultures enriched in retinal neurons or purified Müller glial cells. Retinal cultures were loaded for 40 min with 5 μM Fura-2/AM (Molecular Probes), 0.1% fatty acid-free bovine serum albumin (BSA), and 0.02% pluronic acid F-127 (Molecular Probes) in Krebs solution (132 mM NaCl, 4 mM KCl, 1.4 mM MgCl2, 2.5 mM CaCl2, 6 mM glucose, 10 mM HEPES, pH 7.4), in an incubator with 5% CO2 and 95% atmospheric air at 37°C. After a 10 min post-loading period at room temperature in Krebs solution, to obtain a complete hydrolysis of the probe, the glass coverslip with the cells was mounted on an RC-20 chamber in a PH3 platform (Warner Instruments, Hamden, CT) on the stage of an inverted fluorescence microscope (Axiovert 200; Carl Zeiss). Cells were continuously perfused with Krebs solution and stimulated following the protocol shown in Fig. 1f. Solutions were added to the cells by a fast-pressurized (95% air, 5% CO2 atmosphere) system (AutoMate Scientific, Inc., Berkeley, CA). The variations in [Ca2+]i were evaluated by quantifying the ratio of the fluorescence emitted at 510 nm following alternate excitation (750 ms) at 340 and 380 nm, using a Lambda DG4 apparatus (Sutter Instrument, Novato, CA) and a 510 nm long-pass filter (Carl Zeiss) before fluorescence acquisition with a 40× objective and a CoolSNAP digital camera (Roper Scientific, Trenton, NJ). Acquired values were processed using the MetaFluor software (Universal Imaging Corp., West Chester, PA). Values for Fura-2 fluorescence ratio were calculated based on a cut-off of 10% increase in the [Ca2+]i level induced by the stimulus. Single cell calcium variation profiles were later matched with the cell phenotype by immunolabeling to determine the functional differentiation pattern of retinal cells.
Immunocytochemical Staining for Phenotype Discrimination
Differentiated retinal spheres, enriched neurons or purified Müller glia cultures were fixed in 4% paraformaldehyde (PFA), for 30 min at room temperature, and then washed twice with PBS, for 5 min. Cultures were permeabilized, and nonspecific binding sites were blocked with 0.25% Triton X-100 and 3% BSA, dissolved in phosphate buffer saline (PBS) for 30 min at room temperature. Retinal cells were subsequently incubated overnight at 4°C with the following primary antibodies prepared in PBS containing 0.1% Triton X-100 and 0.3% BSA: mouse monoclonal anti-microtubule-associated protein 2 (MAP-2) (1:200; Sigma-Aldrich), mouse monoclonal anti-Tuj-1 (1:200; Abcam), mouse monoclonal anti-polysialated neural cell adhesion molecule (PSA-NCAM) (1:300; Sigma), mouse monoclonal anti-glial fibrillary acidic protein (GFAP) (1:300; Cell Signaling), mouse monoclonal anti-nestin (1:300; Chemicon), rabbit polyclonal anti-glutamine synthetase (GS, 1:800; Abcam), rabbit polyclonal anti-GFAP (1:500; Cell Signaling Technology, Danvers, MA), rabbit polyclonal anti-MAP-2 (1:300, Santa Cruz Biotechnology), rabbit polyclonal anti-GFAP (1:300, Abcam), rabbit polyclonal anti-CD133 (1:300, Abcam), goat polyclonal anti-Sox-2 (1:200, Santa Cruz Biotechnology), goat polyclonal anti-doublecortin (DCX; 1:200, Santa Cruz Biotechnology).
Thereafter, the coverslips were rinsed in PBS and incubated for 2 h at room temperature with the appropriate secondary antibodies: anti-rabbit IgG labeled with Alexa Fluor 488 (1:500) or with Alexa Fluor 594 (1:500) or anti-mouse IgG labeled with Alexa Fluor 594 (1:500) (all from Molecular Probes). After an additional rinse in PBS, retinal cell nuclei were stained with Hoechst 33342 (2 μg/ml in PBS containing 0.25% BSA), for 5 min at room temperature. Finally, the preparations were mounted using Dako-Cytomation fluorescent medium. Fluorescent images were recorded using a confocal microscope (LSM 510 Meta; Carl Zeiss) or an Axioskope 2 Plus fluorescent microscope (Carl Zeiss). For the purpose of matching the phenotype expression of single cells with their SCCI profile, retinal neurospheres were plated on 12 mm photoetched coverslips containing alphanumeric coded square grids (EMS, Hatfield, PA, www.emsdiasum.com).
Proliferation Assay
5-Bromo-2′-deoxyuridine (BrdU; 10 μM; Sigma-Aldrich) was added in the last 4 h of the culture session as described elsewhere (Agasse et al. 2008). BrdU was unmasked and labeled following successive passages in 1% Triton X-100, ice-cold 0.1 M HCl, 2 M HCl at 37°C, borate buffer (0.1 M Na2B4O7·10H2O, pH 8.5) and then the cells were incubated with the primary rat monoclonal anti-BrdU antibody (1:50; Oxford Biotechnology, Raleigh, NC), overnight at 4°C. Subsequently, cells were incubated with secondary anti-rat IgG labeled with Alexa Fluor 594 (1:200; Molecular Probes), 1 h at room temperature. Nuclei counterstaining and mounting were performed with Hoechst 33342 and Dako mounting medium, respectively.
Data Analysis
Values for Fura-2 ratio were calculated on the basis of a 10% increase in the [Ca2+]i level induced by the stimulus. SCCI profiles were later correlated with cell identity by immunocytochemistry, as described above. Data were expressed as mean ± SEM. The statistical significance of differences between values was determined by one-way ANOVA followed by Tukey’s test. A two-tailed P < 0.05 was considered significant.
Results
Postnatal retina from C57Bl/6 mice were dissociated (Fig. 1a) and plated in the presence of 20 ng/ml EGF for 5–7 days, in order to obtain retinal progenitor-derived neurospheres (Fig. 1b). Secondary neurospheres were also obtained following trypsinization of primary spheres and later grown in proliferating conditions (Fig. 1c). Neurospheres were differentiated as described on coated coverslips following the schedule illustrated in Fig. 1d. Under these conditions, retinal neurospheres originate a pseudo-monolayer of cells, the “carpet,” containing glia-like cells and differentiating neurons (Fig. 1e). In the absence of laminin, the differentiation properties of neurospheres were significantly impaired (data not shown). These cells were later stimulated with selective agonists following the scheme illustrated in Fig. 1f for SCCI analysis.
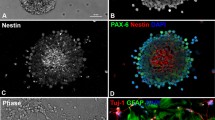
It has been repeatedly shown that cells in the retinal neurospheres express markers of progenitors and eventually neural stem cells (Das et al. 2006a). Sox-2 and CD133, markers of undifferentiated and proliferative cells were found at the core of cytospin-submitted neurospheres (Fig. 2, left). These markers were also found in secondary spheres (not shown), highlighting their self-renewal capacity. Retinal progenitors in the neurospheres were also labeled with an antibody recognizing nestin, an intermediate filament marker present in stem and immature cells. Markers for young and mature neurons (DCX, βIII-tubulin also named Tuj1, PSA-NCAM, and MAP-2) were also detected (Fig. 2, center and top panel, right). Glial phenotypes (GS and GFAP) were found in differentiated neurospheres (Fig. 2, center and bottom panel, right).
Expression of neural cell lineage markers in retinal neurospheres. Expression of Sox-2, CD133, or nestin in cytospin-prepared retinal neurospheres grown under proliferative conditions, for 5 days (left column). Retinal neurospheres were plated on glass coverslips and allowed to differentiate in the absence of EGF. Under these conditions, immature migratory (DCX, PSA-NCAM, and Tuj-1) neuroblasts emerge in culture (center column). Expression of mature neurons (MAP-2) or Müller glial cells (GS and GFAP) (right column). Nuclei were counterstained with Hoechst 33342. Scale bar 50 μm
In order to evaluate the functional responses of single cells in differentiating conditions, intracellular Ca2+ variations were measured following application of 50 mM KCl, 10 μM AMPA, 1 mM ATP, or 100 μM muscimol, a GABAA agonist. The assumption was that KCl and AMPA depolarize neurons (Bloodgood and Sabatini 2008), ATP should activate purinergic receptors in Müller glia cells/astrocytes (Metea and Newman 2006), while muscimol stimulates immature neurons due to activation of GABAA receptors of cells with high intracellular Cl− levels (Zhang et al. 2006). With this principle in mind, a functional platform could be built in order to rapidly characterize a mixed culture of cells differentiating from retinal neurospheres.
Retinal neurospheres, grown under differentiating conditions, generate a large population of cells stimulated by KCl as well as by AMPA (54% of cells responded to KCl and 46% of cells responded to AMPA, Fig. 3a-c). Moreover, we also found that 4.7% of the cell population was stimulated by ATP. Interestingly, some cells that responded to AMPA or to KCl were also stimulated by muscimol. This cell population of immature-like depolarizing cells accounted for 14% of total cells (Fig. 3c). Immunocytochemistry experiments reveal that differentiated retinal neurospheres display Tuj-1 positive neurons and GFAP-positive Müller cells (Fig. 3a, left panel). As positive controls, we used cultures enriched in retinal neurons (Fig. 3d) and cultures enriched in retinal Müller glia cells (Fig. 3g). In agreement with our working hypothesis, retinal neurons responded exclusively to stimulation with AMPA or KCl, but not to ATP or muscimol (Fig. 3d–f). On the other hand, GFAP-positive cells in cultured retinal Müller glia were stimulated by ATP, but not by AMPA, KCl, or muscimol (Fig. 3g–h).
Functional evaluation of retinal cell phenotypes by single cell calcium imaging. a Retinal neurospheres grown under differentiation conditions; immunocytochemistry recognizing Tuj-1 (red) and GFAP (green) show a diversity of cells increasing their [Ca2+]i in different magnitudes (b, c) on application of KCl (50 mM), AMPA (10 μM), ATP (1 mM), or muscimol (100 μM). d Cultures enriched in retinal neurons; Tuj-1 positive retinal neurons (red) show a typical profile of excitable cells with robust response to KCl or AMPA and no response to ATP or muscimol (e, f). g Purified Müller glial cultures; GFAP expressing Müller cells (green) show a unique response to ATP, but no response to KCl, AMPA or to muscimol (h, i). Nuclei were counterstained with Hoechst 33342. Scale bar 50 μm
We used microgrid-labeled coverslips in order to correlate the functional responses of the differentiated retinal cells with cell phenotype. For this, neuronal and glial markers were revealed by immunocytochemistry after performing SCCI experiments in the same cells. Figure 4a illustrates neuronal (MAP-2 labeled in red) and glial cells (GS labeled in green) differentiated from retinal neurospheres treated with 50 ng/ml CNTF. In Fig. 4c, neuronal-like profile of [Ca2+]i transients from cells expressing MAP-2 are shown. These cells are activated by KCl and AMPA, but not by ATP or muscimol. On the other hand, GS expressing Müller cells are activated exclusively by ATP (among the tested stimuli) and are insensitive to KCl, AMPA or to muscimol. Out of 367 cells (n = 7, 10 coverslips) identified by SCCI analysis, 196 were activated by KCl and 187 were activated by AMPA. As shown in Fig. 4a–c, these cells were MAP-2 positive and GS negative. On the other hand, 106 cells were activated by ATP and all of them were GS positive and MAP-2 negative. Interestingly, in control cultures (prepared in the absence of CNTF), we also found that migrating neuroblasts (DCX and PSA-NCAM positive cells) responded to the exposure of depolarizing agents (KCl or AMPA), but also showed an increase in the [Ca2+]i induced by muscimol (Fig. 4d–i). No cells expressing PSA-NCAM or DCX responded to ATP. Therefore, the majority of control cell cultures show functional profile of responses and immunocytochemistry phenotype compatible with neuronal lineage (PSA-NCAM, DCX, and MAP-2) and not glia (GS).
Association of functional response with phenotypic determination in cells differentiating from retinal neurospheres. Cells were grown in differentiation conditions on the top of microgrid-labeled coverslips and then were incubated with Fura-2/AM. After loading, the cells were stimulated with 50 mM KCl, 10 μM AMPA, 1 mM ATP, and 100 μM muscimol (following the same order as shown in Fig. 3), under continuous observation and recording of fluorescence changes. At the end of the SCCI experiment, the coverslips were treated for immunocytochemistry, using antibodies specific for different phenotypic markers. Row a, d, g shows immunocytochemistry pictures against different phenotypic markers, as indicated; row b, e, h shows the same microscope field (row a, d, g) under Fura-2 fluorescence, in SCCI experiments; row c, f, i shows SCCI traces of emission fluorescence (ratio 340/380 nm excitation) of correspondent cells indicated in panels a, d, g. a–c CNTF treated culture show MAP-2 positive neurons (red) that respond to the application of KCl and AMPA, whereas GS positive glial cells (green) respond to ATP. Out of 57 cells shown, 23 were MAP-2 positive and GS negative cells (activated by KCl or by AMPA), while 26 were GS positive and MAP-2 negative (activated by ATP). In control cultures, it is possible to observe PSA-NCAM (d–f) and DCX positive cells (g–h) that respond to the application of KCl, AMPA, and muscimol. c, f, g—scale bar in X axis (200 s)—scale bar in Y axis (arbitrary units of emission fluorescence following 340/380 nm alternate excitation). Scale bar 50 μm
In order to validate the suitability of the presently described technological platform to detect the impact of cell fate determination in the functional outcome of retinal neural cell cultures, we exposed retinal neurosphere cultures to putative proneurogenic (50 ng/ml BDNF) or progliogenic (50 ng/ml CNTF) factors during the differentiation period. Representative SCCI response of 529 retinal cells from neurospheres differentiated under control conditions (Fig. 5a) shows that 54% of the cells are responsive to KCl, 46% are responsive to AMPA, 4.7% respond to ATP, while 14% responded to muscimol (nine coverslips, n = 6 experiments). Addition of 50 ng/ml CNTF increased the number of cells responding to ATP (29%, 106 out of 367 cells from eight coverslips, P < 0.01, n = 6) and decreased the number of cells (13) responding to muscimol (3.5%, Fig. 5b). On the other hand, addition of 50 ng/ml BDNF increased the percentage of cells that respond to KCl (73%, 176 out of 242 cells, five coverslips, n = 4 experiments) and AMPA (70% corresponding to 170 cells; Fig. 5c) and leads to differentiation of fewer cells (42) responding to ATP (17%). Taken together, these data support the general idea that CNTF is mainly gliogenic, while BDNF is proneurogenic in the postnatal retina cells.
Effect of BDNF and CNTF on SCCI responses in cells differentiating from retinal neurospheres. Cultures were stimulated with 50 mM KCl, 10 μM AMPA, 1 mM ATP, and 100 μM muscimol as shown in Fig. 3. Neurospheres grown under differentiation conditions, in the presence of control medium, display many cells responding to KCl and AMPA thus harboring many neurons, and still a significant number of muscimol responsive immature cells (a). Addition of 50 ng/ml CNTF increases the percentage of cells responding to the stimulation with ATP, which is accompanied by a decrease in muscimol responsive population (b). On the other hand, treatment with 50 ng/ml BDNF not only reduces the response to muscimol, but also increases the number of cells responding to KCl or AMPA
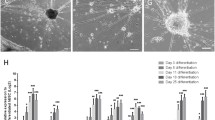
It is known that trophic factors induce differentiation and/or proliferation of retinal neurospheres. Addition of 50 ng/ml BDNF or CNTF increased the percentage of Tuj1 labeled neurons after 4–5 days in vitro (Fig. 6a, b, *P < 0.05). Retinal cells derived from neurospheres in the presence of CNTF had 30% more nuclei labeled with BrdU (added for the last 4 h before fixation) compared with control or BDNF conditions (control 45.97 ± 3.5, 1655 nuclei counted; CNTF 61.46 ± 3.9, 2179 nuclei counted; BDNF 41.33 ± 3.2, 1847 nuclei counted; mean ± SEM, P < 0.05). The number of non-stained glia nuclei was also determined (Müller glia nuclei are bigger than neuronal nuclei) (Fig. 6d). There was a 30% increase in the glia content for CNTF (2196 nuclei counted) treated cultures as compared to BDNF or control (Fig. 6c).
Effect of BDNF and CNTF on phenotypic differentiation of neurons and glia from retinal neurospheres. Expression of Tuj-1 positive cells differentiated from retinal neurospheres in control conditions or in the presence of 50 ng/ml CNTF or 50 ng/ml BDNF (a). Addition of 50 ng/ml CNTF or 50 ng/ml BDNF increases the percentage of Tuj-1 positive neurons (b). The number of Müller glia nuclei, counted under 400× magnification, is increased after 50 ng/ml CNTF treatment as compared to control, after 4 days in culture (c). Representative image of retinal cultures under differentiation conditions after staining with BrdU immunocytochemistry (arrow head) and Hoechst 33342 (asterisk). Müller glia nuclei are significantly bigger as compared to neuronal nuclei (the asterisk is surrounded by 4 Müller cell nuclei) (d). Scale bar 50 μm
Discussion
The neural retina is a well-conserved model to investigate the mechanisms related to differentiation of early and late-born progenitors and cell fate diversification. Retinal progenitors are similar to the ones found in other areas of the CNS, as they respond to mitogenic factors such as EGF, basic fibroblast growth factor (bFGF), and sonic hedgehog, among others (Das et al. 2006a). The mature retina is made of seven major cell types, including six classes of neurons and one type of glial cell, the Müller glia. In the retina, as elsewhere in the CNS, neurogenesis precedes gliogenesis. Therefore, Müller cells, are born when the majority of neurons have already been generated (Cayouette et al. 2006).
In this work, a functional SCCI platform was used to allow the simultaneous identification of a diversity of cell phenotypes differentiating from early postnatal mice retina neurospheres. In the proliferative state, several cells express molecular markers of neural stem/progenitor cells such as the transcription factor sox2, a SoxB1-box transcription factor (Graham et al. 2003) and CD133, a marker of undifferentiated (Lee et al. 2005) and, in some cases, tumor cells (Singh et al. 2003). Also, the intermediate filament marker, nestin, was found (Lendahl et al. 1990) in several cells located in the center of retinal neurospheres (Fig. 2). Sox2 and CD133+ cells are linked to retinal proliferative capacity, neurosphere generation, and differentiation (Carter et al. 2009). Sox2 also seems to play an essential role as an immaturity transcription factor, acting to generate Müller glial and amacrine cells through the upregulation of Notch1 (reviewed in Jadhav et al. 2009). On the other hand, nestin is expressed in Müller cells derived from spheres (Monnin et al. 2007), highlighting the relative differentiating potential of these cells (Ramirez and Lamas 2009).
To associate the functional differentiation of retinal cells with their phenotypic pattern, variations of [Ca2+]i shifts were analyzed in single cells following stimulation with selective agents. In order to accomplish this objective, we developed a protocol designed to selectively stimulate neurons, neuroblasts, progenitors, and glial cells. The information collected in SCCI experiments was then associated with the phenotypic characterization of the same cells by immunocytochemical analysis.
The presence of ionotropic glutamate receptors in retina neurospheres has been previously examined by whole cell patch recording and Ca2+ imaging (Ahmad et al. 1999; Bhattacharya et al. 2003). Here, we show that cells that responded only to AMPA or KCl, expressed MAP-2, marker of a mature neuron. Response to AMPA, KCl, and muscimol were found in neuroblasts expressing DCX and PSA-NCAM, markers of immature neuroblasts involved in migration (Bédard et al. 2006). The majority of the cells expressing PSA-NCAM or DCX (Fig. 5d, g) were in the edge of the neurospheres forming chains suggesting migratory patterns of the neuroblast population. The olfactory bulb under normal and ischemic conditions also displays DCX and nestin-positive cells as migrating neuroblasts (Zhao and Nam 2007).
In the last decade, Müller glia has been recognized as a retinal progenitor cell based on gene profiling techniques (Bonfanti and Peretto 2007). Previous work has shown that Müller glia has a central and selective role on migration and integration of transplanted cells in host tissue (Small et al. 1993). Evidence also suggests that Müller glia are a form of late stage retinal progenitor (Fischer and Reh 2001; Das et al. 2006b), that possess self-renewal capacity, multipotentiality, and regenerative capacity in Zebra fish retina (Hitchcock and Raymond 1992). Therefore, Müller glia seems to be able to dedifferentiate, acquire progenitor-like phenotypes, and produce new neurons and glia. Although these late stage progenitor cells acquire Müller specialized functions, they seem not to, irreversibly, leave the progenitor state.
Although in our experiments calcium shifts in glia cells were only observed after ATP stimulation, purified cultured Müller glia display several characteristics that are seen in retina neurons. For instance, these cells in culture accumulate cyclic AMP induced by catecholamines (Kubrusly et al. 2008), display GABA uptake properties (De Sampaio Schitine et al. 2007) and PACAP-induced activation of adenylyl cyclase (Kubrusly et al. 2005). They also express glutamic acid decarboxylase, beta 2 nicotine acetylcholine receptors, enzymes of catecholamine metabolism, including tyrosine hydroxylase (Kubrusly et al. 2005, 2008; Seki et al. 2006), glutamate (Ramirez and Lamas 2009), and muscarinic acetylcholine receptors (Wakakura et al. 1998). Chick Müller cells in culture express several excitatory amino acids (EAAs) receptors that are coupled to inositol triphosphate mobilization and 45Ca2+ influx (López-Colomé et al. 1993). In this particular study, the order of potency for inositol phosphate accumulation induced by EAA was quisqualate > l-glutamate = kainate (KA) > N-methyl-l-aspartate (NMDA) > l-aspartate with an EC50 in the range of 1–100 μM. However, AMPA had no effect on the basal or stimulated accumulation of inositol phosphate mobilization.
ATP is also described as a functional gliotransmitter in the retina mediating Ca2+ waves among Müller cells and astrocytes (Newman 2003). Indeed, in our hands, ATP was found to stimulate calcium influx in GS and GFAP-positive cells, possibly through ionotropic P2X7 receptors, since these effects were blocked by brilliant blue G (not shown). Several purinergic ionotropic (Pannicke et al. 2000) and metabotropic (Fries et al. 2005) receptors have been identified in Müller glia and in retinal progenitors possibly mediating proliferation induced by ATP (Sholl-Franco et al. 2010). Also, ATP is known to activate microglia in other areas of the CNS (Bernardino et al. 2008).
It was recently described that CNTF has a dual role in the neurospheres. At low CNTF concentrations differentiation of bipolar cells is induced, while high CNTF concentrations promote Müller cell differentiation (Bhattacharya et al. 2008). This dual effect was based on a concentration-dependent recruitment of Mitogen-Activated Protein Kinase and Janus Kinase-Signal Transducer and Activator of Transcription pathways in conjunction with Notch Signaling. CNTF has also additional properties in the retina since it has potent axiogenic (Jo et al. 1999) and regenerative effects (Cui et al. 2003) on RGC. Indeed, both CNTF and BDNF have been co-injected as a possible strategy to protect photoreceptors in the rd1 mouse retina (Azadi et al. 2007). Here, we show that CNTF increases the number of cells responding to ATP (Müller glia cells), and that BDNF and CNTF have important roles in neuronal versus glial cell decision in retinal neurospheres differentiation. Future work will be devoted to match the development of the phenotypic expression and SCCI of the other neuronal classes (rod, cone bipolar, amacrine, RGC, and horizontal). This might be a valuable tool to understand the functional development of strategic cells in the retina and help to open new avenues for “personalized” retinal cell pharmacology and regenerative medicine.
Abbreviations
- AMPA:
-
α-Amino-3-hydroxyl-5-methyl-4-isoxazole-propionate
- BDNF:
-
Brain-derived neurotrophic factor
- CNTF:
-
Ciliary neurotrophic factor
- DCX:
-
Doublecortin
- EAA:
-
Excitatory amino acid
- EGF:
-
Epidermal growth factor
- bFGF:
-
Basic fibroblast growth factor
- GFAP:
-
Glial fibrillary acidic protein
- GS:
-
Glutamine synthetase
- MAP-2:
-
Microtubule-associated protein 2
- PBS:
-
Phosphate buffer saline
- PFA:
-
Paraformaldehyde
- PSA-NCAM:
-
Polysialated neural cell adhesion molecule
- RGC:
-
Retinal ganglion cells
- SCCI:
-
Single cell calcium imaging
References
Agasse F, Bernardino L, Kristiansen H, Christiansen SH, Ferreira R, Silva B, Grade S, Woldbye DP, Malva JO (2008) Neuropeptide Y promotes neurogenesis in murine subventricular zone. Stem Cells 26:1636–1645
Ahmad I, Dooley CM, Thoreson WB, Rogers JA, Afiat S (1999) In vitro analysis of a mammalian retinal progenitor that gives rise to neurons and glia. Brain Res 831:1–10
Azadi S, Johnson LE, Paquet-Durand F, Perez MT, Zhang Y, Ekström PA, van Veen T (2007) CNTF + BDNF treatment and neuroprotective pathways in the rd1 mouse retina. Brain Res 1129:116–129
Bédard A, Gravel C, Parent A (2006) Chemical characterization of newly generated neurons in the striatum of adult primates. Exp Brain Res 170:501–512
Bernardino L, Balosso S, Ravizza T, Marchi N, Ku G, Randle JC, Malva JO, Vezzani A (2008) Inflammatory events in hippocampal slice cultures prime neuronal susceptibility to excitotoxic injury: a crucial role of P2X7 receptor-mediated IL-1beta release. J Neurochem 106:271–280
Bhattacharya S, Jackson JD, Das AV, Thoreson WB, Kuszynski C, James J, Joshi S, Ahmad I (2003) Direct identification and enrichment of retinal stem cells/progenitors by Hoechst dye efflux assay. Invest Ophthalmol Vis Sci 44:2764–2773
Bhattacharya S, Das AV, Mallya KB, Ahmad I (2008) Ciliary neurotrophic factor-mediated signaling regulates neuronal versus glial differentiation of retinal stem cells/progenitors by concentration-dependent recruitment of mitogen-activated protein kinase and Janus kinase-signal transducer and activator of transcription pathways in conjunction with notch signaling. Stem Cells 26:2611–2624
Biehlmaier O, Makhankov Y, Neuhauss SC (2007) Impaired retinal differentiation and maintenance in zebrafish laminin mutants. Invest Ophthalmol Vis Sci 48:2887–2894
Bloodgood BL, Sabatini BL (2008) Regulation of synaptic signalling by postsynaptic, non-glutamate receptor ion channels. J Physiol 586:1475–1480
Bonfanti L, Peretto P (2007) Radial glial origin of the adult neural stem cells in the subventricular zone. Prog Neurobiol 83:24–36
Bringmann A, Iandiev I, Pannicke T (2009) Cellular signaling and factors involved in Müller cell gliosis: neuroprotective and detrimental effects. Prog Retin Eye Res 28:423–451
Carter DA, Dick AD, Mayer EJ (2009) CD133+ adult human retinal cells remain undifferentiated in leukaemia inhibitory factor (LIF). BMC Ophthalmol 9:1
Cayouette M, Poggi L, Harris WA (2006) Lineage in the vertebrate retina. Trends Neurosci 29:563–570
Cui Q, Yip HK, Zhao RCH, So KF, Harvey AR (2003) Intraocular elevation of cyclic AMP potentiates ciliary neurotrophic factor-induced regeneration of adult rat retinal ganglion cell axons. Mol Cell Neurosci 22:49–61
Das AV, James J, Edakkot S, Ahmad I (2006a) Retinal stem cells. In: Rao MS (ed) Neural development and stem cells, vol 10, 2nd edn. Humana Press Inc., Totowa, pp 235–247
Das AV, Mallya KB, Zhao X, Ahmad F, Bhattacharya S, Thoreson WB, Hegde GV, Ahmad I (2006b) Neural stem cell properties of Müller glia in the mammalian retina: regulation by Notch and Wnt signaling. Dev Biol 299:283–302
De Melo Reis RA, Ventura ALM, Schitine CS, de Mello MC, de Mello FG (2008a) Müller glia as an active compartment modulating nervous activity in the vertebrate retina: neurotransmitters and trophic factors. Neurochem Res 33:1466–1474
De Melo Reis RA, Cabral-da-Silva MC, de Mello FG, Taylor JS (2008b) Müller glia factors induce survival and neuritogenesis of peripheral and central neurons. Brain Res 1205:1–11
De Sampaio Schitine C, Kubrusly RC, De Melo Reis RA, Yamasaki EN, De Mello MC, De Mello FG (2007) [3H] GABA uptake by avian Müller retinal glia in culture. Neurotox Res 12:1–9
Fischer AJ, Reh TA (2001) Müller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci 4:247–252
Fischer AJ, Reh TA (2003) Potential of Müller glia to become neurogenic retinal progenitor cells. Glia 43:70–76
Fries JE, Goczalik IM, Wheeler-Schilling TH, Kohler K, Guenther E, Wolf S, Wiedemann P, Bringmann A, Reichenbach A, Francke M, Pannicke T (2005) Identification of P2Y receptor subtypes in human Müller glial cells by physiology, single cell RT-PCR, and immunohistochemistry. Invest Ophthalmol Vis Sci 46:3000–3007
Gamm DM, Wang S, Lu B, Girman S, Holmes T, Bischoff N, Shearer RL, Sauvé Y, Capowski E, Svendsen CN, Lund RD (2007) Protection of visual functions by human neural progenitors in a rat model of retinal disease. PLoS One 2(3):e338
Graham V, Khudyakov J, Ellis P, Pevny L (2003) Sox2 functions to maintain neural progenitor identity. Neuron 39:749–765
Hatakeyama J, Kageyama R (2004) Retinal cell fate determination and bHLH factors. Semin Cell Dev Biol 15:83–89
Hitchcock PF, Raymond PA (1992) Retinal regeneration. Trends Neurosci 15:103–108
Jadhav AP, Roesch K, Cepko CL (2009) Development and neurogenic potential of Müller glial cells in the vertebrate retina. Prog Retin Eye Res 28:249–262
Jo SA, Wang E, Benowitz LI (1999) Ciliary neurotrophic factor is an axogenesis factor for retinal ganglion cells. Neuroscience 89:579–591
Kubrusly RC, da Cunha MC, Reis RA, Soares H, Ventura AL, Kurtenbach E, de Mello MC, de Mello FG (2005) Expression of functional receptors and transmitter enzymes in cultured Müller cells. Brain Res 1038:141–149
Kubrusly RC, Panizzutti R, Gardino PF, Stutz B, Reis RA, Ventura AL, de Mello MC, de Mello FG (2008) Expression of functional dopaminergic phenotype in purified cultured Müller cells from vertebrate retina. Neurochem Int 53:63–70
Lee A, Kessler JD, Read TA, Kaiser C, Corbeil D, Huttner WB, Johnson JE, Wechsler-Reya RJ (2005) Isolation of neural stem cells from the postnatal cerebellum. Nat Neurosci 8:699–701
Lendahl U, Zimmerman LB, McKay RD (1990) CNS stem cells express a new class of intermediate filament protein. Cell 60:585–595
López-Colomé AM, Ortega A, Romo-de-Vivar M (1993) Excitatory amino acid-induced phosphoinositide hydrolysis in Müller glia. Glia 9:127–135
Loureiro Dos Santos NE, Reis RA, Kubrusly RC, de Almeida OM, Gardino PF, de Mello MC, de Mello FG (2001) Inhibition of choline acetyltransferase by excitatory amino acids as a possible mechanism for cholinergic dysfunction in the central nervous system. J Neurochem 77:1136–1144
Martins RA, Pearson RA (2008) Control of cell proliferation by neurotransmitters in the developing vertebrate retina. Brain Res 1192:37–60
Metea MR, Newman EA (2006) Calcium signaling in specialized glial cells. Glia 54:650–655
Monnin J, Morand-Villeneuve N, Michel G, Hicks D, Versaux-Botteri C (2007) Production of neurospheres from mammalian Müller cells in culture. Neurosci Lett 421:22–26
Newman E (2003) Glial cell inhibition of neurons by release of ATP. J Neurosci 23:1659–1666
Pannicke T, Fischer W, Biedermann B, Schädlich H, Grosche J, Faude F, Wiedemann P, Allgaier C, Illes P, Burnstock G, Reichenbach A (2000) P2X7 receptors in Müller glial cells from the human retina. J Neurosci 20:5965–5972
Prada C, Puga J, Pérez-Méndez L, López R, Ramírez G (1991) Spatial and temporal patterns of neurogenesis in the chick retina. Eur J Neurosci 3:559–569
Ramirez M, Lamas M (2009) NMDA receptor mediates proliferation and CREB phosphorylation in postnatal Müller glia-derived retinal progenitors. Mol Vis 15:713–721
Sarthy V, Ripps H (2001) The retinal Müller cell–structure and function. Kluwer, New York, pp 1–33
Seki T, Hinohara Y, Taki C, Nakatani M, Ozawa M, Nishimura S, Takaki A, Itho H, Takenoya F, Shioda S (2006) PACAP stimulates the release of interleukin-6 in cultured rat Müller cells. Ann NY Acad Sci 1070:535–539
Sholl-Franco A, Fragel-Madeira L, Macama AD, Linden R, Ventura AL (2010) ATP controls cell cycle and induces proliferation in the mouse developing retina. Int J Dev Neurosci 28:63–73
Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB (2003) Identification of a cancer stem cell in human brain tumors. Cancer Res 63:5821–5828
Small RK, Watkins BA, Munro PM, Liu D (1993) Functional properties of retinal Müller cells following transplantation to the anterior eye chamber. Glia 7:158–169
Turner DL, Cepko CL (1987) A common progenitor for neurons and glia persists in rat retina late in development. Nature 328:131–136
Wakakura M, Utsunomiya-Kawasaki I, Ishikawa S (1998) Rapid increase in cytosolic calcium ion concentration mediated by acetylcholine receptors in cultured retinal neurons and Müller cells. Graefe’s Arch Clin Exp Ophthalmol 236:934–939
Zhang LL, Pathak HR, Coulter DA (2006) Shift of intracellular chloride concentration in ganglion and amacrine cells of developing mouse retina. J Neurophysiol 95:2404–2416
Zhao LR, Nam SC (2007) Multiphoton microscope imaging: the behavior of neural progenitor cells in the rostral migratory stream. Neurosci Lett 425:83–88
Acknowledgments
This work was supported by a CAPES-Grices protocol No. 3294-07-2, a Brazil–Portugal bilateral agreement, FAPERJ, INCT-INNT, and CNPq funds (Brazil) and by PTDC/SAU-NEU/68465/2006, Portuguese Foundation for Science and Technology and FEDER (Portugal).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Melo Reis, R.A., Schitine, C.S., Kofalvi, A. et al. Functional Identification of Cell Phenotypes Differentiating from Mice Retinal Neurospheres Using Single Cell Calcium Imaging. Cell Mol Neurobiol 31, 835–846 (2011). https://doi.org/10.1007/s10571-011-9673-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-011-9673-6