Abstract
Ependymal cells (EC) in the spinal cord central canal (CC) are believed to be responsible for the postnatal neurogenesis following pathological or stimulatory conditions. In this study, we have analyzed the proliferation of the CC ependymal progenitors in adult rats processed to compression SCI or enhanced physical activity. To label dividing cells, a single daily injection of Bromo-deoxyuridine (BrdU) was administered over a 14-day-survival period. Systematic quantification of BrdU-positive ependymal progenitors was performed by using stereological principles of systematic, random sampling, and optical Dissector software. The number of proliferating BrdU-labeled EC increased gradually with the time of survival after both paradigms, spinal cord injury, or increased physical activity. In the spinal cord injury group, we have found 4.9-fold (4 days), 7.1-fold (7 days), 4.9-fold (10 days), and 5.6-fold (14 days) increase of proliferating EC in the rostro-caudal regions, 4 mm away from the epicenter. In the second group subjected to enhanced physical activity by running wheel, we have observed 2.1–2.6 fold increase of dividing EC in the thoracic spinal cord segments at 4 and 7 days, but no significant progression at 10–14 days. Nestin was rapidly induced in the ependymal cells of the CC by 2–4 days and expression decreased by 7–14 days post-injury. Double immunohistochemistry showed that dividing cells adjacent to CC expressed astrocytic (GFAP, S100beta) or nestin markers at 14 days. These data demonstrate that SCI or enhanced physical activity in adult rats induces an endogenous ependymal cell response leading to increased proliferation and differentiation primarily into macroglia or cells with nestin phenotype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For a long time, the medical science believed that adult mammalian central nervous system (CNS) could not repair itself and that the numbers of postnatal neurons are final, showing a decreasing tendency with physiological aging or after various pathological insults (Mercier et al. 2002). However, the last 30 years of research has documented that the creation of new functional neurons in the adult mammalian, including human CNS actually occurs in two discrete locations: hippocampus and subventricular zone (SVZ) (Gage 2000; Lie et al. 2004). Moreover, stem cells with the potential to give rise to new neurons reside also in other different areas of the adult CNS, indicating to the possibility that endogenous sources of neural stem cells (NSCs) can be mobilized from different regions to replace the dying neurons in neurodegenerative diseases or CNS injuries (Palmer et al. 1999; Yamamoto et al. 2001; Nunes et al. 2003; Minger et al. 2007; Koson et al. 2008).
Despite extreme effort given to research of basic stem cell biology, it has been disscussed for a long time whether ependymal cells adjacent to the SVZ of the lateral ventricles retain the characteristics of NSCs (Johansson et al. 1999; Doetsch et al. 1999). Importantly, a recent study of Coskun et al. (Coskun et al. 2008) documents, that this may be the case, since the subpopulation of ependymal cells, CD133+/CD24−, exhibit features of quiescent NSCs in vitro, i.e., self-renewal and multipotency as well as participation in neurogenesis in vivo after various pathological conditions. Thus, this finding throws a new light to the knowledge of the regenerative capacity and possible importance of the periventricular progenitors in the CNS. Furthermore, the occurrence of ependymal cell layer covering CNS ventricular system including the areas around the third, fourth ventricles, and the CC of the spinal cord supports suggestion, that also these regions may retain similar quiescent NSCs as those which were identified in the lateral ventricles (Weiss et al. 1996). Therefore, the posible appearance of the quiescent ependymal neural stem cells within the CC and understanding their biological regulations may explain their function and open new approaches toward treating spinal cord injury.
Many previous studies have documented that ependyma in the spinal cord plays a crucial role in regenerative processes after injury in lower vertebrates (Nordlander and Singer 1978), but not in adult mammals, where activated ependymal cells contribute primarly to the glial scar formation (Mothe and Tator 2005). However, under in vitro conditions, stem cells isolated from spinal ependyma give rise to functional neurons or oligodendroglia (Yamamoto et al. 2001). These contradictory findings of ependymal neurogenic fate are often explained in regard to beneficial (in vitro) or inhibitory (in vivo) conditions directly influencing neuronal differentiation (Weiss et al. 1996). Furthermore, many previous studies provide evidence that in adult rat spinal cord a significant number of neural progenitors are activated after several types of injury not only in ependyma, but also in other regions of the parenchyma (Yamamoto et al. 2001; Horner et al. 2000; Kehl et al. 1997).
On the other hand, compelling evidence has shown that also physiological stimulation as enrichment enviroment or physical activity may be beneficial for neurogenesis in the CNS. Previous experiments have shown that mice providing systematic excercise in a running wheel, had twice more new hippocampal neurons than controls (Gomez-Pinilla et al. 2001). Beside this, it has been shown that voluntary exercise can increase levels of brain-derived neurotrophic factor (BDNF) and other growth factors, which stimulate neurogenesis, and improve learning and mental performance (Gomez-Pinilla et al. 2001). Furthermore, active exercise may mobilize gene expression profiles that could be beneficial for CNS plasticity processes (Neeper et al. 1995).
Thus, the goal of this study was to assess the proliferative potential of the CC ependymal progenitors in adult rats processed to compression SCI or enhanced physical activity and compare the impact of those two different pathological vs physiological paradigms on ependymal proliferation. The existence of dividing cells was determined by administration of bromodeoxyuridine (BrdU) over a 14-day-survival period. Systematic quantification of BrdU-positive ependymal progenitors was performed by using an unbiased stereological principles and optical Dissector of Elipse software. In addition, we have examined the differentiation fate of BrdU-positive cells. Our data indicate, that the number of proliferating BrdU-labeled EC increased gradually with the time of survival after both paradigms, spinal cord injury, or increased physical activity. However, dividing population of cells revealed macroglial or immature neural or unknown phenotype.
Materials and Methods
Spinal Cord Injury
The spinal cord injury (SCI) was induced by modified balloon-compression technique in adult male Wistar rats (n = 16) weighting between 290 and 310 g, according to our previous study (Vanicky et al. 2001). In brief, animals were anesthetized with 2% halothane in room atmosphere in an induction box and were maintained with 1–1.5% halothane delivered by an inhalation mask. For the core temperature measurements, a thermal probe was inserted into the rectum. Afterward, the shaved skin was cut and the paravertebral muscle overlying the L1-Th8 vertebrae were freed from the attachments, and the soft tissue and corresponding spinous processes were removed. Using a dental drill a small hole was performed in vertebral arch of Th10, and a 2-French Fogarty catheter was inserted into the dorsal epidural space through this hole, aiming cranially to Th8-9 of the spinal level. After stabilization of the body temperature at 37°C, the balloon was inflated with 11.0 μl of saline for 5 min. Then, the catheter was deflated, removed from epidural space, the soft tissue and skin were sutured in anatomical layers, and finally animals were disconnected from anesthesia. All experimental rats were hydrated with cc 5–10 ml of Ringer’s solution and were allowed to recover in warmed cages and kept in pairs during survival. Manual bladder expression was required for 7–14 days after injury, until bladder reflex was established. The antibiotic treatment was performed in all rats by four daily applications of gentamicin in low dose 2 mg/kg to prevent urinary tract infections. The SCI group contained 16 rats (n = 16: n = 4/4d, n = 4/7d, n = 4/10d, n = 4/14d), and the control group (C-sham operated SCI) contained 12 rats (n = 12: n = 3/3d, n = 3/7d, n = 3/10d, n = 3/14d).
Behavioral Testing of Motor Function (BBB Scoring)
Animals were evaluated before surgery and at 1, 4, 7, 10, and 14 days after surgery. Each rat was tested for 5 min by two blinded examiners. The motor performance was tested by using the Basso–Beattie–Bresnahan (BBB) 21-point open field locomotor scale. BBB scores, which categorize combinations of rat hindlimb movements, trunk position and stability, stepping, coordination, paw placement, toe clearance, and tail position, where 0 represents no locomotion and 21-normal motor function was analyzed.
Excercise Experiment
Adaptation to Running Wheels
Rats (n = 18) were placed in the standard large cage wih running wheel (RW) 2 days before the onset of the experiment. Each rat was placed to the RW, which was closed, to prevent rats from escaping from RW. Afterward, they were observed for 15 min for ability to adjust to new enviroment, learn to move forward on mashed surface, and begin voluntary physical activity such as walking and running. This procedure was repeated next day, and only those rats that showed adaptation and running activity were selected for the next experiment.
Running Experiment
Selected rats n = 16 were placed in standard housing conditions with free access to runnin wheel. Afterward, the rats were placed to the RW, which was closed for 30 min, during the time 3.00–3.30 pm, daily during 14 days' survival. Besides this involuntary period, for the rest of the time, the entrance of the RW was opened, and thus the rats had free access to RW at anytime, especially at night, when rats showed highest physiological activity. The excercise group contained 16 rats (n = 16: n = 4/4d, n = 4/7d, n = 4/10d, n = 4/14d). The control group (C-PhA) (no exercise), which was housed in pairs under standard conditions contained 12 rats (n = 12: n = 3/4d, n = 3/7d, n = 3/10d, n = 3/14d).
In Vivo Labeling of Dividing Spinal Progenitors
In all the experimental animals: spinal injury (SCI), excercise (E) and control (C-sham, C-PhA) group, 50 mg/kg i.p. of 5-bromo-2-deoxyuridine (BrdU, Sigma, St. Louis, MO) was administered once daily, using a sterile stock solution of 10 mg BrdU dissolved in 0.9% (w/v) NaCl solution, for each survival interval (4 days, 7 days, 10 days and 14 days).
Tissue Processing
At the end of the survival, all the animals were deeply anesthetized with a ketamine–xylozine cocktail (6 mg/kg of xylozine, 60 mg/kg of ketamine) and transcardially perfused with saline, followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB). Spinal cords were removed, post-fixed in the same fixative overnight at 4°C, and cryoprotected in PB containing 30% sucrose.
SCI and C-Sham Group
Frozen spinal cords sections (40 μm thick) were taken from a 1.2-cm-long spinal cord centered on the injury epicenter, embedded in tissue teck, dissected into three blocks (rostral/A, epicenter/B, caudal/C each 0.4 cm thick) and stored at −20°C. Cryostat spinal cord sections cut from rostral (A) and caudal (C) regions were immersed in PB solutions (floating–staining method); the sections cut from epicenter (B-lesion site) were mounted directly onto slides (Color Frost/Plus; Fisher, Pittsburgh, PA) (Fig. 1). Sections from each segment in 1-mm distance were processed for Luxol Fast Blue histochemistry to visualize spared white and gray matter tissue (Fig. 3A–C).
Spinal cords processed to SCI were cut into three 4-mm coronal segments (Arostral/ R, B central lesion /L, C-caudal to the lesion site/C, complete 1.2-cm-long segment) and embedded in O.C.T. mounting medium. Blocked spinal cord segments were serially sectioned at 200-μm intervals. Coronally cut cryostat 40-μm-thick sections taken from B segment were mounted on slides, whereas sections from A, C segments were immersed in PB solutions and serially stored in a 24-well plates. Slides representative of all groups were selected for both BrdU immunohistochemistry and immunofluorescence labeling
Excercise Group and C-PhA
Frozen cryostat spinal cords sections (40 μm thick) were taken from a 1.2-cm-long spinal cord centered at the thoracic segments (Th8-10), dividing into 3 × 4 mm segments (A, B, and C similar as SCI group), and serially cut sections (200-μm intervals) were immersed in PB solutions (floating–staining method).
Representative slides (10 sections/per each block/4 mm, 30 sections/1.2 cm segment/rat) of all the groups were selected for both BrdU immunohistochemistry and immunofluorescence labeling for neural markers. For the stereological quantitation of BrdU-labeled cells, cryostat sections were stained for light microscopy, using diaminobenzadine (DAB) immunohistochemistry.
Immunohistochemistry
For immunohistological detection of the incorporation of BrdU, pretreatment of tissues was performed as described in Cizkova et al. (2006). For immunocytochemical detection of BrdU, DNA was denaturated to expose the antigen. Free floating spinal cord sections were incubated in 1 N HCl at 37°C for 30 min. Sections were subsequently rinsed for 10 min at 25°C in 0.1 M boric acid (pH 8.5), followed by appropriate rinsing in PBS and incubation with 0.6% H2O2 in PBS for 30 min in the dark. Tissue was rinsed in PBS and incubated with primary antibody, rat anti-BrdU (1:500, AbD Serotec) for 24 h at 4°C. All the blocking steps were performed in 1 × PBS/0.3% Triton-X (TX-100) 1–10% normal goat serum. After primary antibody incubation, sections were rinsed in PBS and secondary antibody goat anti-rat biotinilated antibody (1:250 Vector Laboratories, Burlingame, CA) was applied for 2 h at room temperature. Sections were then rinsed three times in PBS for 5 min and 1-h incubation with avidin–biotin complex (ABC–Elite) was perfomed. BrdU labeling was then visualized using DAB (0.25 mg DAB, 0.009% H2O2, and 0.04% NiCl in PBS) for up to 7 min. DAB incubation was terminated by rinsing in tap water, and slides were then dehydrated through alcohols and coverslipped. Sections were processed for multiple markers to determine the cellular phenotype of BrdU-labeled nuclei. Primary antibodies were chosen, that recognize immature and mature astrocytes (S-100b polypeptide), immature neurons, mature neurons, (βIII tubulin, NeuN), mature astrocytes glial fibrillary acidic protein (GFAP), and oligodendrocytes (NG2, O4, RIP). Control sections were processed to identical staining procedure, but the primary antibodies were omitted. After staining, sections were dried at RT and covered by Prolong—antifade mounting medium. Stained slides were analyzed using Olympus Light or Fluorescence microscope (Olympus BX 40) and captured by Olympus digital camera. Overlaid images were prepared with Photoshop 7.0 and digitally stored for further processing procedure. Stained slides were analyzed using Olympus Light or Fluorescence microscope (Olympus BX 50) and captured by Olympus digital camera.
Stereological Quantification of BrDU-Positive Profile
Quantitative Analysis
We applied stereological technique on every tenth section taken from each SC segment (4 mm). Available 30 sections/each rat were processed to dissector and a stack of ten optical sections (OS) obtained by controlled Motorized Z-stage movement (2 μm) for each section were used for stereological analysis.
The number of proliferating BrdU nuclei was counted within CC area frame (100 × 80 μm)/10 OS/40 μm section/10 sections within each SC segment (A, B, and C,) for SCI and C-sham group and within thoracic segments for PhA group and controls, by using stereological principles of, systematic random sampling and optical Dissector Elipse software (Kakinohana et al. 2004). The BrdU-positive nuclei were counted within the wall of the ependymal cell layer forming CC, in the ependymal cells with more than 50% of their perimeters in apposition to cell-forming periventricular wall. The optical images (2-μm thick) were obtained by Olympus microscope using immerse 100× oil objective with numeric aperture 1.3. The optical images were captured using digital camera Olympus DP 50 and Olympus DP software supplied with controlled motorized Z stage (Ellipse) (Fig. 2).
The principle of BrdU nuclei stereological quantification method by using optical Dissector Ellipse software. Stack of ten optical sections (OS) obtained by controlled Motorized Z-stage movement (2 μm) for each 40-μm section were used for stereological analysis. The BrdU--positive nuclei were counted within the wall of the ependymal cell layer forming CC, outlining constant area frame (100 × 80μm)/10 OS applied with optical Dissector, which counted complete BrdU nuclei-green dots and excluded incomplete nuclei-red dots. The 3D reconstruction of BrdU-positive nuclei shows the distribution of counted-green bars vs excluded-red bars representing BrdU nuclei within 40-μm section
We have estimated total number of BrdU-positive nuclei counted on each 40-μm section/10 sections/1 segment/4000 μm (complete 30 sections/3 segments/rat); the total counts of positive profiles were multiplied by the ratio of reference volume to sampling volume in order to obtain the estimated number of BrdU-positive cells.
Statistical Analysis
Behavioral data determined by BBB scores were averaged across hind limbs and differences between the experimental groups were analyzed by using student t-test and one-way ANOVA tests. All the data are presented as mean values ± SEM. Differences between groups were considered statistically significant if *P < 0.1.**P < 0.05.
Results
Tissue Damage and Functional Outcome in SCI Group
The balloon-induced contusion SCI model used in this study is characterized by a central hemorrhagic necrosis that spreads radially and rostrocaudally, resulting in variously shaped cystic cavities. The lesion conditions (volume of 11.0 μl) that were applied to generate injury mainly preserved distribution of the CC ependymal zone and adjacent area, but resulted in extensive destruction of remaining surrounding tissue. The morphological and pathological changes at site of injury (Fig. 3B), and adjacent to the epicenter cranially (Fig. 3A) and caudally (Fig. 3C) of the lesioned spinal cord are shown by the Luxol Fast Blue (LFB) staining, commonly used to observe myelin, where myelin fibers appear blue, neurophil pink, and nerve cells purple (Fig. 3A, B, C). The balloon compression created a lesion at the Th 8–9 vertebral level with a central lesion area spanning to the gray matter (pink and purple). However, the CC ependymal layer was preserved and surrounded by residual gray matter. In addition, the spinal cord section from the lesion site (B segment) processed for BrdU immunoreactivity showed preserved CC ependymal cell layer containing proliferating progenitors (Fig. 3D, D′).
The morphological changes in coronal spinal cord sections at the central lesion (B), rostral (A), and caudal (C) segments processed to Luxol Fast Blue (LFB) staining, The balloon compression creates a lesion at the Th9 vertebral level with a central lesion area spanning most of the gray matter (pink and purple) with CC ependymal layer surrounded by residual gray matter (blue). In addition, the spinal cord section from the lesion site (B segment) processed for BrdU immunoreactivity showed preserved CC ependymal cell layer containing proliferating progenitors. Scale bar = 500 μm in A–D, D′ = 50 μm
Locomotor Recover (BBB Score)
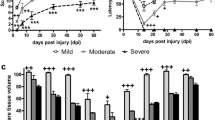
Animals tested prior to surgery did not show any functional deficits, BBB score = 21. However, spinal cord injury caused in all the rats flaccid hindlimb paralysis with BBB score = 0 at 24 h; however, a gradual recovery of hindlimb locomotion was seen over the next 2 weeks in all the SCI rats (n = 15) (BBB = 0/1D, BBB = 3.5 ± 0.7/4D, BBB = 7.7 ± 0.8/7D, BBB = 10.7 ± 1.4/10D, BBB = 12.2 ± 1.1/14D (Fig. 4). Function recovery of hindpaws evaluated with open field test (BBB score) correlated with our behavioral data obtained in similar groups, which were processed to identical SCI model (volume 10.0–12.5 μl), but without BrdU injections. This confirmed that systematic daily dose of BrdU, used in this experiment did not cause side effects or toxicity, which could influence functional recovery.
Locomotor function after moderate spinal cord injury (generated by 11 μl volume of epidural balloon) indicated by black full line and sham operation indicated by intermitted black line during 14 days' of survival (BBB score). Note a progressive recovery of hindlimb motor function over the next 2 weeks in all SCI rats. Sham-operated controls showed minimal divergence from the reference line (score 21) indicating normal locomotor performance with no observable neurological deficits
Adaptation to Voluntary Physical Activity
The rats selected for running experiment showed some variations in regard to voluntary physical activity. However, most of them (14 out of 16 rats) were enjoying the environment of RW and were running or walking during 30-min forced period, with short-resting interruptions. Although, the rats under constantly applied forced periods had unlimited access to the running wheels, it appeared that use of the wheels mainly occurred during the active, night phase of the animals.
BrdU Labeling and Immunohistochemistry
To assess the proliferative activity of ependymal cells, we have administered BrdU in the two control and two experimental groups, at 4 h after SCI and 4 h after applied forced physical activity in the RW. The daily injection of BrdU (50 mg/kg, i.p.) during 14 days had no side effects; we did not register anorexia, loss of weight, or apathy (data not shown).
SCI Experiment
BrdU Positivity in the Ependymal Cells of Central Canal in Control Rats
In the sham-operated (control) spinal cord, dividing, BrdU-positive cells in the ependymal zone constitute a very minor population: 807 ± 205/1.2 cm segments (4 days); 960 ± 385/1.2 segments (7 days); 1195 ± 230/1.2 cm segments (10 days); 1200 ± 278/1.2 cm segments (14 days). As shown in Fig. 5A, 10A only few BrdU-positive nuclei per 40-μm thick section within the ependymal cell layer could be identified.
The representative coronal sections taken from Th8 spinal cord (A segment) showing BrdU positive ependymal labeling in control-sham SCI rats at 4 days (A), in rats after spinal cord injury at 4 days (B), 7 days (C), 10 days (D), and 14 days (E). Note, only few BrdU-positive nuclei in the CC region of sham controls (A). SCI significantly increased BrdU positivity limited to the CC ependyma at 4 days, followed by progressive upregulation at 7–14 days (C–E). The counterstained sections with the nuclear dye hematoxylin in combination with DAB (D) or with DAB enhancer (NiCl) (E) revealed round, oval, or elongated BrdU-positive nuclei restricted to the basal ependymal layer (A′, C′, arrow) or BrdU-positive nuclei found outside of the CC (B′, D′ arrowhead). (A′, B′, C′, D′ reveal details of BrdU nuclei in E). Scale bar = 50μm, Abbreviations: SCI, spinal cord injury; BrdU, 5-bromodeoxyuridine; CC, central canal
BrdU Positivity in the Ependymal Cells of Central Canal After SCI
In the spinal injury group, the BrdU-positive nuclei increased gradually with the time, 4.9 fold (4 days), 7.1-fold (7 days), 4.9 fold (10 days), and 5.6-fold (14 days) when compared with sham operated group (Fig. 5B–E). The first significant upregulation of BrdU-positive nuclei was seen at 4 days (4032 ± 695), when increased number of proliferating cells were moreover restricted to the CC ependyma (Fig. 5B). Furthermore, a progressive enhancement of BrdU-positive profiles was detected at longer survival, at 7–14 days, when some of the proliferating nuclei were tending to migrate out from the ependymal layer Fig. 5C–E. Serial Z-projections of the optical sections (2-μm distance) were stacked to reveal cellular profile and to quantify number of BrdU-positive nuclei in counterstained sections with the nuclear dye hematoxylin and DAB (Fig. 5D) or with DAB enhancer (NiCl) (Fig. 5E). Round, oval, or elongated BrdU-positive nuclei (diameter 4 × 4 μm, 4 × 8 μm) were found either within (A′, C′, D′) or out (A′, B′) of the ependymal layer. This confirms the medio-lateral migratory stream of dividing BrdU-positive cells after longer survival. Furthermore, the majority of BrdU-labeled nuclei tend to migrate toward lession site (Johansson et al. 1999). Thus, the highest concentration of BrdU-labeled cells analyzed by means of stereological quantification was restricted to the ependymal zone of the CC at 7–14 days time points, as depicted in the bar graph in Fig. 6. Furthermore, utilizing the method of segmental sampling (A, B, and C segments) and applying the stereological principles of quantification of BrdU–IR profiles, we have detected the time-dependent gradual increase of proliferating ependymal cells in the rostral-A segment (4 mm) away from the lesion epicenter Fig. 7, 8 (A segment). On the contrary, the caudal C segment moreover showed an opposite effect of epedymal proliferation, when number of BrdU-positive nuclei in ependyma from day 7 progressively decreased with survival time Fig. 7, 8 (C segment). The proliferation activity of ependyma in the central lesion B segment showed constant high levels during 7–14 days Fig. 7, 8 (B segment). In addition, the presence of labeled cells near the CC as well as in the pial layers was also noted, but their numbers were not included in the quantitative assessments.
SCI significantly increases the number of BrdU-positive nuclei in the CC ependymal layer compared with sham-operated control in time dependent manner. The highest concentration of BrdU-labeled cells analyzed by means of stereological quantification was restricted to the ependymal zone of the CC at 7–14 days time points (n = 4 rats, 12 sections per rat, *P < 0.1 and **P < 0.05, Student’s t test). Abbreviations: SCI, spinal cord injury; BrdU, 5-bromodeoxyuridine; CC, central canal
Quantification of BrdU nuclei in the ependyma of the rostral (A), central lesion (B), and caudal (C) spinal cord segments (4 mm long) after SCI. Note, the gradual increase of proliferating ependymal cells in the rostral-A segment (4–14 days), whereas decreasing tendency in the caudal C segment from (7–14 days). The central lesion segment B showed stable significant elevation of BrdU-positive cells from 7–14 days compared to sham-controls *P < 0.1 and **P < 0.05, Student’s t-test. Abbreviations: SCI, spinal cord injury; BrdU, 5-bromodeoxyuridine; CC, central canal
Coronal spinal cord sections taken from A, B, and C segments at 14 days after SCI, revealing the highest number of BrdU nuclei in A rostral segment, slight decrease in B segment, and the lowest number of BrdU profiles found in C segment. Scale bar = 50μm. Abbreviations: SCI, spinal cord injury; BrdU, 5-bromodeoxyuridine; CC, central canal
BrdU Positivity in the Ependymal Cells of Central Canal After Increased Physical Activity
In the second group, which was subjected to enhanced physical activity by running wheel, we have observed a significant 2.1-and 2.6-fold increases of BrdU IR cells in the thoracic spinal cord at 4 and 7 days. However, at longer period of survival, no significant increase (1.5–1.7 fold) of BrdU IR at 10–14 days in comparison to controls was detected (Fig. 9). The immunohistochemical detection of BrdU by specific antibodies showed a prominent number of BrdU-positive nuclei localized bilaterally within the ependymal lining in running group at 4 and 7 days (Fig. 10E–H) when compared with controls (not exposed to running wheel) (Fig. 10A–D). The distribution of densely stained elongated BrdU-positive nuclei (2 × 8–12 μm of diameter) was particularly concentrated to the medial portion of the ependymal region (Fig. 10E, F, indicated by intermitted line and arrows). The ventral and dorsal tip (floor and roof plate region referred to the embryonic stage) of the ependymal lining was mainly free of BrdU labeling, revealing only occasional BrdU nuclei (Fig. 10E). The anatomical appearance of enhanced number of BrdU positive nuclei within the medial region of the CC ependymal layer may resemble proliferative activity of neural progenitors localized in similar area of the neural tube, most probably giving rise to interneurons and motoneurons (Fig. 11).
Quantitative analysis of the BrdU nuclei in the ependyma of the thoracic spinal cord segments Th7-9 (1.2 cm long) after physical activity. Note, the significant increase of proliferating ependymal cells labeled with BrdU at 4 days and the maximal significant elevation of BrdU incorporation observed at 7 days. At the longer period of survival, 10-14 days, no significant increase of BrdU positive nuclei occurred, when compared to sham-controls. *P < 0.1 and **P < 0.05, Student’s t-test. Abbreviations: SCI, spinal cord injury; BrdU, 5-bromodeoxyuridine; CC, central canal
Representative coronal spinal cord sections cut throughout the thoracic segment Th8 in control rats at 4–14 days (A, B, C, and D), and rats after physical activity (running) at 4–14 days (E–H). Only few occasional BrdU nuclei could be seen in controls (A, B, C, and D, indicated by arrows). After physical activity, a significantly higher number of BrdU labeled nuclei at 4 and 7 days were seen mainly within the lateral, or occasional dorsal portion of the neuroepithelium (E, F, indicated by arrows) compared with controls. Lower number of BrdU-positive nuclei was seen at 10 days (G) and 14 days (H). Scale bar = 20 μm. Abbreviations: SCI, spinal cord injury; BrdU, 5-bromodeoxyuridine; CC, central canal
Comparison of the BrdU–IR in the CC of the thoracic spinal cord segment after physical activity at 4 days (A), or 7 days (C), with the schematic drawing of the main neuroepithelial zones, the basal plate (bp), intermediate plate (ip), and alar plate (ap), where rat neurons during embryogenesis are generated. Abbreviations: roof plate (rp) floor plate (fp). Scale bar = 10 μm
Phenotype of BrdU-Positive Cells After SCI
Nestin positivity was detected around the CC by 4 days, and it further increased at 7 days, spreading toward medial dorsal horn margins, surrounding the lesion site. (Fig. 12). Afterward, nestin positivity slightly declined by 10 D, thus only limited Nestin labeling around lesion site could be seen at 14 D (Fig. 13).
Time course of nestin expression following spinal cord injury at 4, 7, or 10 days. Immunofluorescence of nestin positivity (green) was detected in the region around the CC (circle) by 4 days that further increased at 7 days, spreading toward medial dorsal horn margins, surrounding the lesion site. Nestin immunoreactivity declined by 10 days, thus a limited nestin labeling was found, around CC. Arrows indicate the highest nestin labeling. CC, central canal. Scale bars at 10 days as indicated at 4 and 7 days
Nestin expression after spinal cord injury at 14 days in A segment (A) at 14 days. Note, the absence of nestin labeling (green) around the CC (B); however, limited amount of positive fibers and cell bodies around the medial dorsal horn margins occurred (B), higher magnifications taken from boxed area from (A) were detected. Scale bars: A = 100 μm, B = 50 μm
Despite the high density of BrdU labeling in the CC and in close vicinity, a careful search for BrdU and NeuN colocalization yielded no definitive associations (data not shown). However, some nestin-positive cells coexpressed GFAP, while others did not (Fig. 14C). However, many nestin-positive cells with undefined phenotype occurred in the close vicinity to the CC region at the most rostral spinal cord segments (Fig. 15A–D and 16). These data demonstrate that SCI in adult rats induces an endogenous ependymal cell response leading to their increased proliferation and differentiation primarily into macroglial phenotype as well as into cells of unknown phenotype.
Double labeling of SC section taken from A segment, following spinal cord injury at 14 days with GFAP (A, C), nestin (B), antibodies (C). Note, colocalization of GFAP/Nestin immunoreactivity at the lesion site (white matter of dorsal funiculi, single-bolted arrow), while adjacent nestin-positive cell body wilth long process did not colocalize with GFAP labeling (C, two arrows pointing to long process). Scale bars: A–C = 50 μm
Schematic illustration of BrdU IR in the thoracic spinal cord section (Th8) of the control, SCI or PhyA group. Note, the highest BrdU expression in the CC canal, and around the lesion site of SCI group, compare different distribution patterns of BrdU-positive nuclei in the ependyma between SCI and PhA group, and increased BrdU response in the parenchyma of the SC in both groups. Below each schematic drawing, a panel revealing BrdU–IR in the corresponding ventral white matter is performed. Scale bars: A–C = 100 μm
Discussion
In this study, we have compared the impact of two different paradigms: pathological spinal cord injury vs physiological stimulation on the proliferation activity of the CC ependymal progenitors in adult rats. Our results confirm and extend those of a set of previous studies indicating that the number of proliferating BrdU-labeled EC increases gradually with the time of survival after both paradigms; however, some important differences were documented and will be further clarified in the discussion (Zai and Wrathall 2005; Mothe and Tator 2005; Yamamoto et al. 2001; Danilov et al. 2006).
The most prominent response of ependymal progenitor cells was found after spinal cord injury at 7–14 days. This confirms that the selected mild spinal cord injury seems to be more powerful stimuli for proliferation of spinal NSC than physiological activation. Although, voluntary running or exposure to an enriched environment may increase neurogenesis in distinct regions of the adult mammalian CNS (Brown et al. 2003; van Praag et al. 1999), the spontaneous adaptation may lead to contradictory effect. We believe that this might be the explanation for rapid decrease of mitogenic activity, observed in the PhA group. Rats, that were performing daily exercise seemed to adaptate after 4 days, showing lower physical activity, mainly resting in the running wheel. Therefore, this might be a simple explanation for a spontaneous attenuation of the mitogenic activity in the spinal cord. This obstacle could be prevented by applying special treadmill equipment, with adjustable speed, thus performing standard running conditions for each rat.
It is well documented that the multipotent neural stem cells are found in many regions of the adult CNS, but the fundamental question of their precise origin (subventricular, ependymal, parenchymal), the induction signals essential for proliferation, migration, or differentiation remain still unresolved, and in many experimental studies apparently contradictory results have been reported (Rice et al. 2003; Horner et al. 2000; Ke et al. 2006). Therefore, due to growing evidence that these cells may originate from the periventricular region along the entire ventricular axis, particularly including the forebrain SVZ (Weiss et al. 1996; Coskun et al. 2008), the ependymal layer of the CC has been considered as the target anatomical region in this study. Thus, we have confirmed, that majority of equally distributed BrdU-labeled nuclei occurred in the ependymal layer after SCI (Mothe and Tator 2005). Furthermore, some dividing cells migrate from the CC ependyma laterally, or dorsally towards the lesion site (Johansson et al. 1999), most probably giving rise to macroglial elements (Johansson et al. 1999). However, after physical activity of running, a high number of BrdU-positive nuclei were restricted to the medial region of the CC, while the dorsal or ventral tip of the CC remained almost free of division. This phenomenon resembles the distribution pattern of the neuronal progenitors occurring in the neuroepithelial zones (the basal, intermediate, and alar plates) which are hypothesized to generate neurons during embryogenesis of rat (Altman and Bayer 1984). For example, the basal plate is considered to be the source of macroneurons—motor neurons, the intermediate plate the source of mesoneurons, i.e., intermediate-relay neurons and finally the alar plate, the source of microneurons, i.e., interneurons of the dorsal horn that are produced last. Due to this hypothesis, we might suggest that BrdU-positive profiles located in the medial part of CC after running may correspond rather to the neuronal than macroglial fate (Altman and Bayer 1984). In addition, many BrdU positive profiles were found also in the surrounding gray and white matter after both, SCI and physical activity, confirming that the NSCs occur also in the parenchyma of the spinal cord (Yamamoto et al. 2001). Moreover, an interesting and challenging observation has been documenting that the spinal cord white matter contain not only axonal tracts but also neuronal population (Abbadie et al. 1999).
Furthermore, utilizing the method of segmental sampling and applying the stereological quantification of BrdU–IR profiles, we have detected the time-dependent region-specific difference in the number of BrdU positive cells rostral vs caudal to the injury epicenter. The progressive increase of proliferating ependymal cells was found in the rostral segment at 7 days, <4 mm away from the lesion epicenter. On the contrary, the caudal segment showed moreover an opposite effect, when the number of BrdU-positive nuclei in CC decreased. It is well documented that in the rostral segments, the spared spinal cord tissue still contains intact or partially lesioned descending (ventro-–lateral–medial region) or ascending pathways. Therefore, the endogenously occurring neuregulins or other factors produced by activated axons may provide trophic support, or may directly influence mitotic activity of local progenitors cells (Fernandez et al. 2000; Barres and Raff 1999). This might lead to higher proliferation of NG2 positive cells, which stimulate oligodendroglial production, remyelination of damaged axons, and regeneration (Zai and Wrathall 2005). On the other hand, at the lesion site or at caudal segments, the severed axons release inhibitory molecules, including pro-mitotic factors that induce proliferation in the spared glia and together with macrophages-microglia, may contribute to formation of glial scar (McGee and Strittmatter 2003; Schwab 2004; Yamashita et al. 2005).
Furthermore, although a number of various pools of neural progenitors differing from original stem cells have been found in adult CNS, the lineage phenotypic plasticity, relationship, or independency among them have not yet been completely characterized (Kondo and Raff 2000; Palmer et al. 1999; Palmer et al. 2001). However, one group of studies strongly suggests, that a significant number of both parenchymal and ependymal neural progenitors are present in the adult spinal cord and contribute to de novo neurogenesis following CNS injury (Ke et al. 2006) (Danilov et al. 2006), while others moreover describe restricted gliogenesis and regret the possibility of neuronal differentiation (Zai and Wrathall 2005). Since there is a close relationship between the ependymal cells and type C astrocytes, which both originate from the radial glia, it is essential to suggest that these cells may also function as neural stem cells in adult CNS (Spassky et al. 2005). Another possible mechanism explaining neurogenesis in adult CNS system is unexpected phenotypic plasticity of progenitors, thus non-neurogenic progenitors may change their properties under certain conditions (Horner et al. 2000).
In this study, we have demonstrated the rapid expression of nestin in the ependymal cells of the CC at 1 week, which further decreases by 14 days post-injury. This may correlate particularly with the upregulation of macroglia (Johansson et al. 1999); however, scattered nestin-positive cells with bipolar or multipolar morphology could be found in the close vicinity to the CC region, in the dorsal and in ventral horn at 14 days. Unfortunately, we were not able to identify their phenotype, since they did not coexpress available developmental neuronal (DCX, Tuji, NeuN) or glial markers (NG2, O4, RIP, Iba, and GFAP). Thus, we assume that these nesting-positive cells may represent some developmental stages of NSC. Although the high mitotic activity in the CC was documented after SCI, no BrdU/NeuN colocalization could be confirmed. Thus, the majority of the dividing cells expressed glial phenotype (GFAP) or nestin. Taken together, these data, as do our results, confirm that SCI as well as physical activity in adult rats induces an endogenous ependymal cell response, which deserve further studies.
References
Abbadie C, Skinner K, Mitrovic I, Basbaum AI (1999) Neurons in the dorsal column white matter of the spinal cord: complex neuropil in an unexpected location. Proc Natl Acad Sci USA 96:260–265. doi:10.1073/pnas.96.1.260
Altman J, Bayer SA (1984) The development of the rat spinal cord. Adv Anat Embryol Cell Biol 85:1–164
Barres BA, Raff MC (1999) Axonal control of oligodendrocyte development. J Cell Biol 147:1123–1128. doi:10.1083/jcb.147.6.1123
Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, Kuhn HG (2003) Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J NeuroSci 17:2042–2046. doi:10.1046/j.1460-9568.2003.02647.x
Cizkova D, Rosocha J, Vanicky I, Jergova S, Cizek M (2006) Transplants of human mesenchymal stem cells improve functional recovery after spinal cord injury in the rat. Cell Mol Neurobiol 26:1165–1178. doi:10.1007/s10571-006-9093-1
Coskun V, Wu H, Blanchi B, Tsao S, Kim K, Zhao J, Biancotti JC, Hutnick L, Krueger RC Jr, Fan G, de Vellis J, Sun YE (2008) CD133 + neural stem cells in the ependyma of mammalian postnatal forebrain. Proc Natl Acad Sci USA 105:1026–1031. doi:10.1073/pnas.0710000105
Danilov AI, Covacu R, Moe MC, Langmoen IA, Johansson CB, Olsson T, Brundin L (2006) Neurogenesis in the adult spinal cord in an experimental model of multiple sclerosis. Eur J NeuroSci 23:394–400. doi:10.1111/j.1460-9568.2005.04563.x
Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A (1999) Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97:703–716. doi:10.1016/S0092-8674(00)80783-7
Fernandez PA, Tang DG, Cheng L, Prochiantz A, Mudge AW, Raff MC (2000) Evidence that axon-derived neuregulin promotes oligodendrocyte survival in the developing rat optic nerve. Neuron 28:81–90. doi:10.1016/S0896-6273(00)00087-8
Gage FH (2000) Mammalian neural stem cells. Science 287:1433–1438. doi:10.1126/science.287.5457.1433
Gomez-Pinilla F, Ying Z, Opazo P, Roy RR, Edgerton VR (2001) Differential regulation by exercise of BDNF and NT-3 in rat spinal cord and skeletal muscle. Eur J NeuroSci 13:1078–1084. doi:10.1046/j.0953-816x.2001.01484.x
Horner PJ, Power AE, Kempermann G, Kuhn HG, Palmer TD, Winkler J, Thal LJ, Gage FH (2000) Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci 20:2218–2228
Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J (1999) Identification of a neural stem cell in the adult mammalian central nervous system. Cell 96:25–34. doi:10.1016/S0092-8674(00)80956-3
Kakinohana O, Cizkova D, Tomori Z, Hedlund E, Marsala S, Isacson O, Marsala M (2004) Region-specific cell grafting into cervical and lumbar spinal cord in rat: a qualitative and quantitative stereological study. Exp Neurol 190:122–132
Ke Y, Chi L, Xu R, Luo C, Gozal D, Liu R (2006) Early response of endogenous adult neural progenitor cells to acute spinal cord injury in mice. Stem Cells 24:1011–1019. doi:10.1634/stemcells.2005-0249
Kehl LJ, Fairbanks CA, Laughlin TM, Wilcox GL (1997) Neurogenesis in postnatal rat spinal cord: a study in primary culture. Science 276:586–589. doi:10.1126/science.276.5312.586
Kondo T, Raff M (2000) Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science 289:1754–1757. doi:10.1126/science.289.5485.1754
Koson P, Zilka N, Kovac A, Kovacech B, Korenova M, Filipcik P, Novak M (2008) Truncated tau expression levels determine life span of a rat model of tauopathy without causing neuronal loss or correlating with terminal neurofibrillary tangle load. Eur J NeuroSci 28:239–246. doi:10.1111/j.1460-9568.2008.06329.x
Lie DC, Song H, Colamarino SA, Ming GL, Gage FH (2004) Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol 44:399–421. doi:10.1146/annurev.pharmtox.44.101802.121631
McGee AW, Strittmatter SM (2003) The Nogo-66 receptor: focusing myelin inhibition of axon regeneration. Trends Neurosci 26:193–198. doi:10.1016/S0166-2236(03)00062-6
Mercier F, Kitasako JT, Hatton GI (2002) Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol 451:170–188. doi:10.1002/cne.10342
Minger SL, Ekonomou A, Carta EM, Chinoy A, Perry RH, Ballard CG (2007) Endogenous neurogenesis in the human brain following cerebral infarction. Reg Med 2:69–74
Mothe AJ, Tator CH (2005) Proliferation, migration, and differentiation of endogenous ependymal region stem/progenitor cells following minimal spinal cord injury in the adult rat. Neuroscience 131:177–187. doi:10.1016/j.neuroscience.2004.10.011
Neeper SA, Gomez-Pinilla F, Choi J, Cotman C (1995) Exercise and brain neurotrophins. Nature 373:109. doi:10.1038/373109a0
Nordlander RH, Singer M (1978) The role of ependyma in regeneration of the spinal cord in the urodele amphibian tail. J Comp Neurol 180:349–374. doi:10.1002/cne.901800211
Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann GII, Jiang L, Kang J, Nedergaard M, Goldman SA (2003) Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med 9:439–447. doi:10.1038/nm837
Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH (1999) Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci 19:8487–8497
Palmer TD, Schwartz PH, Taupin P, Kaspar B, Stein SA, Gage FH (2001) Cell culture. Progenitor cells from human brain after death. Nature 411:42–43. doi:10.1038/35075141
Rice AC, Khaldi A, Harvey HB, Salman NJ, White F, Fillmore H, Bullock MR (2003) Proliferation and neuronal differentiation of mitotically active cells following traumatic brain injury. Exp Neurol 183:406–417. doi:10.1016/S0014-4886(03)00241-3
Schwab ME (2004) Nogo and axon regeneration. Curr Opin Neurobiol 14:118–124. doi:10.1016/j.conb.2004.01.004
Spassky N, Merkle FT, Flames N, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A (2005) Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci 25:10–18. doi:10.1523/JNEUROSCI.1108-04.2005
van Praag H, Kempermann G, Gage FH (1999) Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2:266–270. doi:10.1038/6368
Vanicky I, Urdzikova L, Saganova K, Cizkova D, Galik J (2001) A simple and reproducible model of spinal cord injury induced by epidural balloon inflation in the rat. J Neurotrauma 18:1399–1407. doi:10.1089/08977150152725687
Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC, Reynolds BA (1996) Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci 16:7599–7609
Yamamoto S, Yamamoto N, Kitamura T, Nakamura K, Nakafuku M (2001) Proliferation of parenchymal neural progenitors in response to injury in the adult rat spinal cord. Exp Neurol 172:115–127. doi:10.1006/exnr.2001.7798
Yamashita T, Fujitani M, Yamagishi S, Hata K, Mimura F (2005) Multiple signals regulate axon regeneration through the nogo receptor complex. Mol Neurobiol 32:105–112. doi:10.1385/MN:32:2:105
Zai LJ, Wrathall JR (2005) Cell proliferation and replacement following contusive spinal cord injury. Glia 50:247–257. doi:10.1002/glia.20176
Acknowledgments
We express our thanks to Maria Spontakova for her great and valuable assistance in the immunocytochemical analysis. This study was supported by financial grants: APVV 51-002105, VEGA 2-0019-08, VEGA 1-0674-09, APVV/0682/07, AV0Z50450515 (Research Concept of IAPG), MEB0808108, and APVV 0045-07.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cizkova, D., Nagyova, M., Slovinska, L. et al. Response of Ependymal Progenitors to Spinal Cord Injury or Enhanced Physical Activity in Adult Rat. Cell Mol Neurobiol 29, 999–1013 (2009). https://doi.org/10.1007/s10571-009-9387-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-009-9387-1