Abstract
Immunohistochemistry for neuron-specific nuclear protein (NeuN), caspase-3, calcitonin gene-related peptide (CGRP), and calcium-binding proteins was performed on the trigeminal ganglion (TG) in wild type and Brn-3a knockout mice at embryonic days 12.5–16.5 (E12.5–E16.5). In Brn-3a knockout mice, the number of NeuN-immunoreactive (ir) neuron profiles increased at E14.5 (40.0% increase) and decreased at E16.5 (28.3% reduction) compared to wild type mice. Caspase-3-ir neuron profiles were abundant in the TG of wild type mice at E12.5–E16.5. However, the loss of Brn-3a decreased the number of caspase-3-ir neuron profiles at E12.5 (69.7% reduction) and E14.5 (51.7% reduction). At E16.5, the distribution of caspase-3-ir neuron profiles was barely affected by the deficiency. CGRP-ir neuron profiles were observed in the TG of wild type mice but not knockout mice at E12.5. At E14.5 and E16.5, CGRP-ir neuron profiles were abundant in both wild type and knockout mice. Calbindin D-28 k (CB)-ir neuron profiles decreased in the TG of mutant mice at E12.5 compared to wild type mice (56.4% reduction). At E14.5, however, Brn-3a deficiency transiently increased CB-ir neuron profiles (169.4% increase as compared to wild type mice). Calretinin (CR)-ir neuron profiles could not be detected in the TG of wild type mice at E12.5–16.5. However, numerous CR-ir neuron profiles transiently appeared in the knockout mouse at E14.5. Parvalbumin (PV)-ir neurons appeared in wild type and knockout mice at E14.5. At this stage, the number of large (>50 μm2) PV-ir neuron profiles in knockout mice was fewer than that in wild type mice. The number and cell size of PV-ir neuron profiles were barely affected by the deficiency at E16.5. The present study indicates that the loss of Brn-3a causes increase of TG neurons at E14.5 and decrease of TG neurons at E16.5. It is also suggested that Brn-3a deficiency affects the number and cell size of CGRP- and calcium-binding protein-containing neurons at E12.5 and E14.5. Caspase-3-dependent cell death of CB- and CR-ir neurons may be suppressed by the deficiency at E14.5.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brn-3a/Brn-3.0 is a member of the POU family of transcription factors and expressed by neurons in the central and peripheral nervous systems (Xiang et al. 1995; McEvilly et al. 1996; Huang et al. 1999). Targeted deletion of Brn-3a gene causes defect of axonal growth in the trigeminal nerve and reduction of sensory neurons in the trigeminal ganglion (TG) (McEvilly et al. 1996; Xiang et al. 1996; Huang et al. 1999; Eng et al. 2001). Thus, this factor is considered to stimulate outgrowth of neuronal processes and protect neurons from apoptosis during development (McEvilly et al. 1996; Latchman 1998; Smith et al. 1997a, 1997b, 1998; Eng et al. 2001; Ensor et al. 2001).
Recently, we have investigated the distribution of neurochemical substances in TGs of the knockout mice at embryonic day18.5 (E18.5) and postnatal day 0 (P0) (Ichikawa et al. 2002). Small calbindin D-28 k (CB)- and calretinin (CR)-immunoreactive (ir) neurons increase and large parvalbumin (PV)-ir neurons decrease in the knockout mouse. The number of calcitonin gene-related peptide-(CGRP)-ir neurons is similar between wild type and knockout mice. However, loss of Brn-3a causes reduction in the cell size of CGRP-ir neurons. This suggests that Brn-3a deficiency may affect the cell body size of TG neurons and the content of neurochemical substances in TG neurons during embryonic period. However, little is known about the relationship between cell death of TG neurons and their expression of neurochemical substances.
In the present study, the number of TG neurons was examined in Brn-3a knockout and wild type mice during embryonic period by neuron-specific nuclear protein (NeuN) immunohistochemistry. The expression of apoptotic executioner caspase-3 was also examined in the TG of Brn-3a knockout mouse. In addition, the distribution of CGRP and calcium-binding proteins was investigated in the mutant TG.
Methods
Animals
Mice lacking the Brn-3a gene were prepared as described by Xiang et al. (1996). A total of 12 knockout mice were obtained from breeding of Brn-3a heterozygous mice, and used at E12.5, E14.5 and E16.5. Twelve age-matched wild type mice were used for normal control.
Immunohistochemistry
Animals were immersion-fixed overnight in 4% paraformaldehyde at 4°C. Subsequently, tissues were stored at 4°C in phosphate-buffered saline (PBS) containing 0.1 mM sodium azide until use. The head including the TG was immersed in PBS containing 20% sucrose overnight, frozen-sectioned sagittally at 8 μm, and thaw-mounted on gelatin-coated glass slides. Complete series of sections were divided into 10 subsets so that every tenth was mounted on the same slides. Each subseries was stained for one of the 6 antigens to be examined. These sections were incubated overnight with fluorescein isothiocyanate-conjugated monoclonal mouse anti-NeuN antibody, or rabbit antisera against caspase-3 (1:5,000, Cell Signaling Technology, Inc., USA), CGRP (1:50,000, Peninsula, USA), CB (1:50,000, Swant, Switzerland), CR (1:50,000; Winsky et al. 1989) or PV (1:50,000, Swant, Switzerland). The sections were subsequently incubated with biotinylated goat anti-rabbit IgG and avidin–biotin–horseradish peroxidase complex (Vector Laboratories) except for NeuN. All images of the TG were captured into a personal computer. For analysis of the number of TG neurons, the number of all NeuN-ir profiles was counted in all sections of the subseries. For other analysis, the number and cross-sectional area of all round or oval ir profiles >5 μm2 were obtained in all sections of each subseries. Thus, all data were obtained from every tenth of serial sections of four ganglia in four wild type mice and four ganglia in four Brn-3a knockout mice. The data were analyzed for each ganglion (LuminaVision program, Mitani Corporation, Japan) and the difference between wild type and Brn-3a knockout mice was analyzed by ANOVA and Bonferroni-type multiple t-test.
The specificity of the primary antisera used in this study has been described elsewhere (Winsky et al. 1989; Ichikawa et al. 1989, 2002; Sugimoto et al. 2004).
The experiments were carried out under the control of the Animal Research Control Committee in accordance with The Guidelines for Animal Experiments of Okayama University Medical School, Government Animal Protection and Management Law (No. 105), and Japanese Government Notification on Feeding and Safekeeping of Animals (No. 6). All efforts were made to minimize the number of animals used and their suffering.
Results
NeuN
At E12.5, NeuN-ir neuron profiles were abundant in wild type and Brn-3a knockout mice (figure not shown). However, the loss of Brn-3a affected the number of NeuN-ir neuron profiles at E14.5 and E16.5. The number of NeuN-ir neuron profiles increased in the knockout mouse compared to wild type mouse at E14.5, (40.0% increase) (Table 1). At E16.5, however, Brn-3a deficiency decreased the number of NeuN-ir neuron profiles (28.3% reduction) (Table 1).
Caspase-3
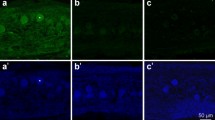
Caspase-3-ir neuron profiles were distributed throughout the TG of wild type mice at E12.5–E16.5 (Figs. 2a–c). They were abundant at E12.5 and E14.5, and decreased at E16.5. The mean numbers ± S.D. of caspase-3-ir neuron profiles/ganglion were 327.3 ± 44.6 at E12.5, 333.5 ± 22.6 at E14.5, and 190.8 ± 27.2 at E16.5 (Fig. 1). On the other hand, loss of Brn-3a drastically decreased the number of caspase-3-ir neuron profiles at E12.5 and E14.5 compared to wild type mice (Figs. 1, 2a, b, d, e, g, h, j, k). The mean numbers ± S.D. of caspase-3-ir neuron profiles/mutant ganglion were reduced to 99.3 ± 14.5 at E12.5 (69.7% decrease as compared to wild type mice) and 143 ± 22.7 at E14.5 (51.7% decrease as compared to wild type mice). At E16.5, however, the number of caspase-3-ir neuron profiles was barely affected by Brn-3a deficiency (204.8 ± 37.3) (Figs. 1, 2c, f i, l). Caspase-3-ir neuron profiles were evenly distributed in the TG of knockout mice at E12.5–E16.5.
Bar graphs showing the mean number ± S.D. of ir cells/ganglion of wild type and Brn-3a knockout mice. The data were obtained from four ganglia in wild type mice and four ganglia in Brn-3a knockout mice. * indicates significant difference between wild type and knockout mice (P < 0.01, ANOVA and Bonferroni-type multiple t-test)
Immunohistochemical microphotographs of capsae-3-ir neurons in the TG of wild type (a–f) and Brn-3a knockout (g–l) mice at E12 (a, d, g, j), E14.5 (b, e, h, k), and E16.5 (c, f, i, l). Brn-3a deficiency decreases the number of caspase-3-ir neurons at E12.5 (a, d, g, j) and E14.5 (b, e, h, k). At E16.5, however, the distribution of caspase-3-ir neurons is barely affected by the deficiency (c, f, i, l). Arrows and arrowheads point to small (<50 μm2) and large (>50 μm2) caspase-3-ir neurons, respectively. Bars = 200 μm (a–c, g–i) and 50 μm (d–f, j–l)
Cell size analysis demonstrated that Brn-3a deficiency significantly decreased the number of both small (<50 μm2) and large (>50 μm2) caspase-3-ir neuron profiles at E12.5 (70.5% [small] and 67.3% [large] decrease as compared to wild type mice) and E14.5 (51.6% [small] and 65.3% [large] decrease as compared to wild type mice) (Table 2, Fig. 2d, e, j, k). At E16.5, however, the number of small and large caspase-3-ir neuron profiles was similar in wild type and knockout mice (Table 2, Fig. 2f, l).
CGRP
Many CGRP-ir neuron profiles were detected in wild type mice at E12.5 (Fig. 3a, d). The mean number ± S.D. of ir neuron profiles/ganglion at E12.5 was 232.8 ± 28.5. These neuron profiles transiently decreased to 108 ± 94.3 at E14.5 and increased to 303.3 ± 34.2 at E16.5 (Fig. 1). CGRP-ir neuron profiles were evenly distributed throughout the ganglion of wild type mice (Figs. 3a–f). In knockout mice, however, CGRP-ir neuron profiles could not be detected at E12.5 (Fig. 3g, j). At E14.5, such neuron profiles appeared in the TG and the mean number ± S.D. of CGRP-ir neuron profiles/mutant ganglion was 65.5 ± 28.5 (Figs. 1, 3h, k). These neuron profiles increased to 275.3 ± 44.5 at E16.5 (Figs. 1, 3i, l). At E14.5 and E16.5, the topographic distribution and number of CGRP-ir neuron profiles were barely affected by Brn-3a deficiency (Figs. 1, 3b, c, e, f, h, i k, l).
Schematic drawings (a–c, g–i) and immunohistochemical microphotographs (d–f, j–l) of CGRP-ir neurons in representative sections of the TG of wild type (a–f) and Brn-3a knockout (g–l) mice at E12 (a, d, g, j), E14.5 (b, e, h, k), and E16.5 (c, f, i, l). CGRP-ir neurons are detected in wild type mice (a, d) but not in knockout mice at E12.5 (g, j). However, the distribution of CGRP-ir neurons is similar in wild type and knockout mice at E14.5 (b, e, h, k) and E16.5 (c, f, i, k). Green and red dots in (a–c, h, i) indicate small (<50 μm2) and large CGRP-ir neurons (>50 μm2), respectively. Arrows and arrowheads in d, e, f, k, and l point to small (<50 μm2) and large (>50 μm2) CGRP-ir neurons, respectively. Bars = 200 μm (a–c, g–i) and 50 μm (d–f, j–l)
The great majority of CGRP-ir neuron profiles were smaller than 50 μm2 in both wild type and knockout mice in the embryonic period (Table 2, Fig. 3a–l).
CB
CB-ir neuron profiles were abundantly scattered throughout the TG of wild type mice at E12.5–E16.5 (Fig. 4a–f). The mean number ± S.D. of CB-ir neuron profiles/ganglion in wild type mice at E12.5 was 688.8 ± 69.4, and gradually decreased toward 284 ± 68.1 at E16.5 (Fig. 1). On the other hand, loss of Brn-3a caused significant changes in the number and topographic distribution of CB-ir neuron profiles. At E12.5, CB-ir neuron profiles were seen only in the ventral portion of the mutant ganglion and the number (300.5 ± 55.9) was reduced by 56.4% as compared to wild type mice (Figs. 1, 4a, d, g, j). At E14.5, CB-ir neuron profiles dramatically but only transiently increased to 1,496 ± 214.1 (169.4% increase as compared to 555.3 ± 85.9 in wild type mice) (Figs. 1, 4b, e, h, k). At E16.5, CB-ir neuron profiles in the mutant TG drastically decreased to 318.3 ± 146.7, which is comparable to the number in wild type mice (284 ± 68.1) (Figs. 1, 4c, f, i, l). At E14.5 and E16.5, CB-ir neuron profiles were evenly distributed throughout the ganglion (Figs. 4b, c, h, i).
Schematic drawings (a–c, g–i) and immunohistochemical microphotographs (d–f, j–l) of CB-ir neurons in representative sections of the TG of wild type (a–f) and Brn-3a knockout (g–l) mice at E12 (a, d, g, j), E14.5 (b, e, h, k), and E16.5 (c, f, i, l). The number of CB-ir neurons in knockout mice (g, j) is fewer than that in wild type mice (a, d) at E12.5. At E14.5, however, Brn-3a deficiency dramatically increases the number of CB-ir neurons (b, e, h, k). The distribution of CB-ir neurons is similar in wild type and knockout mice at E16.5 (c, f, i, k). Green and red dots in (a–c) and (g–i) indicate small (<50 μm2) and large CB-ir neurons (>50 μm2), respectively. Arrows and arrowheads in (d–f) and (j–l) point to small (<50 μm2) and large (>50 μm2) CB-ir neurons, respectively. Bars = 200 μm (a–c, g–i) and 50 μm (d–f, j–l)
Cell size analysis demonstrated that loss of Brn-3a reduced the number of small and large CB-ir neuron profiles by 63.3 and 27.0%, respectively, as compared to wild type mice at E12.5 (Table 2, Fig. 4a, d, g, j). At E14.5, however, the deficiency resulted in 110.1% increase of small ir neuron profiles and 606.6% increase of large ir neuron profiles as compared to wild type mice (Table 2, Fig. 4b, e, h, k). The number of both small and large CB-ir neuron profiles was barely affected by the mutation at E16.5 (Table 2, Fig. 4c, f, i, l).
CR
CR-ir neuron profiles could not be observed in the TG of wild type mice at E12.5–E16.6 (Figs. 1, 5a–f). In knockout mice, the ganglion was also devoid of CR-ir neuron profiles at E12.5 (Fig. 5g, j). At E14.5, however, numerous CR-ir neuron profiles dramatically and transiently appeared in the TG (Figs. 1, 5h, k). The mean number ± S.D. of CR-ir neuron profiles/mutant ganglion was 1467.3 ± 327.1 (Fig. 1). These neuron profiles were observed throughout the mutant ganglion (Fig. 5h, k). They disappeared in the TG of knockout mice at E16.5 (Fig. 5i, l).
Schematic drawings (a–c, g–i) and immunohistochemical microphotographs (d–f, j–l) of CR-ir neurons in representative sections of the TG of wild type (a–f) and Brn-3a knockout (g–l) mice at E12 (a, d, g, j), E14.5 (b, e, h, k) and E16.5 (c, f, i, l). Small (arrows in k) and large (arrowheads in k) CR-ir neurons are detected only in knockout mice at E14.5. (Green and red dots in h indicate small (<50 μm2) and large CB-ir neurons (>50 μm2), respectively. Bars = 200 μm (a–c, g–i) and 50 μm (d–f, j–l)
By cell size analysis, CR-ir neuron profiles had small and large cell bodies in knockout mice at E14.5 (Table 2, Fig. 5h, k).
PV
PV-ir neuron profiles could not be detected in the ganglion of wild type mice at E12.5 (Fig. 6a, d). However, they were abundant and scattered throughout the ganglion at E14.5 and E16.5 (Figs. 1, 6b, c, e, f). The mean numbers ± S.D. of ir neuron profiles/ganglion in wild type mice were 433.8 ± 84.5 at E14.5 and 413.8 ± 78.0 at E16.5 (Fig. 1). The number of PV-ir neuron profiles was barely affected by loss of Brn-3a at E12.5 (0), E14.5 (408 ± 88.9), and E16.5 (370.8 ± 90.8). The topographic distribution of PV-ir neuron profiles was also similar between wild type and knockout mice at E14.5 and E16.5 (Fig. 6b, c, e, f, h, i, k, l).
Schematic drawings (a–c, g–i) and immunohistochemical microphotographs (d–f, j–l) of PV-ir neurons in representative sections of the TG of wild type (a–f) and Brn-3a knockout (g–l) mice at E12 (a, d, g, j), E14.5 (b, e, h, k), and E16.5 (c, f, i, l). The TG of wild type and knockout mice is devoid of PV-ir neurons at E12.5 (a, d, g, j). At E14.5, Brn-3a deficiency reduces large PV-ir neurons (b, e, h, k). The distribution and cell size of PV-ir neurons are similar in wild type (c, f) and knockout (i, l) mice at E16.5. Green and red dots in b, c, h and i indicate small (<50 μm2) and large CB-ir neurons (>50 μm2), respectively. Arrows and arrowheads in e, f, k, and l point to small (<50 μm2) and large (>50 μm2) PV-ir neurons, respectively. Bars = 200 μm (a–c, g–i) and 50 μm (d–f, j–l)
By cell size analysis, loss of Brn-3a decreased the number of large PV-ir neuron profiles at E14.5 (57.4% reduction as compared to wild type mice) (Table 2, Fig. 6b, e, h, k). The number of small and large PV-ir neuron profiles was barely affected by loss of Brn-3a at E16.5 (Table 2, Fig. 6c, f, i, l).
Discussion
The present study demonstrated that the number of NeuN-ir neurons increased in the TG of Brn-3a knockout mouse at E 14.5. The present study also revealed that the number of small and large caspase-3-ir cells reduced in Brn-3a knockout mice at E12.5 and E14.5. Thus, Brn-3a deficiency probably suppresses caspase-3-dependent cell death at E12.5 and E14.5, and increases the number of TG neurons at E14.5. At E16.5, the number of NeuN-ir neurons decreased in the TG of Brn-3a knockout mice at E 16.5. In this study, the distribution of caspase-3 was not examined in the mutant TG between E14.5 and E16.5. However, a previous study by Nissl stain has demonstrated that pyknotic cells markedly increase in the TG of Brn-3a knockout mice at E15.5 (Huang et al. 1999). Thus, Brn-3a deficiency is considered to accelerate cell death of TG neurons at this stage. As a result, the number of TG neurons may decrease in the mutant TG at E16.5.
The present study also investigated the distribution of CGRP and calcium-binding proteins in wild type and Brn-3a-knockout mice. At E12.5, CGRP-ir neurons were observed in the TG of wild type mice whereas such neurons could not be detected in Brn-3a knockout mice. Brn-3a deficiency also decreased the number of CB-ir neurons at this stage. As shown in a previous study, excessive cell death of TG neurons could not be detected in Brn-3a knockout mice until E14.5 (Huang et al. 1999). Therefore, the expression of CGRP and CB in TG neurons may be suppressed by the deficiency at E12.5.
At E14.5, loss of Brn-3a increased the number of small and large CB-ir neurons. This observation is similar to a previous microarray finding that the expression of mRNA for calbindin increased in the TG of Brn-3a knockout mice at E13.5 (Eng et al. 2004). In addition, small and large CR-ir neurons appeared in Brn-3a knockout mice but not in wild type mice at E14.5. The increase of CB- and CR-ir neurons may be correlated to the reduction of caspase-3-ir neurons in mutant mice at E12.5–14.5. It is possible that the loss of Brn-3a suppresses caspase-3-dependent cell death of CB- and CR-ir neurons. However, the possibility that the transient increase of CB- and CR-ir neurons at E14.5 results from transient expression of CB and CR proteins could not be also neglected in this study. On the other hand, large PV-ir neurons decreased in Brn-3a knockout mice compared to wild type mice at E14.5. This reduction is unlikely to be correlated to cell death of TG neurons, because their excess cell death could not be detected at E12.5–E14.5. The loss of Brn-3a may reduce PV protein in large TG neurons.
Conclusion
The present study investigated the effect of Brn-3a deficiency on the expression of NeuN, caspase-3, CGRP, and calcium-binding proteins. We found that the number of NeuN-ir neurons increased at E14.5 and decreased at E16.5 in Brn-3a knockout mice. Caspase-3-ir cells were significantly reduced in the mutant mouse at E12.5 and E14.5 whereas CB- and CR-ir neurons dramatically and transiently increased at E14.5. Thus, loss of Brn-3a may suppress caspase-3-dependent death of CB- and CR-ir neurons.
References
Eng SR, Gratwick K, Rhee JM, Fedtsova N, Gan L, Turner EE (2001) Defects in sensory axon growth precede neuronal death in Brn3a-deficient mice. J Neurosci 21:541–549
Eng SR, Lanier J, Fedtsova N, Turner EE (2004) Coordinated regulation of gene expression by Brn3a in developing sensory ganglia. Development 131:3859–3870. doi:10.1242/dev.01260
Ensor E, Smith MD, Latchman DS (2001) The Brn-3a transcription factor protects sensory but not sympathetic neurons from programmed cell death/apoptosis. J Biol Chem 276:5204–5212. doi:10.1074/jbc.M007068200
Huang EJ, Zang K, Schmidt A, Saulys A, Xiang M, Reichardt LF (1999) POU domain factor Brn-3a controls the differentiation and survival of trigeminal neurons by regulating Trk receptor expression. Development 126:2869–2882
Ichikawa H, Wakisaka S, Matsuo S, Akai M (1989) Peptidergic innervation of the temporomandibular disk in the rat. Experientia 45:303–304. doi:10.1007/BF01951817
Ichikawa H, Yamaai T, Jacobowitz DM, Mo Z, Xiang M, Sugimoto T (2002) Effect of Brn-3a deficiency on parvalbumin-, calbindin D-28 k-, calretinin- and calcitonin gene-related peptide-immunoreactive primary sensory neurons in the trigeminal ganglion. Neuroscience 113:537–546. doi:10.1016/S0306-4522(02)00182-3
Latchman DS (1998) The Brn-3a transcription factor. Int J Biochem Cell Biol 30:1153–1157. doi:10.1016/S1357-2725(98)00090-9
McEvilly RJ, Erkman L, Luo L, Sawchenko PE, Ryan AF, Rosenfeld MG (1996) Requirement for Brn-3.0 in differentiation and survival of sensory and motor neurons. Nature 384:574–577. doi:10.1038/384574a0
Smith MD, Dawson SJ, Latchman DS (1997a) The Brn-3a transcription factor induces neuronal process outgrowth and the coordinate expression of genes encoding synaptic proteins. Mol Cell Biol 17:345–354
Smith MD, Morris PJ, Dawson SJ, Schwartz ML, Schlaepfer WW, Latchman DS (1997b) Coordinate induction of the three neurofilament genes by the Brn-3a transcription factor. J Biol Chem 272:21325–21333. doi:10.1074/jbc.272.34.21325
Smith MD, Ensor EA, Coffin RS, Boxer LM, Latchman DS (1998) Bcl-2 transcription from the proximal P2 promoter is activated in neuronal cells by the Brn-3a POU family transcription factor. J Biol Chem 273:16715–16722. doi:10.1074/jbc.273.27.16715
Sugimoto T, Jin H, Fujita M, Fukunaga T, Nagaoka N, Yamaai T, Ichikawa H (2004) Induction of activated caspase-3-immunoreactivity and apoptosis in the trigeminal ganglion neurons by neonatal peripheral nerve injury. Brain Res 1017:238–243. doi:10.1016/j.brainres.2004.05.069
Winsky L, Nakata H, Martin BM, Jacobowitz DM (1989) Isolation, partial amino acid sequence, and immunohistochemical localization of a brain-specific calcium-binding protein. Proc Natl Acad Sci USA 86:10139–10143. doi:10.1073/pnas.86.24.10139
Xiang M, Zhou L, Macke JP, Yoshioka T, Hendry SH, Eddy RL, Shows TB, Nathans J (1995) The Brn-3 family of POU-domain factors: primary structure, binding specificity, and expression in subsets of retinal ganglion cells and somatosensory neurons. J Neurosci 15:4762–4785
Xiang M, Gan L, Zhou L, Klein WH, Nathans J (1996) Targeted deletion of the mouse POU domain gene Brn-3a causes selective loss of neurons in the brainstem and trigeminal ganglion, uncoordinated limb movement, and impaired suckling. Proc Natl Acad Sci USA 93:11950–11955. doi:10.1073/pnas.93.21.11950
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ichikawa, H., Terayama, R., Yamaai, T. et al. Brn-3a Deficiency Transiently Increases Expression of Calbindin D-28 k and Calretinin in the Trigeminal Ganglion During Embryonic Development. Cell Mol Neurobiol 29, 691–698 (2009). https://doi.org/10.1007/s10571-009-9383-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-009-9383-5