Abstract
The serum/glucose deprivation (SGD)-induced cell death in cultured PC12 cells represents a useful in vitro model for the study of brain ischemia and neurodegenerative disorders. Nigella sativa L. (family Ranunculaceae) and its active component thymoquinone (TQ) has been known as a source of antioxidants. In the present study, the protective effects of N. sativa and TQ on cell viability and reactive oxygen species (ROS) production in cultured PC12 cells were investigated under SGD conditions. PC12 cells were cultured in DMEM medium containing 10% (v/v) fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin. Cells were seeded overnight and then deprived of serum/glucose for 6 and 18 h. Cells were pretreated with different concentrations of N. sativa extract (15.62–250 μg/ml) and TQ (1.17–150 μM) for 2 h. Cell viability was quantitated by MTT assay. Intracellular ROS production was measured by flow cytometry using 2′,7′-dichlorofluorescin diacetate (DCF-DA) as a probe. SGD induced significant cells toxicity after 6, 18, or 24 h (P < 0.001). Pretreatment with N. sativa (15.62–250 μg/ml) and TQ (1.17–37.5 μM) reduced SGD-induced cytotoxicity in PC12 cells after 6 and 18 h. A significant increase in intracellular ROS production was seen following SGD (P < 0.001). N. sativa (250 μg/ml, P < 0.01) and TQ (2.34, 4.68, 9.37 μM, P < 0.01) pretreatment reversed the increased ROS production following ischemic insult. The experimental results suggest that N. sativa extract and TQ protects the PC12 cells against SGD-induced cytotoxicity via antioxidant mechanisms. Our findings might raise the possibility of potential therapeutic application of N. sativa extract and TQ for managing cerebral ischemic and neurodegenerative disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite remarkable advances in the prevention and treatment of cerebral ischemia or stroke, it still remains a leading cause of death and disability in the aged population (Amantea et al. 2009).
Reactive oxygen species (ROS) is presumably involved in ischemia-induced neuronal cell damage as well as neurodegenerative disorders (Amantea et al. 2009; Behl and Moosmann 2002). Serum/glucose deprivation (SGD) has served as an excellent in vitro model for the understanding of the molecular mechanisms of neuronal damage during brain ischemia and for the development of neuroprotective drugs against ischemia-induced brain injury (Chu et al. 2008; Hillion et al. 2005). A well-defined cell system for in vitro studies of SGD-evoked neuronal injury can be provided by the rat pheochromocytoma (PC12) cell line (Woronowicz et al. 2007).
A considerable promising approach to neuroprotection is the use of antioxidants, which suppress the effects of ROS (Ochiaia et al. 2004).
Nigella sativa L. (family Ranunculaceae), commonly known as black seed or black cumin, is an annual plant that has been traditionally used as a natural remedy for a number of illnesses and conditions such as asthma, hypertension, diabetes, inflammation, cough, bronchitis, headache, eczema, fever, dizziness, and influenza (Ali and Blunden 2003). It has been shown that N. sativa, as well as thymoquinone (TQ), as its active constituent, inhibit non-enzymatic lipid peroxidation in liposomes and have appreciable free radical scavenging properties (Houghton et al. 1995).
Nigella sativa extracts have some protective effects against muscle tissue injury caused by lower limb ischemia–reperfusion. It has also been shown that N. sativa and TQ may be useful agents for the prevention of renal ischemia–reperfusion (IR)-induced oxidative damage in rats (Hosseinzadeh et al. 2007; Hosseinzadeh and Montahaei 2007). Generation of free radicals may be, at least partially, the basis of many human diseases and conditions (Sun and Chen 1998; Love 1999). Therefore, the antioxidant action of N. sativa may explain its claimed usefulness in folk medicine. This antioxidant property would explain its action against CCl4 hepatotoxicity (Nagi et al. 1999), liver fibrosis and cirrhosis (Turkdogan et al. 2000), and hepatic damage induced by Schistosoma mansoni infection (Mahmoud et al. 2002; Ali and Blunden 2003).
In order to have a new insight into the medicinal value of N. sativa, and TQ the present study was carried out to investigate whether N. sativa, and TQ was capable of protecting PC12 cells against SGD-induced cytotoxicity.
Materials and Methods
Reagents
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium (MTT), Thymoquinone, 2′,7′-dichlorofluorescin diacetate (DCFH-DA), were purchased from Sigma (St Louis, MO, USA). Other materials mainly included glucose-high DMEM, glucose-free DMEM, and FCS were purchased from Gibco. N. sativa seeds were authenticated by Pharmacognosy Department, School of Pharmacy, MUMS (Mashhad, I.R. Iran).
Preparation of N. sativa Extract
Powdered seeds (100 g) of N. sativa were extracted in a Soxhlet extractor with ethanol (70%). The resulting extract was concentrated under reduced pressure and kept at 4°C until use.
Nigella sativa extract and TQ were dissolved in dimethyl sulfoxide (DMSO) (50 mg/ml and 60 mM, respectively) and kept at −80°C.
Cell Culture
PC12 cells were obtained from Pasteur Institute (Tehran, Iran). Cells were maintained at 37°C in a humidified atmosphere (90%) containing 5% CO2. Cells were cultured in high-glucose Dulbecco’s modified Eagle’s medium (DMEM) (4.5 g/l) with 10% (v/v) fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin.
For the experiments, PC12 cells were seeded overnight and then were subjected to SGD for 6, 18, and 24 h by replacing the culture medium with the glucose-free DMEM supplemented with 100 U/ml penicillin and 100 U/ml streptomycin. PC-12 cells, were then pretreated (2 h) with N. sativa extract (15.62–250 μg/ml) and TQ (1.17–150 μM) and subjected to SDG for 6 and 18 h. For MTT assay, cells were seeded at 5000/well onto 96-well culture plates. For assay of ROS production, cells were seeded at 100,000/well onto a 24-well plate. For each concentration and time course study, there was a control sample which remained untreated and received the equal volume of medium.
Cell Viability
The cell viability was determined using a modified 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium (MTT) assay (Mosmann 1983). Briefly, they were seeded (104 cell/well) onto flat-bottomed 96-well culture plates. After removing the medium, MTT solution (5 mg/ml in PBS) was added for 1.5 h resulting formazan which was solubilized with DMSO (100 μl). The absorption was measured at 570 nm (620 nm as a reference).
Measurement of Intracellular Reactive Oxygen Species
The determination of intracellular reactive oxygen species (ROS) levels was accomplished as described previously with minor modifications (Wang and Joseph 1999; Mosmann 1983). In brief, at 18 h after ischemic insult, the PC12 cells were incubated with 5 μM DCFH-DA at 37°C for 30 min in the dark. The fluorescence of 2′,7′-dichlorofluorescin (DCF), the oxidation product of DCFH-DA, was excited at 480 nm and detected at 530 nm by flow cytometry. Temperature was maintained at 37°C throughout the experiment.
Quantification of TQ in N. sativa Seed Extract
TQ quantification was carried out by HPLC on a reversed-phase C18 analytical column (250 × 4.6 mm, 4.6 mm particle size), using an isocratic mobile phase of water: methanol: 2-propanol (50:45:5% v/v) at a flow rate of 1 ml/min. UV monitoring was carried out at 254 nm. The chromatograms of a sample of N. sativa seed extract and standard TQ were showed in Figs. 3 and 4.
Statistical Analysis
All results were expressed as mean ± SEM. Statistical differences between groups were analyzed by one way analysis of variance with subsequent Tukey’s tests. A probability level of P < 0.05 was considered significant statistically.
Results
N. Sativa or TQ-Protected PC12 Cells Against Serum/Glucose Deprivation-Induced Cytotoxicity
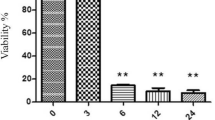
The result showed SGD could decrease cell viability of cultured PC12 cells in a time-dependent manner compared to control (Fig. 1). Exposure to SGD for 6, 18, and 24 h decreased cell viability to 41.7 ± 2.37, 33.5 ± 1.53, and 22.5 ± 4.85%, respectively (P < 0.001). 6 and 18 h SGD were then selected to induce PC12 cell injury in all subsequent experiments.
Effect of serum/glucose deprivation on PC12 cell viability. Cell viability was quantitated by MTT assay. PC12 cells exposed to serum/glucose deprivation for 6, 18, and 24 exhibited toxicity, reaching a maximal effect after 24 h. Results are mean ± SEM (n = 9). The experiments were done as duplicate. *** P < 0.001 SGD versus control
Pretreatment with N. sativa extract (15.62–250 μg/ml) and TQ (1.17–37.5 μM) at different concentrations significantly attenuated SGD-induced toxicity in PC12 cells. The attenuating effect of N. sativa on the survival of cultured PC12 cells displayed a dose dependent pattern (Fig. 2). There was not any toxic effects when PC12 cells were incubated with N. sativa and TQ alone (data were not shown).
Protective effects of N. sativa and TQ on serum/glucose deprivation-induced cytotoxicity in PC12 cells. PC12 cells were pretreated with N. sativa (15.62–250 μg/ml) or TQ (1.17–150 μM) for 2 h, and then were exposed to serum/glucose deprivation for an additional 18 h with respective original concentrations of TQ. The incubation in the high-glucose medium during the whole treatment period served as control group, and the treatment only with serum/glucose free medium for 18 h served as serum/glucose deprivation alone group. The cell viability was expressed as the percent (%) of the control value by using MTT assay. The data presented are means ± SEM of three independent experiments (n = 3). Results are mean ± SEM (n = 9). *** P < 0.001 SGD versus control. # P < 0.05, ## P < 0.01, and ### P < 0.001 compared to SGD
As shown in Fig. 2, the less toxicity was seen following pretreatment of PC12 cells with 250 μg/ml of N. sativa and 4.68 μM of TQ.
Effects of N. sativa and TQ on Serum/Glucose Deprivation-Induced Elevation of ROS Production in PC12 Cells
Molecular probe DCFH-DA was used to monitor alterations in intracellular ROS levels with flow cytometry. ROS production was measured following the exposure of PC12 cells to SGD for 18 h with or without the pretreatment with N. sativa (250 μg/ml) and TQ (2.34, 4.68, 9.37 μM). As shown in Fig. 2 SGD for 18 h could significantly increase the number of DCF-positive cells illustrating an elevation of ROS production compared to control. On the other hand, pretreatment with N. Sativa (250 μg/ml) and TQ (2.34, 4.68, 9.37 μM) resulted in a significant attenuation of ROS production following SGD (Fig. 3).
Flow cytometry with DCFH-DA staining for measuring ROS production for cultured PC12 cells in control group (a), serum/glucose deprivation alone group (b), pretreatment with 250 μg/ml N. sativa plus exposure to serum/glucose deprivation (c), pretreatment with TQ (2.34, 4.68, 9.37 μM) plus exposure to serum/glucose deprivation (d–f), respectively. In the groups (c–f), the PC12 cells were exposure to serum/glucose deprivation in the presence of different drugs for only 18 h. The ROS production was assessed according to changes in the fluorescence intensity of DCF, the oxidation product of DCFH-DA. The values represent 5 independent experiments. *** P < 0.001 SGD versus control. # P < 0.05 and ## P < 0.01 compared to SGD
TQ Quantification
Using the calibration curve, the quantification of TQ in a sample of commercial N. sativa seeds oil was achieved and was about 0.058% w/w (Fig. 4).
Discussion
Dysfunction of oxidative metabolism and depression of energy metabolism occurs in brain ischemia are linked to neuron degeneration (Johnson et al. 1995). Oxidative stress is a condition induced by oxygen and oxygen-derived free radicals commonly known as ROS.
There are a number of processes that have been implicated in the pathogenesis of cell injury, mainly include the production of toxic lipid metabolites, proteases and endonucleases, and generation of free radicals (ROS) that can cause oxidative damage to cellular macromolecules including lipids, proteins, and nucleic acids. Lipid peroxidation makes cell membranes particularly vulnerable to the large numbers of polyunsaturated fatty acids (PUFAs). The oxidation of PUFAs increases hydrophilic nature of the membrane resulting alteration in fluidity and permeability. The inhibition of the function of membrane bound receptors and enzymes also occurs (Fisher 2001; Love 1999). The basis of many neurological and neurodegenerative disorders such as ischemia–reperfusion, seizure, Parkinson and Alzheimer’s disease, at least partially, is the generation of free radicals. It has been well-documented that antioxidant therapy protect against CNS injuries (Gilgun-Sherki et al. 2002; Love 1999; Sun and Chen 1998).
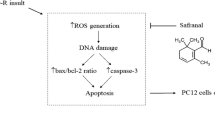
In the present study, we used the SGD-induced insult in PC12 cells as a model of the pathological process of cerebral ischemia (Hillion et al. 2005) in an attempt to explore for naturally occurring drugs with neuroprotective effects. The results obtained in the present investigation suggest that N. sativa and TQ, its active constituent, have an overall protective effect against SGD-induced cell death via antioxidant mechanisms.
Nigella sativa displays a broad spectrum of pharmacological properties. N. sativa and its main active component, TQ have been shown to possess antioxidant activities (Houghton et al. 1995; Daba and Abdel-Rahman 1998; Nagi and Mansour 2000; Burits and Bucar 2000). Recently, the compound was reported to be capable of protecting gentamicin-induced nephrotoxicity in rats (Sayed-Ahmed and Nagi 2007) and the neuroprotective effect of N. sativa oil and its fractions against beta amyloid (Aβ)-induced cell death in primary rat cerebellar granule neurons was investigated (Ismail et al. 2008).
MTT assay confirmed that the the cell viability was significantly decreased following SGD, while N. sativa and TQ pretreatment reserved these alterations.
The increase in ROS production may also be responsible for SGD-induced cytotoxicity (Liu et al. 2003). In agreement with these findings, we found that intracellular ROS production was significantly increased following SGD. The ROS are found to mediate much of the damage that occurs after transient brain ischemia and in the penumbral region of infarcts caused by permanent ischemia (Love 1999), and are considered possible candidates for elucidating the pathogenesis of acute CNS injury, becoming an important therapeutic target (Bromont et al. 1989; Hall and Braughler 1989; Oliver et al. 1990). In the present study, pretreatment with N. sativa and TQ shown to effectively block SGD-induced ROS production, indicating that an inhibition of intracellular ROS generation might be involved in the neuroprotective effects of N. sativa and TQ thereby confirming their antioxidant role in ischemic cell injury.
It has been shown that non-enzymatic lipid peroxidation in liposomes can be inhibited with both the N. sativa and TQ (Houghton et al. 1995). N. sativa as well as TQ, have appreciable antioxidant and free radical scavenger properties. The antioxidant action of N. sativa and/or TQ may explain the protective effect of these agents against various hepatotoxic and nephrotoxic models in vivo and in vitro as well as liver fibrosis and cirrhosis (Badary et al. 1997; Daba and Abdel-Rahman 1998; El-Dakhakhny et al. 2000; El Daly 1998; Mansour et al. 2001; Nagi et al. 1999; Turkdogan et al. 2000).
The protective effect of TQ against carbon tetrachloride-induced hepatotoxicity might be related to the ability of this agent to inhibit lipid peroxidation (Al Gharably et al. 1997). Decreased tissue MDA and protein carbonyl levels and prevented inhibition of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) enzyme activities following experimental spinal cord injury in rats by N. sativa treatment are also reported. (Kanter et al. 2006).
Recently, El-Abhar et al. (2003) showed that N. sativa and TQ have a marked protective action against IR-induced gastric mucosal lesions. It has been reported that the aqueous extract of N. sativa seeds exhibits an inhibitory effect on nitric oxide production by murine macrophages and the active component(s) is/are non-protein in nature (Mahmood et al. 2003).
The strong cytotoxic, chemopreventive, anticarcinogenic, and antimutagenic activity and reduction in the toxic effects of standard antineoplastic agents that seen with N. sativa were accompanied by restoration of the concentrations of reduced glutathione (GSH), lipid peroxides and the activities of some enzymes (Ali and Blunden 2003).
The anti-inflammatory and analgesic actions N. sativa and TQ (Khanna et al. 1993; Mutabagani and El Mahdi 1997; Abdel-Fattah et al. 2000; Al-ghamdi et al. 2001) have also may be related to inhibition of thromboxane B2 and leucotrienes B4 generation (by inhibiting cyclooxygenase and 5-lipooxygenase, respectively), and membrane lipid peroxidation (Houghton et al. 1995).
We found that N. sativa contains 0.05% w/w TQ. The different compounds in the extract were found to act in a synergistic manner (i.e., more than the mere summation of the actions of the individual compounds). This stresses the importance of using the whole the crude extract (or oil) of the seeds in pharmacological studies. This property has been noted before with a number of spices (Beckstrom-Sternberg and Duke 1994).
To conclude, the present study indicates that N. sativa and TQ pretreatment ameliorates SGD-induced cell toxicity in cultured PC12 cells, and its protective effects might be mediated by inhibition of the intracellular ROS production. This study on the neuroprotective effects of N. sativa and TQ may suggest the possible application of N. sativa and TQ in clinical setting to prevent and treat the common neurological insults.
References
Abdel Fattah AFM, Matsumoto K, Watanabe H (2000) Antinociceptive effects of Nigella sativa oil and its major component, thymoquinone, in mice. Eur J Pharmacol 400:89–97
Al Gharably NM, Badary OA, Nagi MN, Al Sawaf HA, Al Rikabi AC, Al Bekairi AM (1997) Protective effect of thymoquinone against carbon tetrachloride-induced hepatotoxicity in mice. Res Commun Pharmacol Toxicol 2:41–50
Al-Ghamdi MS (2001) The anti-inflammatory, analgesic and antipyretic activity of Nigella sativa. J Ethnopharmacol 76:45–48
Ali BH, Blunden G (2003) Pharmacological and toxicological properties of Nigella sativa. Phytother Res 17:299–305
Amantea D, Marrone MC, Nisticò R, Federici M, Bagetta G, Bernardi G, Mercuri NB (2009) Chapter 25 Oxidative stress in stroke pathophysiology. Validation of hydrogen peroxide metabolism as a pharmacological target to afford neuroprotection. Int Rev Neurobiol 85:363–374
Badary OA, Nagi MN, Al Shabanah OA, Al Shawaf HA, Al Sohaibani MO, Al Bekairi AM (1997) Thymoquinone ameliorates the nephrotoxicity induced by cisplatin in rodents and potentiates its antitumor activity. Can J Physiol Pharmacol 75:1356–1361
Beckstrom-Sternberg SM, Duke JA (1994) Potential for synergistic action of phytochemicals in spices. In: Charalambous G (ed) Spices herbs and edible fungi. Elsevier Science, Oxford, pp 201–223
Behl C, Moosmann B (2002) Antioxidant neuroprotection in Alzheimer’s disease as preventive and therapeutic approach. Free Radic Biol Med 33:182–191
Bromont C, Marie C, Bralet J (1989) Increased lipid peroxidation in vulnerable brain regions after transient forebrain ischemia in rats. Stroke 20:918–924
Burits M, Bucar F (2000) Antioxidant activity of N. sativa essential oil. Phytother Res 14:323–328
Chu LF, Wang WT, Ghanta VK, Lin CH, Chiang YY, Hsueh CM (2008) Ischemic brain cell-derived conditioned medium protects astrocytes against ischemia through GDNF/ERK/NF-kB signaling pathway. Brain Res 1239(C):24–35
Daba MH, Abdel Rahman MS (1998) Hepatoprotective activity of TQ in isolated rat hepatocytes. Toxicol Lett 16:23–29
El Dakhakhny M, Mady NI, Halim MA (2000) Nigella sativa L. protects against induced hepatotoxicity and improves serum lipid profile in rats. Arzneimittelforsch 50:832–836
El Daly ES (1998) Protective effect of cysteine and vitamin E, Crocus sativus and Nigella sativa extracts on cisplatin-induced toxicity in rats. J Pharm Bel 53:87–95
El-Abhar HS, Abdallah M, Saleh S (2003) Gastroprotective activity of Nigella sativa oil and its constituent, thymoquinone, against gastric mucosal injury induced by ischaemia-reperfusion in rats. J Ethnopharmacol 84:251–258
Fisher M (2001) Stroke therapy, 2nd edn. Butter worth-Heinemann, Boston, pp 25–50
Gilgun-Sherki Y, Rosenbaum Z, Melamed E, Offen D (2002) Antioxidant therapy in acute central nervous system injury: current state. Pharm Rev 54:271–284
Hall ED, Braughler JM (1989) Central nervous system trauma and stroke. II. Physiological and pharmacological evidence for involvement of oxygen radicals and lipid peroxidation. Free Radic Biol Med 6:303–313
Hillion JA, Takahashi K, Maric D, Ruetzler C, Barker JL, Hallenbeck JM (2005) Development of an ischemic tolerance model in a PC12 cell line. J Cereb Blood Flow Metab 25:154–162
Hosseinzadeh H, Montahaei R (2007) Protective effect of N. sativa L. extracts and thymoquinone, its active constituent, on renal ischemia reperfusion induced oxidative damage in rats. Pharmacologyonline 1:176–189
Hosseinzadeh H, Moghim FF, Mansouri SMT (2007) Effect of N. sativa seed extracts on ischemia reperfusion in rat skeletal muscle. Pharmacologyonline 2:326–335
Houghton PJ, Zarka R, Heras B, Hoult RS (1995) Fixed oil of N. sativa and derived TQ inhibit eicosanoid generation in leucocytes and membrane lipid peroxidation. Planta Med 61:33–36
Ismail N, Ismail M, Latiff LA, Mazlan M, Mariod AA (2008) Black cumin seed (N. sativa linn.) oil and its fractions protect against beta amyloid peptide-induced toxicity in primary cerebellar granule neurons. J Food Lipids 15(4):519–533
Johnson EM Jr, Greenlund LJ, Akins PT, Hsu CY (1995) Neuronal apoptosis: current understanding of molecular mechanisms and potential role in ischemic brain injury. J Neurotrauma 12:843–852
Kanter M, Coskun O, Kalayci M, Buyukbas S, Cagavi F (2006) Neuroprotective effects of Nigella sativa on experimental spinal cord injury in rats. Hum Exp Toxicol 25:127–133
Khanna T, Zaidi FA, Dandiya PC (1993) CNS and analgesic studies on Nigella sativa. Fitoterapia 5:407–410
Liu Y, Song XD, Liu W, Zhang TY, Zuo J (2003) Glucose deprivation induces mitochondrial dysfunction and oxidative stress in PC12 cell line. J Cell Mol Med 7:49–56
Love S (1999) Oxidative stress in brain ischemia. Brain Pathol 9:119–131
Mahmood MS, Gilani AH, Khwaja A, Rashid A, Ashfaq MK (2003) The in vitro effect of aqueous extract of Nigella sativa seeds on nitric oxide production. Phytother Res 17:921–924
Mahmoud MR, El-Abhar HS, Saleh S (2002) The effects of N. sativa oil against the liver damage induced by Schistosoma mansoni in mice. J Ethnopharmacol 79:1–11
Mansour MA, Ginawi OT, El Hadiyah T, El Khatib AS, Al Shabanah OA, Al Sawaf HA (2001) Effects of volatile oil constituents of Nigella sativa on carbon tetrachloride induced hepatotoxicity in mice: evidence for antioxidant effects of thymoquinone. Res Commun Mol Pathol Pharmacol 110:239–251
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 16:55–63
Mutabagani A, El Mahdi SM (1997) A study of the anti-inflammatory activity of Nigella sativa L. and thymoquinone in rats. Saudi Pharmacol J 5:110–113
Nagi MN, Mansour MA (2000) Protective effect of TQ against doxorubicin-induced cardiotoxicity in rats: a possible mechanism of protection. Pharmacol Res 41:283–289
Nagi MN, Alam K, Badary OA (1999) TQ protects against carbon tetrachloride hepatotoxicity in mice via an antioxidant mechanism. Biochem Mol Biol Int 47:143–159
Ochiaia T, Ohnoa S, Soedaa S, Tanakab H, Shoyamab Y, Shimenoa H (2004) Crocin prevents the death of rat pheochromyctoma (PC12) cells by its antioxidant effects stronger than those of a-tocopherol. Neurosci Lett 362:61–64
Oliver CN, Starke-Reed PE, Stadtman ER, Liu GJ, Carney JM, Floyd RA (1990) Oxidative damage to brain proteins, loss of glutamine synthetase activity, and production of free radicals during ischemia/reperfusion-induced injury to gerbil brain. Proc Natl Acad Sci USA 87:5144–5147
Sayed-Ahmed MM, Nagi MN (2007) Thymoquinone supplementation prevents the development of gentamicin-induced acute renal toxicity in rats. Clin Exp Pharmacol Physiol 34:399–405
Sun AY, Chen YM (1998) Oxidative stress and neurodegenerative disorders. J Biomed Sci 5:401–414
Turkdogan MK, Agaoglu Z, Yener Z, Sekeroglu R, Akkan HA, Avci ME (2000) The role of antioxidant vitamins (C and E), selenium and N. sativa in the prevention of liver fibrosis and cirrhosis in rabbits, new hopes. Dtscch Tierarzt Wschr 108:71–73
Wang H, Joseph JA (1999) Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med 27:612–616
Woronowicz A, Amith SR, Davis VW, Jayanth P, De Vusser K, Laroy W, Contreras R, Meakin SO, Szewczuk MR (2007) Trypanosome trans-sialidase mediates neuroprotection against oxidative stress, serum/glucose deprivation, and hypoxia-induced neurite retraction in Trk-expressing PC12 cells. Glycobiology 17(7):725–734
Acknowledgments
The authors would like to thank Research Affairs of Mashhad University of Medical Sciences for financially supporting this work and Miss Aghaee for her assistance in preparation of N. sativa extract. We are also grateful of Dr. H. Nasirli for her assistance in flow cytometry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mousavi, S.H., Tayarani-Najaran, Z., Asghari, M. et al. Protective Effect of Nigella sativa Extract and Thymoquinone on Serum/Glucose Deprivation-Induced PC12 Cells Death. Cell Mol Neurobiol 30, 591–598 (2010). https://doi.org/10.1007/s10571-009-9484-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-009-9484-1