Abstract
Inappropriate vascular remodeling is thought to be the main cause of restenosis following angioplasty. Migration of vascular smooth muscle cells (VSMC) into lumina, which is promoted by degradation of the extracellular matrix by matrix metalloproteinases (MMPs) plays a causal role in pathological vascular remodeling. The aim of the present research is to explore the effects of a novel cytokine, IL-17, on migration of VSMC and MMP-9 secretion. Carotid artery VSMC was isolated from Sprague–Dawley rats. Expression of MMP-9 and cell migration induced by IL-17 and its related signal pathway were detected. The results showed that IL-17-induced migration of VSMC in an MMP-9-dependent manner. IL-17-induced MMP-9 expression was via p38 MAPK and ERK1/2 dependent NF-κB and AP-1 activation. The present results demonstrated that IL-17 may play a role in vascular remodeling and targeting IL-17 or its specific downstream mediators is a potentially novel therapeutic pathway for attenuating the post-angioplastic restenosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vascular remodeling, which is defined as a response to a variety of stimuli including hemodynamic changes, inflammation, and injury, leads to change in the size and/or composition of an adult blood vessel. However, inappropriate remodeling is currently thought to be the main cause of most prevalent vascular pathologies of arteries, such as atherosclerosis and restenosis following angioplasty (Gibbons and Dzau 1994).Smooth muscle cell (SMC) migration plays a causal role in pathological remodeling of the vessel walls (Ross 1999a, b; Schwartz 1997). Migration of SMC and neointimal hyperplasia are promoted by degradation of the extracellular matrix (ECM) by matrix metalloproteinases (MMPs), which are major components of the enzyme cascade responsible for degradation of the ECM and basement membrane proteins.
MMP-9, which belongs to the collagenases according to the substrate specificity, is expressed in many cell types including SMC, and induced by proinflammatory cytokines, such as IL-1β, IL-18 and IL-17 (Cortez et al. 2007; Chandrasekar et al. 2006; Liang et al. 2007). IL-17 is a member of a novel group of proinflammatory cytokines which share little homology with other interleukins. Many cell types in the body express its receptors (IL-17R) and are therefore targets of IL-17 (Yao et al. 1995). Recently, it was found that IL-17 was involved in myocardial remodeling and atherosclerosis (Cortez et al. 2007; Patel et al. 2007). However, few studies have been done to observe the effects of IL-17 in vascular remodeling after balloon injury of carotid artery. Since migration of SMC plays a key role in the vascular remodeling, we target effects of IL-17 on SMC migration in the present research.
Materials and Methods
Recombinant human IL-17 (rhIL-17), anti-IL-1β, IL-6, and TNF-α neutralizing antibodies, IL-1β,IL-6 and TNF-α ELISA kit, and normal goat IgG were purchased from R & D Systems. Functional grade purified anti-human IL-17 antibodies and normal mouse IgG antibodies were obtained from eBioscience (San Diego, CA). Anti-MMP-9 monoclonal antibody was purchased from NeoMarkers (Fremont, CA). Cycloheximide (CHX), a protein synthesis inhibitor (10 μg/ml in DMSO) and Actinomycin D (ActD), an RNA synthesis inhibitor (2.5 μg/ml in DMSO) were from Sigma; GM-6001, a nonspecific MMP inhibitor with potent inhibitory activity against collagenase, gelatinases, and stromelysin (10 μM in DMSO), was from Biomol. MEK1 inhibitor (PD98059) was from Cell Signaling and p38 MAPK inhibitor (SB203580) was from Promega.
Isolation, Culture and Characterization of CAVSMC
The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996). Isolated CAVSMC from the common carotid arteries of Sprague–Dawley rats were prepared using a method described previously (Mnjoyan et al. 2008). Isolated CAVSMC was seeded on a chamber slide, allowed to differentiate in differentiation media (0.4% FCS and 50 μg/ml heparin in DMEM) for 10 days, and stained with anti-α-actin antibody. Nuclei were counterstained with 4, 6-diamidino-2-phenylindole (DAPI). Only those batches of CAVSMC that showed a 90% or higher α-actin positivity were used for further analyses.
Detection of IL-17RA Expression in CAVSMC
Expression of IL-17RA in CAVSMC was determined by reverse trancriptional PCR (RT-PCR). Total RNA was isolated with Trizol (Invitrogen) and cDNA was synthesized. The primers used were as follows: Sense: 5′-CACGTTGAGTGGACCTTG-3′; Antisense: 5′-CCAGCAGGATCTGGTA-3′. (Genebank no. NM 001107883, product length: 421 bp).
Cell Migration Analysis
CAVSMC migration was detected with the commercial kit as per the manufacturer’s directions as reported previously (Chandrasekar et al. 2006). Cultured CAVSMC treated with trypsin were suspended in modified DMEM with 1% bovine serum albumin, and concentration of cells was adjusted to 4.0 × 105/ml. Cells were layered on the coated insert filters and stimulated with IL-17 (20 ng/ml). Same concentration of IL-17 was added to the lower chamber. After incubation at 37°C for 24 h, we washed the membrane with PBS and removed the non-invading cells on the upper surface with cotton swabs. The Discovery Labware BD BioCoat™ invasion chambers (catalog no. 354481, BD Biosciences) and 8.0 μm pore polyethylene terephthalate membranes with a thin layer of Matrigel basement membrane matrix were applied. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay was used to detect cells migrating to the lower surface of the membrane at A 540 nm. A broad-spectrum MMP inhibitor GM-6001 (10 μM) (Cortez et al. 2007) was added to the culture 15 min prior to stimulation with IL-17 to determine the role of MMPs in IL-17-mediated cell migration.
RNA Interference
siRNA targeting rat MMP-2 (NM 031054), MMP-9 (NM 031055), NF-κB(XM 342346) and c-Jun (NM 021835) were synthesized commercially (Sigma). Efficiency of these siRNA was tested with Northern blotting and quantitive real-time RT-PCR in HEK293 cells, only those with inhibitory efficiency of mRNA expression >80% were used in the further experiments (data not shown). About 48 h prior to the addition of IL-17, CAVSMCs were transfected with siRNA using Lipofectamine 2000 (Invitrogen) following the manufacture’s instructions. And a siRNA that did not target any genes was used as a negative control.
Northern Blotting
Total RNA was isolated by lysing cells in TRIzol reagent (GIBCO) and quantified using spectrophotometry at 260 nm. RNA (10 μg) was separated by formaldehyde-containing 1% agarose gel electrophoresis, and gels were stained with SYBR green (Sigma) to visualize 28S/18S ribosomal RNA bands. Gels were transferred overnight to a nylon membrane (GeneScreen Plus; NEN Life Science Products) and probed with 32P-labeled MMP-9 cDNA. The membrane was exposed to film for 1 h to 7 days at −80°C. The film was developed and scanned, and densitometry was performed using Alphaease software. Loading was normalized to the 28S band.
Real-time Quantitative RT-PCR
DNA-free total RNA was extracted using RNAqueous-4 PCR kit (Ambion). RNA quality was assessed by capillary electrophoresis using the Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). Real time quantitative PCR was performed using Quanti-Tect SYBR-Green Probe RT-PCR Kit (Qiagen) and Gene 3000 sequence detection system (Gene Corp.). Each sample was assayed in triplicate. For relative quantification, the C t method: ratio = 2-[(C t (MMPs)-C t (GAPDH)] was used, with GAPDH as a control. For copy number determination, a calibration curve was obtained using serial dilutions of linearized GAPDH cDNA as template and the primers used in the experiment were as follows: GAPDH primers 5′- GAAGGTGAAGGTCGGAGTC-3′ and 5′-GAAGATGGTGATGGGATTTC-3′; MMP-9: 5′-GATGGTCTACTGGCACACG-3′ and 5′-GTCCCTCGAAGGTGAAGG-3′. The mRNA expression levels of target genes were expressed relative to the appropriate control, which was set to 1.0.
Western Blot Analysis
Nuclear cell extracts with the ReadyPrep Protein Extraction Kit (Cytoplasmic/Nuclear) were used for assessing nuclear import of NF-κB (p65) and c-Jun. IκB was detected in cytoplasmic fraction by Western blot. Antibodies (Santa Cruz Inc, CA, USA) used were as follows: anti-phospho-p65 (sc-101749), anti-IκB (sc-74452), anti-phospho-c-Jun (sc-16312, specific to ser63/73) and anti-c-Jun (sc-45, specific to N-terminal).
MMP-1, 2, 9 and TIMP-1, 2 levels in supernatant and cell extractions were confirmed by Western blotting. Proteins were separated by 10% PAGE and electroblotted onto a Hybond-P polyvinylidene difluoride membrane (Amersham Biosciences).The membrane was incubated with rabbit anti-rat MMP 1,2,9 and TIMP 1,2 antibody (Boster Biological Technology Ltd. Wuhan, China. 1:1000 dilution) and subsequently with horseradish peroxidase-conjugated goat/donkey anti-rabbit/goat immunoglobulin G. The immunoreactive bands were detected by chemiluminescence (ECL Plus, GE Healthcare). The β-tubulin antibodies were used as internal control to confirm equal protein loading. The results were quantified using the Quantity One Software (Bio-Rad).
Stimulation of CAVSMC
Cells were treated with various concentrations (0-100 ng/ml) of IL-17 for 12 h to clarify the optimizational dose for inducing MMP-9 expression. In another experiment, cells were stimulated with IL-17 (20 ng/ml) for 24 h and MMP-9 was determined at various time. To examine if the MMP-9 mRNA expression was dependent on IL-17, we used the IL-17(20 ng/ml) or IL-17RA (10 μg/ml) neutralizing antibodies incubated with CAVSMC for 1 h before addition of IL-17 (Cortez et al. 2007). IL-17 has pleiotropic activities, including induction of other cytokines, such as IL-1β, IL-6 and TNF-α (Shen et al. 2006). Since these cytokines are known to stimulate MMP-9 expression (Eberhardt et al. 2000; Yao et al. 2007; Moon et al. 2004), we determined whether IL-17-mediated MMP-9 expression is dependent on IL-1β, IL-6, or TNF-α. CAVSMC was incubated with IL-1β, IL-6, or TNF-α neutralizing antibodies (10 μg/ml for 1 h; Boster Corp. Wuhan, China) 1 h before the addition of IL-17 (Cortez et al. 2007).
Protein synthesis inhibitor (CHX) or transcriptional inhibitor (ActD) was used to determine whether IL-17-mediated MMP-9 expression was regulated at the transcriptional and/or translational level. CAVSMC was treated with CHX or ActD for 1 h and then IL-17 (20 ng/ml for 12 h) was added, and RNA was isolated and analyzed for MMP-9 expression by quantitative RT-PCR.
We explored whether IL-17 induces TF activation via p38 MAPK and ERK1/2. Quiescent CAVSMC were treated with SB-203580 or PD-98059 before treating with IL-17. TF activation was detected after 2 h (C-jun) or 6 h (NF-κB) by Western blotting of nuclear protein extracts. mRNA expression of MMP-9 was detected with quantitative RT-PCR at 12 h.
Statistical Analysis
All values are expressed as mean ± SEM. For statistical analysis, a paired t-test was used for comparison between two groups. One-way ANOVA and Tukey–Kramer multiple range test were used for comparisons among multiple groups. P < 0.05 was considered significant.
Results
Effects of IL-17 in Stimulating MMP-9 Expression
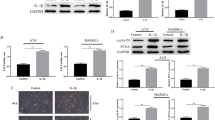
First, we detected whether CAVSMC could express IL17-RA using RT-PCR. As shown in Fig. 1a, CAVSMC expresses IL-17RA under basal conditions. CAVSMC expresses MMP-9 mRNA at low levels under basal conditions, and treatment with IL-17 for 12 h significantly increased MMP-9 expression, with peak levels obtained at 20 ng/ml (Fig. 1b). Time course studies revealed that IL-17 induced MMP-9 expression in a time-dependent manner. The peak levels of mRNA were observed at 12 h, with no further increases detected at 24 h (Fig. 1c). MMP-9 mRNA expression was blocked by IL-17 and IL-17RA neutralizing antibody was detected by real-time RT-PCR (Fig. 1d). These results demonstrated that IL-17 can induce MMP-9 mRNA expression in a time- and dose-dependent manner in CAVSMC.
IL-17 stimulates MMP-9 expression in CAVSMC. a Expression of IL-17RA was detected by RT-PCR in CAVSMC. b Dose-dependent manner of MMP-9 expression induced by IL-17. c Time-dependent manner of MMP-9 expression induced by IL-17. d Anti-IL-17 and IL-17RA neutralizing antibody attenuated mRNA expression of MMP-9. (*, P < 0.05 versus IL-17 group, AVONA)
MMP-9 protein expression was significantly increased when stimulated with IL-17. When incubated with IL-17 or IL-17RA neutralizing antibody, the expression of MMP-9 was decreased (Fig. 2a). Elevated levels of IL-1β, IL-6 and TNF-α were detected in the supernatant after CAVSMC was treated with IL-17 at 20 ng/ml for 12 h. (data not shown). Pretreatment with neutralizing antibodies against IL-1β, IL-6, or TNF-α failed to block IL-17-mediated MMP-9 expression. (Fig. 2b).
Role of AP-1 and NF-kB in IL-17-Induced MMP-9 mRNA Expression
IL-17-mediated MMP-9 expression was significantly inhibited by the RNA synthesis inhibitor ActD, but not by the protein synthesis inhibitor CHX (Fig. 3a), which showed that IL-17-induced MMP-9 expression was regulated at transcriptional level. Treatment of CAVSMC with IL-17 for 6 h caused an increase in nuclear p65 protein levels, whereas no significant changes could be observed at a very early time point (30 min). Degradation of IkB in cytoplasmic fraction was observed at the same time. Similar to p65, nuclear import of c-Jun protein was efficiently increased by IL-17 at both time points tested (Fig. 3b). The possible regulation of MMP-9 mRNA transcription by AP-1 and NF-kB were investigated using selective pharmacological inhibitors or siRNA. NF-kB inhibitor (helenalin) significantly attenuated IL-17-induced MMP-9 mRNA accumulation assessed by real-time RT-PCR. Transfection with siRNA targeting c-Jun and NF-kB also decreased the IL-17-induced MMP-9 mRNA expression (Fig. 3c).
IL-17-induced MMP-9 expression was dependent on NF-kB and AP-1. a Act D, while not CHX, inhibited IL-17 induced MMP-9 mRNA expression. b Nuclear transport of NF-kB and C-Jun was obtained with IL-17 stimulation. c Inhibition of NF-kB and AP-1 reduced expression of MMP-9 mRNA. (*, P < 0.05 versus IL-17 group, AVONA)
Role of p38 MAPK and ERK1/2 in IL-17-Induced Transcription Factor (TF) Activation and MMP-9 Expression
IL-17-induced activation of p38 MAPK phosphorylation and activity, which were blocked by the inhibitor SB-203580 (1 μM) and IL-17 neutralizing antibodies. Similarly, phosphorylation and activity of ERK1/2 were induced by IL-17 and blunted by PD-98059 (10 μM) and IL-17 neutralizing antibodies (Fig. 4a).
Role of p38 MAPK and ERK1/2 in TF activation and MMP-9 expression. a IL-17-induced activation of p38 MAPK and ERK1/2 was blunted by SB-203580 and PD-98059, respectively. b Nuclear transport of TF induced by IL-17 was blocked by SB-203580 and PD-98059, respectively. c mRNA expression of MMP-9 was attenuated by MAPK inhibitors (*, P < 0.05 versus IL-17 group, AVONA)
As shown in Fig. 4b, nuclear translocation of NF-κB p65 was significantly attenuated by SB-203580 and PD-98059. Similarly, IL-17-mediated AP-1 activation was attenuated by SB-203580 and PD-98059. Furthermore, SB-203580 and PD-98059 attenuated IL-17-mediated MMP-9 mRNA expression (Fig. 4c).
Effects of IL-17 on MMPs/TIMPs Balance
MMP-1, 2, 9 and TIMP-1, 2 were determined by ELISA in the supernatant and Western blotting from cell extractions after incubation with IL-17 (20 ng/ml) for 24 h. IL-17 induces MMPs expression in a time-dependent manner, with peak levels obtained at 12 h, but has no obvious effects on TIMPs expression (data not shown).
Effects of IL-17 in Stimulating CAVSMC Migration
Because IL-17 is a potent inducer of IL-1β, IL-6 and TNF-α production, we first investigated whether IL-17-induced CAVSMC migration is mediated by these cytokines. Pretreatment with the respective neutralizing antibodies for 1 h prior to IL-17 treatment failed to modulate cell migration. In contrast, incubation with anti-IL-17 or anti-IL-17RA neutralizing antibody for 1 h blocked IL-17-induced cell migration. These results indicate that IL-17 is a potent inducer of CAVSMC migration and mediates its effect independent of the proinflammatory cytokines IL-1β, IL-6 and TNF-α. (Fig. 5a).
Role of IL-17 in migration of CAVSMC. a IL-17 induced migration of CAVASMC was independent of IL-1β, IL-6 and TNF-α. Anti-1L-17 and IL-17RA neutralizing antibody reduced the cell migration stimulated by IL-17. b MMP inhibitor (GM-6001) and siRNA targeting MMP2/9 reduced the IL-17-induced cell migration. The effects of inhibition of MMP-9 was more pronounced than that of MMP-2. (*, P < 0.05 versus IL-17 group. #, P < 0.05 versus MMP-9 group, AVONA)
Whether IL-17 induces CAVSMC migration in an MMP-9-dependent manner was studied by using MMPs inhibitor or siRNA targeting MMP-2/MMP-9. Pretreatment with the broad-spectrum MMPs inhibitor GM-6001 attenuated IL-17-dependent CAVSMC migration. The effect of GM-6001 appeared to be more pronounced than was MMP-9 knockdown, which indicated that MMP-9 was not the sole mediator for CAVSMC migration. The siRNA mediated knockdown of both MMP-2 and MMP-9 attenuated IL-17-mediated CAVSMC migration (Fig. 5b). Inhibition of MMP2/9 expression was confirmed by Western blotting and RT-PCR (data not shown). However, knockdown of MMP-9 was more effective in inhibiting cell migration than that of MMP-2. Treatment with control siRNA had no obvious effect on CAVSMC migration.
Discussion
In the present study, induction of MMP-9 expression in CAVSMC by IL-17 was observed. The effects of IL-17 in stimulating MMP-9 expression are independent of IL-1β, IL-6, and TNF-α. IL-17 regulates MMP-9 expression at the transcriptional level and is dependent on p38 MAPK and ERK1/2 mediated AP-1 and NF-κB activation. IL-17 induces CAVSMC migration in an MMP-9-dependent manner. Since migration of SMC to lumina is a key process in neointimal formation and restenosis, MMPs degrade ECM and facilitate migration, our results suggest that IL-17 may be potentially important in neointimal formation and restenosis after balloon-injury induced vascular remodeling.
IL-17 s constitute a unique family of cytokines that has no structural homology to other ILs (Bettelli et al. 2007) and is expressed mainly by a subset of CD4+ T cells, i.e., Th17 cells. The IL-17 group is composed of six major isoforms, IL-17A, -B, -C, -D, -E, and -F (Moseley et al. 2003). IL-17 signals via IL-17 receptors (IL-17R), which included IL-17RA, -B, -C, -D, and -E (Moseley et al. 2003). Many cell types express IL-17Rs and are targets of IL-17. In present research, expression of IL-17RA in CAVSMC was observed, which indicated that CAVSMC is target of IL-17.
Clinical and experimental data showed that IL-17 was involved in cardiovascular diseases. In unstable angina and acute myocardial infarction patients, increased IL-17 levels were detected in plasma (Hashmi and Zeng 2006). IL-17 stimulates MMP-1 expression in primary human cardiac fibroblasts (HCP) and mediates HCP migration via MMP-1-dependent pathway, which suggest that IL-17 plays a critical role in myocardial remodeling (Cortez et al. 2007). Upregulation of MMP-2 and MMP-9 by IL-17 was also observed in that research, which was consistent with our results. IL-17 stimulates C-reactive protein (CRP) expression via p38 MAPK and ERK1/2-dependent NF-κB and C/EBP β activation in coronary artery smooth muscle cells (CASMC), which suggest that IL-17 may mediate chronic inflammation, atherosclerosis, and thrombosis (Patel et al. 2007). However, it is unknown whether IL-17 plays a role in CAVSMC migration in carotid artery after balloon injury.
SMC migration and proliferation are important features of both normal physiological vessel growth and vascular pathology (Ross 1999a, b). In normal vessels, SMCs are largely confined to the tunica media. The proliferation of SMCs in the tunica media and their migration into the luminal surface are important factors which determined the intimal thickening and hyperplasia in post-angioplastic restenosis. Migration of SMCs were regulated by MMPs/TIMPs balance in ECM. The disruption of this delicate balance in favor of MMPs activation results in ECM degradation and induction of SMC motility and migration (Cortez et al. 2007; Galis and Khatri 2002; Ikeda and Shimada 2003; Cho and Reidy 2002). Among the MMPs, MMP-9 plays a key role in vascular remodeling by degradation and reorganization of ECM (Galis and Khatri 2002; Cho and Reidy 2002). Inhibition of MMP-9 increases the collagen content of arteries and potentially their mechanical stability, decreases intimal hyperplasia and the lumen loss. Both MMP-2 and MMP-9 are expressed in SMC and excess activation of MMP-2 and MMP-9 results in destruction of the ECM and leads to pathological remodeling and vascular restenosis (Ikeda and Shimada 2003; Cho and Reidy 2002; Galis et al. 2002).
In the present study, MMPs (MMP-1,2 and 9) were induced by IL-17 in time- and dose-dependent manner, while TIMPs (TIMP-1, 2) were not significantly affected by IL-17, suggesting that IL-17 alters the MMPs/TIMPs balance in favor of MMPs expression and induces ECM degradation. Although the knockdown of MMP-2 and MMP-9 significantly inhibits IL-17-mediated CAVSMC migration, the effects are more pronounced when MMP-9 expression is targeted, indicating that MMP-9 might play a more dominant role in IL-17-mediated CAVSMC migration.
Our results also show that IL-17 stimulates MMP-9 expression at the transcriptional level. IL-17 stimulated MMP-9 mRNA expression and secretion. P65 and AP-1 protein (c-Jun) were markedly elevated after stimulation with IL-17. Selective inhibitor of NF-κB or knockdown of NF-κB and c-Jun using siRNA attenuated MMP-9 expression. These results suggest that both AP-1 and NF-κB are critical mediators of IL-17-mediated MMP-9 gene transcription.
It has been reported previously that ERK1/2 was involved in IL-17-mediated MMP-1 expression in HCF (Cortez et al. 2007). The signal transduction pathways involved in IL-17-mediated MMP-9 expression in CAVSMC were determined in the present research. As shown in the results, IL-17 induced p38 MAPK and ERK1/2 activation, SB-203580 and PD-98059 blunted IL-17-mediated transcription factor activation and MMP-9 expression. These results demonstrated that IL-17 induced MMP-9 expression was dependent on MAPK-mediated NF-κB and AP-1 activation. C-Jun N-terminal kinase (JNK) is an important member of MAPK family. In human airway smooth muscle cells, IL-17A mediated rapid phosphorylation of MAPK including JNK (Rahman et al. 2006). JNK was also involved in IL-1beta induced MMP-9 expression in human tracheal smooth muscle cells (Liang et al. 2007). This MAPK signal may also participate in the MMP-9 expression stimulated by IL-17 in CAVSMC, while it was not tested in the current research. It requires further study to clarify the role of JNK in IL-17-induced MMP-9 expression and cell migration. This will make the mechanisms related to the effects of IL-17 in migration of CAVSMC more precise.
Conclusions
Taken together, IL-17 induces CAVSMC migration through the induction of MMP-9 expression, which is mediated by p38 MAPK and ERK1/2 dependent AP-1 and NF-κB activation. Targeting expression of IL-17 or its specific downstream mediators is a potentially novel therapeutic pathway for attenuating the post-angioplastic restenosis.
References
Bettelli E, Oukka M, Kuchroo VK (2007) T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol 8:345–350. doi:10.1038/ni0407-345
Chandrasekar B, Mummidi S, Mahimainathan L, Patel DN, Bailey SR, Imam SZ, Greene WC, Valente AJ (2006) Interleukin-18-induced human coronary artery smooth muscle cell migration is dependent on NF-kappaB- and AP-1-mediated matrix metalloproteinase-9 expression and is inhibited by atorvastatin. J Biol Chem 281:15099–15109. doi:10.1074/jbc.M600200200
Cho A, Reidy MA (2002) Matrix metalloproteinase-9 is necessary for the regulation of smooth muscle cell replication and migration after arterial injury. Circ Res 91:845–851. doi:10.1161/01.RES.0000040420.17366.2E
Cortez DM, Feldman MD, Mummidi S, Valente AJ, Steffensen B, Vincenti M, Barnes JL, Chandrasekar B (2007) IL-17 stimulates MMP-1 expression in primary human cardiac fibroblasts via p38 MAPK- and ERK1/2-dependent C/EBP-beta, NF-kappaB, and AP-1 activation. Am J Physiol Heart Circ Physiol 293:H3356–H3365. doi:10.1152/ajpheart.00928.2007
Eberhardt W, Huwiler A, Beck KF, Walpen S, Pfeilschifter J (2000) Amplification of IL-1 beta-induced matrix metalloproteinase-9 expression by superoxide in rat glomerular mesangial cells is mediated by increased activities of NF-kappa B and activating protein-1 and involves activation of the mitogen-activated protein kinase pathways. J Immunol 165:5788–5797
Galis ZS, Khatri JJ (2002) Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res 90:251–262
Galis ZS, Johnson C, Godin D, Magid R, Shipley JM, Senior RM, Ivan E (2002) Targeted disruption of the matrix metalloproteinase-9 gene impairs smooth muscle cell migration and geometrical arterial remodeling. Circ Res 91:852–859. doi:10.1161/01.RES.0000041036.86977.14
Gibbons GH, Dzau VJ (1994) The emerging concept of vascular remodeling. N Engl J Med 330:1431–1438. doi:10.1056/NEJM199405193302008
Hashmi S, Zeng QT (2006) Role of interleukin-17 and interleukin-17-induced cytokines interleukin-6 and interleukin-8 in unstable coronary artery disease. Coron Artery Dis 17:699–706. doi:10.1097/01.mca.0000236288.94553.b4
Ikeda U, Shimada K (2003) Matrix metalloproteinases and coronary artery diseases. Clin Cardiol 26:55–59. doi:10.1002/clc.4960260203
Liang KC, Lee CW, Lin WN, Lin CC, Wu CB, Luo SF, Yang CM (2007) Interleukin-1beta induces MMP-9 expression via p42/p44 MAPK, p38 MAPK, JNK, and nuclear factor-kappaB signaling pathways in human tracheal smooth muscle cells. J Cell Physiol 211:759–770. doi:10.1002/jcp.20992
Mnjoyan ZH, Doan D, Brandon JL, Felix K, Sitter CL, Rege AA, Brock TA, Fujise K (2008) The critical role of the intrinsic VSMC proliferation and death programs in injury-induced neointimal hyperplasia. Am J Physiol Heart Circ Physiol 294:H2276–H2284. doi:10.1152/ajpheart.91527.2007
Moon SK, Cha BY, Kim CH (2004) ERK1/2 mediates TNF-alpha-induced matrix metalloproteinase-9 expression in human vascular smooth muscle cells via the regulation of NF-kappaB and AP-1: Involvement of the ras dependent pathway. J Cell Physiol 198:417–427. doi:10.1002/jcp.10435
Moseley TA, Haudenschild DR, Rose L, Reddi AH (2003) Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev 14:155–174. doi:10.1016/S1359-6101(03)00002-9
Patel DN, King CA, Bailey SR, Holt JW, Venkatachalam K, Agrawal A, Valente AJ, Chandrasekar B (2007) Interleukin-17 stimulates C-reactive protein expression in hepatocytes and smooth muscle cells via p38 MAPK and ERK1/2-dependent NF-kappaB and C/EBPbeta activation. J Biol Chem 282:27229–27238. doi:10.1074/jbc.M703250200
Rahman MS, Yamasaki A, Yang J, Shan L, Halayko AJ, Gounni AS (2006) IL-17A induces eotaxin-1/CC chemokine ligand 11 expression in human airway smooth muscle cells: role of MAPK (Erk1/2, JNK, and p38) pathways. J Immunol 177:4064–4071
Ross R (1999a) Atherosclerosis is an inflammatory disease. Am Heart J 138:S419–S420. doi:10.1016/S0002-8703(99)70266-8
Ross R (1999b) Atherosclerosis–an inflammatory disease. N Engl J Med 340:115–126. doi:10.1056/NEJM199901143400207
Schwartz SM (1997) Perspectives series: cell adhesion in vascular biology. Smooth muscle migration in atherosclerosis and restenosis. J Clin Invest 99:2814–2816. doi:10.1172/JCI119472
Shen F, Hu Z, Goswami J, Gaffen SL (2006) Identification of common transcriptional regulatory elements in interleukin-17 target genes. J Biol Chem 281:24138–24148. doi:10.1074/jbc.M604597200
Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, Cohen JI, Spriggs MK (1995) Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity 3:811–821. doi:10.1016/1074-7613(95)90070-5
Yao JS, Zhai W, Fan Y, Lawton MT, Barbaro NM, Young WL, Yang GY (2007) Interleukin-6 upregulates expression of KDR and stimulates proliferation of human cerebrovascular smooth muscle cells. J Cereb Blood Flow Metab 27:510–520. doi:10.1038/sj.jcbfm.9600365
Acknowledgments
We would like to express our appreciations to Sun Zhidan for his excellent technical assistance and Li Wen for her revision of the draft. This research was sponsored partly by the Heilongjiang Youth Fund (QC07C100) and Special Fund of The First Affiliated Hospital of Harbin Medical University (2007035).
Author information
Authors and Affiliations
Corresponding author
Additional information
G. Cheng and L. Wei have contributed equally.
Rights and permissions
About this article
Cite this article
Cheng, G., Wei, L., Xiurong, W. et al. IL-17 Stimulates Migration of Carotid Artery Vascular Smooth Muscle Cells in an MMP-9 Dependent Manner via p38 MAPK and ERK1/2-Dependent NF-κB and AP-1 Activation. Cell Mol Neurobiol 29, 1161–1168 (2009). https://doi.org/10.1007/s10571-009-9409-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-009-9409-z