Abstract
It is well established that the involvement of reactive species in the pathophysiology of several neurological diseases, including phenylketonuria (PKU), a metabolic genetic disorder biochemically characterized by elevated levels of phenylalanine (Phe). In previous studies, we verified that PKU patients (treated with a protein-restricted diet supplemented with a special formula not containing l-carnitine and selenium) presented high lipid and protein oxidative damage as well as a reduction of antioxidants when compared to the healthy individuals. Our goal in the present study was to evaluate the effect of Phe-restricted diet supplemented with l-carnitine and selenium, two well-known antioxidant compounds, on oxidative damage in PKU patients. We investigated various oxidative stress parameters in blood of 18 treated PKU patients before and after 6 months of supplementation with a special formula containing l-carnitine and selenium. It was verified that treatment with l-carnitine and selenium was capable of reverting the lipid peroxidation, measured by thiobarbituric acid-reactive species, and the protein oxidative damage, measured by sulfhydryl oxidation, to the levels of controls. Additionally, the reduced activity of glutathione peroxidase was normalized by the antioxidant supplementation. It was also verified a significant inverse correlation between lipid peroxidation and l-carnitine blood levels as well as a significant positive correlation between glutathione peroxidase activity and blood selenium concentration. In conclusion, our results suggest that supplementation of l-carnitine and selenium is important for PKU patients since it could help to correct the oxidative stress process which possibly contributes, at least in part, to the neurological symptoms found in phenylketonuric patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The participation of reactive oxygen species (ROS) on the pathophysiology of a crescent number of pathologies, including cancer, neurodegenerative disorders, and inherited metabolic diseases is well established (Halliwell and Gutteridge 2007).
ROS are continuously produced during normal physiologic events and are capable of easily initiating deleterious cascades inducing peroxidation of membrane lipids. In order to handle ROS production, organisms possess an efficient antioxidant system which includes non-enzymatic antioxidant defenses and antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px). However, when the balance between the generation of ROS and the inactivation of ROS by the antioxidant system is lost, oxidative stress occurs, leading to oxidative damage to cellular membrane or intracellular molecules, and causing pathological conditions (Halliwell and Gutteridge 2007; Chaudière 1994; German 1999).
Phenylketonuria (PKU) is an inborn error of phenylalanine (Phe) metabolism, caused by deficiency of the enzyme phenylalanine hydroxylase activity, which converts Phe to tyrosine (Tyr). Although Phe is thought to be neurotoxic in PKU, no single mechanism has been identified as being responsible for the central nervous system-related problems, and the pathophysiology of the disease still remains unclear (Scriver and Kaufman 2001; Van Spronsen et al. 2001).
In this context, there is strong evidence that oxidative stress contributes for the neurological damage in PKU, especially in untreated patients or in patients who do not comply with the low-Phe diet. It is presumed that Phe and/or its metabolites induce excessive production of free radicals and/or deplete the tissue antioxidant capacity (Colome et al. 2003; Schulpis et al. 2005; Sierra et al. 1998, 2006, 2009a; Sirtori et al. 2005). In addition, protein-restricted diet for PKU patients may lead to a decrease of the antioxidants intake, contributing to the oxidative damage verified in the disease (Artuch et al. 2004; van Backel et al. 2000).
We previously demonstrated that PKU patients submitted to a protein-restricted diet presented lipid and protein oxidative damage and a decrease in the antioxidant status (Sitta et al. 2006, 2009a). Additionally, we verified in PKU patients a positive correlation between the plasma antioxidant reactivity and l-carnitine (LC) concentrations and a negative correlation between malondialdehyde levels and LC levels, a substance with a potential antioxidant effect that is found to be reduced in PKU patients under strict diet (Sitta et al. 2009b). Therefore, in the present work we evaluated the effect of a long-term supplementation with LC and selenium (Se) on various oxidative stress parameters in PKU patients in order to test for the efficacy of this treatment on the antioxidant status of phenylketonuric patients.
Methods
Patients and Controls
Eighteen patients (mean age 17.2 ± 2.6 years; range 15–22 years-old) with classical PKU under treatment were studied. The average blood Phe levels calculated from the various measurements obtained at every 2 months was 686 ± 315 μmol/l. The dietary treatment consisted of a restricted protein diet supplemented with a special formula not containing LC and Se (PKU 3—Support®). The diet contained 220–450 mg/(kg day) Phe and 2.55–4.00 g/(kg day) Tyr according to patients’ age. Oxidative stress parameters were analyzed in blood of PKU patients before and after at least 6 months of supplementation with Se and LC (PKU 3 Advanta—Support®—Se: 31.5 mcg/day; LC: 98 mg/day). Eighteen healthy children (mean age 19.4 ± 3.7 years; range 18–23 years-old) were used as the control group.
The study was approved by the Ethics Committee of the Hospital de Clínicas de Porto Alegre.
Erythrocyte and Plasma Preparation
Erythrocytes and plasma were prepared from whole blood samples obtained from fasting individuals (controls and PKU patients) by venous puncture with heparinized vials. Fifty microliters of the whole blood was spotted onto specialized paper cards for posterior analysis of free l-carnitine. The additional whole blood was centrifuged at 1,000×g, plasma was removed by aspiration and frozen at −80°C until determinations. Erythrocytes were washed three times with cold saline solution (0.153 mol/l sodium chloride). Lysates were prepared by the addition of 1 ml of distilled water to 100 μl of washed erythrocytes and frozen at −80°C until analysis.
Oxidative Stress Parameters
Determination of Thiobarbituric Acid-Reactive Species (TBARS)
Thiobarbituric acid-reactive species were determined according to the method described by Esterbauer and Cheeseman (1990). Briefly, 300 μl of 10% trichloroacetic acid was added to 150 μl of plasma and centrifuged at 1,000×g for 10 min at 4°C. Three hundred microliters of the supernatant was transferred to a test tube and incubated with 300 μl 0.67% thiobarbituric acid (7.1% sodium sulfate) at 100°C for 1 h. The resulting pink stained TBARS were determined at 535 nm wavelength in a spectrophotometer. Calibration curve was performed using 1,1,3,3-tetramethoxypropane subjected to the same treatment as that for the supernatants. TBARS were calculated as nanomoles per milligram protein.
Glutathione Peroxidase (GSH-Px)
GSH-Px activity was measured using the RANSEL kit (Randox Laboratories, UK). The method is based on Paglia and Valentine (1967). Glutathione peroxidase catalyses the oxidation of glutathione by cumene hydroperoxide. In the presence of glutathione reductase and NADPH, oxidized glutathione is immediately converted to its reduced form with a concomitant oxidation of NADPH to NADP+. The decrease in absorbance at 340 nm is measured. One GSH-Px unit is defined as 1 μmol of NADPH consumed per minute and the specific activity was represented as units per milligram protein.
Determination of Protein Carbonyl Content
Protein carbonyl formation was measured spectrophotometrically according to Reznick and Packer (1994). One hundred microliters of plasma was treated with 1 ml of 10 mM 2,4-dinitrophenylhidrazine (DNPH) dissolved in 2.5 N HCl or with 2.5 N HCl (blank) and left in the dark for 90 min. Samples were then precipitated with 500 μl 20% TCA and centrifuged for 5 min at 10,000×g. The pellet was then washed with 1 ml ethanol:ethyl acetate (1:1, V/V) and dissolved in 200 μl 6 M guanidine prepared in 2.5 N HCl at 37°C for 5 min. The difference between the DNPH-treated and HCl-treated samples (blank) was used to calculate the carbonyl content determined at 370 nm. The results were calculated as nanomoles of carbonyl goups per milligram protein.
Determination of Sulfhydryl Content
This assay is based on the reduction of 5,5′-dithio-bis (2-nitrobenzoic acid) (DTNB) by thiols, generating a yellow derivative (TNB) whose absorption is measured spectrophotometrically at 412 nm (Aksenov and Markesbery 2001). Thirty microliters of plasma was incubated with an equal volume of DTNB at room temperature for 30 min in a dark room. The sulfhydryl content is inversely correlated to oxidative damage to proteins. Results were reported in nanomoles of TNB per milligrams of protein.
Catalase Assay (CAT)
CAT activity was assayed by the method of Aebi (1984) measuring the absorbance decrease at 240 nm in a reaction medium containing 20 mM H2O2, 10 mM potassium phosphate buffer, pH 7.0, and 0.1–0.3 mg protein/ml. One unit of the enzyme is defined as 1 μmol of H2O2 consumed per minute and the specific activity was reported in units per milligram of protein.
Superoxide Dismutase (SOD)
SOD activity was determined using the RANSOD kit (Randox, United Kingdom). The method is based on the formation of red formazan from the reaction of 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride and superoxide radical (produced in incubation medium from the xanthine–xanthine oxidase reaction system), which is assayed spectrophotometrically at 505 nm. The inhibition of the produced chromogen is proportional to the activity of the SOD present in the sample. A 50% inhibition is defined as one unit of SOD and the specific activity was represented in units per milligram protein.
Free l-Carnitine Determination
Free LC levels were determined in blood spots by liquid chromatography electrospray tandem mass spectrometry (LC–MS/MS), using the multiple reaction monitoring (MRM) mode (Chace et al. 1997). Results were reported in micromoles/liter.
Selenium Determination
Atomic absorption spectrophotometry with hydride generation was used for plasma Se determination. Results were reported in micrograms/liter.
Protein Determination
Erythrocyte protein concentrations were determined by the method of Lowry et al. (1951), using bovine serum albumin as standard. Plasmatic protein concentrations were determined by the Biuret method using a diagnostic kit (Labtest Diagnóstica, MG, Brazil).
Statistical Analysis
Data were expressed as mean ± standard deviation, and were analyzed using repeated measures analysis of variance. Correlations between variables were calculated using the Spearman correlation coefficient. A P value lower than 0.05 was considered significant. All analyses were performed using the Statistical Package for Social Sciences (SPSS) software in a PC-compatible computer.
Results
Table 1 shows blood Phe, free LC, and Se concentrations in PKU patients before and after supplementation with LC and Se and also in controls. Both LC and selenium levels were significantly reduced in PKU patients before supplementation when compared to controls. It can also be seen in the table that the antioxidant treatment was capable of reverting this deficiency and that Phe values were higher than expected.
The evaluation of oxidative damage to lipids (TBARS) and proteins (sulfhydryl content and carbonyl formation) in PKU patients and controls is displayed in Fig. 1. TBARS were significantly increased in PKU patients before antioxidant supplementation, reflecting an elevated amount of malondialdehyde, an end product of membrane fatty acid peroxidation. The supplementation with Se and LC was capable of reverting this process. In addition, plasma protein sulfhydryl groups were significantly reduced while plasma carbonyl formation was significantly increased in PKU patients without supplementation of antioxidants when compared to controls. The administration of Se and LC corrected the oxidation of sulfhydryl groups but no effect was observed upon carbonyl formation.
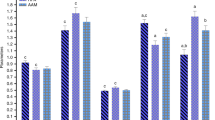
Figure 2 shows the enzymatic antioxidant defenses (SOD, GSH-Px, and CAT) in erythrocytes from PKU patients and controls. It can be seen that GSH-Px and SOD activities were significantly reduced in PKU patients before antioxidant supplementation when compared to controls. The Se and LC treatment increased GSH-Px activity to the levels of controls, but did not alter SOD activity. On the other hand, CAT activity in PKU patients showed no significant difference from the controls.
Enzymatic antioxidant activities in erythrocytes from PKU patients and controls (n = 10). a Superoxide dismutase activity (SOD). b Glutathione peroxidase activity (GSH-Px) c Catalase activity (CAT). Data represent mean ± S.D. * P < 0.05, ** P < 0.01, different from controls (Analysis of variance for repeated measures)
As can be seen in Fig. 3, a significant negative correlation was verified between free l-carnitine levels and lipid peroxidation (r = −0.560; P < 0.01). On the other hand, a highly significant positive correlation between plasma selenium levels and erythrocyte glutathione peroxidase activity had been verified in treated PKU patients (r = 0.945; P < 0.01).
Discussion
PKU is one of the most common inborn errors of metabolism (IEM) and is also considered the first successfully treated IEM (Bickel et al. 1953). Dietary therapy is the main treatment for PKU patients. To maintain plasma levels of phenylalanine within nearly normal levels, the recommended therapy is a low-protein diet poor in animal products including controlled amounts of cereal, fruit, and vegetables, in addition to a protein supplementation with phenylalanine-free synthetic formulas (Start 1998). Untreated PKU patients usually present with severe mental retardation, seizures, microcephaly, spasticity, and developmental problems, whereas a well-controlled diet prevents these clinical manifestations (Huttenlocher 2000).
Although necessary to avoid mental retardation in PKU patients, the low ingestion of proteins with a high biologic value decreases the bioavailability of essential nutrients, including antioxidant compounds (Acosta 1996). Thus, it was reported that PKU patients under restricted diet present low levels of selenium, l-carnitine, and coenzyme Q10 substances that are necessary for a normal antioxidant capacity (Sitta et al. 2009b; Wilke et al. 1992; Artuch et al. 1999, 2001; Schulpis et al. 1990). In this context, recent studies carried out in animal models and also in PKU patients have emphasized the role of oxidative stress in the pathophysiology of PKU, which represent a disequilibrium between tissue antioxidant and reactive species formation in favor of the latter (Sierra et al. 1998; Sirtori et al. 2005; Sitta et al. 2006, 2009a, b; Ercal et al. 2002; Hagen et al. 2002; Martinez-Cruz et al. 2002). In this study, we evaluated the effect of supplementation with a special formula containing l-carnitine and selenium on various oxidative stress parameters in patients with classic PKU under treatment.
Initially, we verified that lipid peroxidation was significantly increased in PKU patients treated with low-protein diet and Phe-free synthetic formula not containing LC and Se. Furthermore, the supplementation of antioxidants LC and Se was capable of correcting this pathological process, reducing malondialdehyde levels, measured by the thiobarbituric acid-reactive species assay. Lipid peroxidation has received special attention since this process may damage cell structures by altering the integrity, fluidity, permeability, and functional loss of biomembranes, modifying low density lipoprotein and generating potentially toxic products (Greenberg et al. 2008) being, therefore, associated to a crescent number of pathological conditions, including neurological diseases (Adibhatla and Hatcher 2010). In this context, lipid oxidative damage has been reported in various neurodegenerative disorders including various inborn errors of metabolism (Deon et al. 2007; Ribas et al. 2010; Barschak et al. 2009; Mc Guire et al. 2009). This is probably because brain is particularly vulnerable to lipid oxidation since it contains high concentrations of polyunsaturated fatty acids and has relatively low antioxidant capacity compared to other organs (Smith et al. 2007; Markesbery and Lovell 2007).
Enhanced lipid peroxidation in PKU patients could be associated to high levels of the toxic metabolites accumulating in the disease, particularly Phe that could lead to an increased production of free radicals. The increase in plasma lipid peroxides could also result from a reduction in enzymatic and non-enzymatic antioxidant defenses, common in patients under restricted diets.
Our findings showing a marked diminution of plasma l-carnitine levels and significantly increased TBARS (lipid oxidation) in PKU patients that were reestablished with l-carnitine supplementation, as well as a significant inverse correlation between l-carnitine and malondialdehyde (MDA) blood levels, indicate that lipid peroxidation in PKU patients was mainly due to shortage of l-carnitine. In particular, it is presumed that l-carnitine has a protective role against ROS by scavenging hydroxyl radicals, formed in the Fenton reaction system (Pietta 2000; Derin et al. 2004). l-Carnitine can also reduce MDA levels by facilitating fatty acid transport thereby lowering the availability of lipids for peroxidation (Rajasekar et al. 2005).
In addition to lipid peroxidation, high levels of protein oxidative damage (high levels of carbonyl formation and reduced levels of sulfhydryl groups) were also observed in treated PKU patients not receiving LC and Se. Oxidative damage to proteins, lipids, or DNA may all be deleterious, however, proteins are the most important targets for ROS and secondary by-products of oxidative stress when these are formed in vivo, as they are the major component of most biological systems and can scavenge 50–75% of reactive radicals such as hydroxyl (Davies et al. 1999). For this reason, in the last decade, there has been a considerable growth in the number of articles reporting increased levels of protein damage in various human diseases often correlating well with the progression of the disease (Dalle-Donne et al. 2003). Oxidative damage to proteins is induced either directly by reactive species or indirectly by reaction of secondary products of oxidative stress. Some ROS-induced protein modifications can result in unfolding or alteration of protein structure, and some are essentially harmless events. However, not all proteins are equally sensitive to oxidative damage, and oxidation susceptibility depends on the structure of the protein (Dalle-Donne et al. 2005).
Furthermore, l-carnitine and selenium treatment reversed oxidation of thiol groups but did not alter the increase of carbonyls in PKU patients. This is probably because thiol groups are easily oxidized and reduced pending on the redox status of the cell. These groups can be oxidized by reactive species, especially at protein cysteine residues that may mediate regulatory processes of protein, potentially leading to alterations of protein function. In this context, mild oxidation of cysteines can generate sulfenic acid, inter- or intra-molecular disulfides, protein-mixed disulfides with low molecular weight thiols, and S-nitrosothiols, all reversible modifications (Woo et al. 2003). On the other hand, carbonylation of proteins, a widespread indicator of severe oxidative damage and disease-derived protein dysfunction is irreversible since carbonyls tend to form high-molecular-weight aggregates that are resistant to degradation and accumulate as damaged or unfolded proteins (Dalle-Donne et al. 2006).
In our study, we found a decrease of the enzymatic antioxidant capacity in erythrocytes of PKU patients not receiving Se and LC, as verified by a decrease of SOD and GSH-Px activities. As regard to GSH-Px activity, it is possible that Se deficiency found in PKU patients could be responsible for the decrease in this activity, which is dependent on this micronutrient. In fact, Se supplementation restored the activity of this enzyme and the concentrations of plasma selenium were strongly correlated with the GSH-Px activity in erythrocytes, which reinforces this presumption and is in agreement with previous studies (Sierra et al. 1998; van Backel et al. 2000). On the other hand, it may be speculated that because of the reduction of these antioxidant activities, these PKU patients might have a lower capacity to scavenge hydrogen peroxide and superoxide reactive species. On the other hand, LC and Se supplementation did not alter SOD activity, which does not depend on Se.
We used erythrocytes in the present study because selenium-dependent glutathione peroxidase is expressed in these cells and they are highly susceptible to oxidative stress because their membranes are rich in polyunsaturated fatty acids and they have a large content of oxygen and iron (Carmagnol et al. 1983). Furthermore, selenium has a higher affinity for erythrocyte glutathione peroxidase than for plasma glutathione peroxidase (Lombeck et al. 1996).
In conclusion, this report corroborates previous studies showing that PKU patients are susceptible to oxidative stress caused by an increase in free radical production and a depletion in antioxidant capacity. More importantly, to the best of our knowledge this is the first report describing that the supplementation of a mixture containing selenium and l-carnitine to PKU patients for a long period was capable of correcting lipid and protein oxidative damage and restoring the GSH-Px activity. For this reason, selenium and l-carnitine supplementation might be an adjuvant therapy for PKU patients consuming artificial low-protein diets.
References
Acosta PB (1996) Nutrition studies in treated infants and children with phenylketonuria: vitamins, minerals, trace elements. Eur J Pediatr 155:136–139
Adibhatla RM, Hatcher JF (2010) Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 12:125–169
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Aksenov MY, Markesbery WR (2001) Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci Lett 302:141–145
Artuch R, Vilaseca MA, Moreno J, Lambruschini N, Cambra FJ, Campistol J (1999) Decreased serum ubiquinone-10 concentration in phenylketonuria. Am J Clin Nutr 70:892–895
Artuch R, Colome C, Vilaseca MA, Sierra C, Cambra FJ, Lambruschini N, Campistol J (2001) Plasma phenylalanine is associated with decreased serum ubiquinone-10 concentrations in phenylketonuria. J Inherit Metab Dis 24:359–366
Artuch R, Colome C, Sierra C, Brandi N, Lambruschini N, Campistol J, Ugarte D, Vilaseca MA (2004) A longitudinal study of antioxidant status in phenylketonuric patients. Clin Biochem 37:198–203
Barschak AG, Sitta A, Deon M, Busanello EN, Coelho DM, Cipriani F, Dutra-Filho CS, Giugliani R, Wanjer M, Vargas CR (2009) Amino acids levels and lipid peroxidation in maple syrup urine disease patients. Clin Biochem 42:462–466
Bickel H, Gerrard J, Hickmans EM (1953) Influence of phenylalanine intake on phenylketonuria. Lancet 265:812–813
Carmagnol F, Sinet PM, Jerome H (1983) Selenium-dependent and nonselenium-dependent glutathione peroxidases in human tissue extracts. Biochim Biophys Acta 759:49–57
Chace DH, Hillman SL, Van Hove JLK, Naylor EW (1997) Rapid diagnosis of MCAD deficiency: quantitative analysis of octanoylcarnitine and other acylcarnitines in newborn blood spots by tandem mass spectrometry. Clin Chem 43:2106–2113
Chaudière J (1994) Some chemical and biochemical constraints of oxidative stress in living cells. In: Riee-Evans CA, Burdon RH (eds) Free radical damage and its control. Elsevier, Amsterdam, pp 25–66
Colome C, Artuch R, Vilaseca MA, Sierra C, Brandi N, Lambruschini N, Cambra FJ, Campistol J (2003) Lipophilic antioxidants in patients with phenylketonuria. Am J Clin Nutr 77:185–188
Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 329:23–38
Dalle-Donne I, Scaloni A, Giustarini D, Cavarra E, Tell G, Lungarella G, Colombo R, Rossi R, Milzani A (2005) Proteins as biomarkers of oxidative/nitrosative stress in diseases: the contribution of redox proteomics. Mass Spectr Rev 24:55–99
Dalle-Donne I, Aldini G, Carini M, Colombo R, Rossi R, Milzani A (2006) Protein carbonylation, cellular dysfunction, and disease progression. J Cell Mol Med 10:389–406
Davies MJ, Fu S, Wang H, Dean RT (1999) Stable markers of oxidant damage to proteins and their application in study of human disease. Free Radic Biol Med 27:1151–1161
Deon M, Sitta A, Barschak AG, Coelho DM, Pigatto M, Schmitt GO, Jardim LB, Giugliani R, Wajner M, Vargas CR (2007) Induction of lipid peroxidation and decrease of antioxidant defenses in symptomatic and asymptomatic patients with X-linked adrenoleukodystrophy. Int J Dev Neurosci 25:441–447
Derin N, Izgut-Uysal VN, Agac A, Aliciguzel Y, Demir N (2004) l-carnitine protects gastric mucosa by decreasing ischemia reperfusion induced lipid peroxidation. J Physiol Pharmacol 55:595–606
Ercal N, Aykin-Burns N, Gurer-Orhan H, Mcdonald JD (2002) Oxidative stress in a phenylketonuria animal model. Free Radic Biol Med 32:906–911
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421
German B (1999) Free radical and antioxidant protocols. Humana Press, Totowa
Greenberg ME, Li XM, Gugiu BG, Gu X, Qin J, Salomon RG, Hazen SL (2008) The lipid Whisker model of the structure of oxidized cell membranes. J Biol Chem 283:2385–2396
Hagen MEK, Pederzolli CD, Sgaravatti AM, Bridi R, Wajner M, Wanmacher CMD, Wyse ATS, Dutra-Filho CS (2002) Experimental hyperphenylalaninemia provokes oxidative stress in rat brain. Biochim Biophys Acta 1586:344–352
Halliwell B, Gutteridge JMC (2007) Oxidative stress: adaptation, damage, repair and death. In: Halliwell B, Gutteridge JMC (eds) Free radicals in biology and medicine. Oxford University Press, Oxford, pp 246–350
Huttenlocher PR (2000) The neuropathology of phenylketonuria: human and animal studies. Eur J Pediatr 159:102–106
Lombeck I, Jochum F, Terwolbeck K (1996) Selenium status in infants and children with phenylketonuria and in maternal phenylketonuria. Eur J Pediatr 155:140–144
Lowry OH, Rosebrough NJ, Lewis-Farr A, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Markesbery WR, Lovell MA (2007) Damage to lipids, proteins, DNA, and RNA in mild cognitive impairment. Arch Neurol 64:954–956
Martinez-Cruz F, Pozo D, Osuna C, Espinar A, Marchante C, Guerrero JM (2002) Oxidative stress induced by phenylketonuria in the rat: prevent by melatonin, vitamin E and vitamin C. J Neurosci Res 69:550–558
Mc Guire PJ, Parikh A, Diaz GA (2009) Profiling of oxidative stress in patients with inborn errors of metabolism. Mol Genet Metab 98:173–180
Paglia DE, Valentine WN (1967) Glutathione peroxidase. J Lab Clin Med 70:158
Pietta PG (2000) Flavonoids as antioxidants. J Nat Prod 63:1035–1042
Rajasekar P, Kaviarasan S, Anuradha CV (2005) l-carnitine administration prevents oxidative stress in high fructose-fed insulin resistant rats. Diab Croat 34:21–28
Reznick AZ, Packer L (1994) Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 233:357–363
Ribas GS, Manfredini V, de Mari JF, Wayhs CY, Vanzin CS, Biancini GB, Sitta A, Deon M, Wajner M, Vargas CR (2010) Reduction of lipid and protein damage in patients with disorders of propionate metabolism under treatment: a possible protective role of l-carnitine supplementation. Int J Dev Neurosci 28:127–132
Schulpis KH, Nounopoulos C, Scarpalezou A, Bouloukos A, Missiou-Tsagarakis S (1990) Serum carnitine level in phenylketonuric children under dietary control in Greece. Acta Paediatr Scand 79:930–934
Schulpis KH, Tsakiris S, Traeger-Synodinos J, Papassotiriou I (2005) Low total antioxidant status is implicated with high 8-hydroxy-2-deoxyguanosine serum concentrations in phenylketonuria. Clin Biochem 38:239–242
Scriver CR, Kaufman S (2001) Hyperphenylalaninemia: phenylalanine hydroxylase deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease. McGraw-Hill, New York, pp 1667–1724
Sierra C, Vilaseca MA, Moyano D, Brandi N, Campistol J, Lambruschini N, Cambra FJ, Deulofeu R, Mira A (1998) Antioxidant status in hyperphenylalaninemia. Clin Chim Acta 276:1–9
Sirtori LR, Dutra-Filho CS, Fitarelli D, Sitta A, Haeser A, Barschak AG, Wajner M, Coelho DM, Llesuy S, Belló-Klein A, Giugliani R, Deon M, Vargas CR (2005) Oxidative stress in patients with phenylketonuria. Biochim Biophys Acta 1740:68–73
Sitta A, Barschak AG, Deon M, Terroso T, Pires R, Giugliani R, Dutra-Filho CS, Wajner M, Vargas CR (2006) Investigation of oxidative stress parameters in treated phenylketonuric patients. Metab Brain Dis 21:287–296
Sitta A, Barschak AG, Deon M, Barden AT, Biancini GB, Vargas PR, de Souza CF, Netto C, Wajner M, Vargas CR (2009a) Effect of short- and long-term exposition to high phenylalanine blood levels on oxidative damage in phenylketonuric patients. Int J Dev Neurosci 27:243–247
Sitta A, Barschak AG, Deon M, De Mari JF, Barden AB, Vanzin C, Biancini GB, Schwartz IVD, Wajner M, Vargas CR (2009b) l-Carnitine blood levels and oxidative stress in treated phenylketonuric patients. Cell Mol Nurobiol 29:211–218
Smith DG, Cappai R, Barnham KJ (2007) The redox chemistry of the Alzheimer’s disease amyloid-b peptide. Biochim Biophys Acta 1768:1976–1990
Start K (1998) Treating phenylketonuria by a phenylalanine-free diet. Prof Care Mother Child 8:109–110
van Backel MME, Printzen G, Wermuth B, Wiesmann UN (2000) Antioxidant and thyroid hormone status in selenium-deficient phenylketonuric and hyperphenylalaninemic patients. Am J Clin Nutr 72:976–981
Van Spronsen FJ, Smit PG, Koch R (2001) Phenylketonuria: tyrosine beyond the phenylalanine-restricted diet. J Inherit Metab Dis 24:1–4
Wilke BC, Vidailhet M, Favier A, Guillemin C, Ducros V, Arnaud J, Richard MJ (1992) Selenium, glutathione peroxidase (GSH-Px) and lipid peroxidation products before and after selenium supplementation. Clin Chim Acta 207:137–142
Woo HA, Chae HZ, Hwang SC, Yang KS, Kang SW, Kim K, Rhee SG (2003) Reversing the inactivation of peroxiredoxin caused by cysteine sulfinic acid formation. Science 300:53–656
Acknowledgments
This work was supported in part by grants from FAPERGS, CNPq, and FIPE/HCPA-Brazil.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sitta, A., Vanzin, C.S., Biancini, G.B. et al. Evidence that l-Carnitine and Selenium Supplementation Reduces Oxidative Stress in Phenylketonuric Patients. Cell Mol Neurobiol 31, 429–436 (2011). https://doi.org/10.1007/s10571-010-9636-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-010-9636-3