Abstract
Free radicals play an important role in the pathogenesis of brain injury. This study evaluates the potential relationship between ischaemia/reperfusion (I/R)-induced brain injury, peripheral oxidative stress (lymphocyte DNA damage), plasma antioxidant potential and uric acid levels. We observed that 15 min of ischaemia were sufficient to significantly increase lymphocyte DNA damage that remained elevated at the end of early (3 h) reperfusion and at later (72 h) reperfusion time; this parameter was not significantly increased, when compared to preoperated levels. In parallel, antioxidant potential was elevated after 15 min of ischaemia, remained high at early (3 h) reperfusion and decreased again with longer (72 h) reperfusion. A close association between the plasma antioxidant status and the uric acid content has been confirmed by findings that changes in TRAP values positively correlate with uric acid concentration in rat plasma after ischaemic injury. Moreover, results of in vitro experiments with extra uric acid addition to control plasma have shown that uric acid contributes to a greater part of TRAP values. These results indicate a similar time course of brain I/R-associated oxidative stress and peripheral antioxidant defence status and/or oxidative stress in animal experiments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain ischaemia and reperfusion (I/R) injury is associated with an increased production of free radicals in the brain, causing injury that can lead to significant morbidity and mortality (Valko et al. 2007). Following brain ischaemia, free radicals are generated from several potential sources, such as the mitochondrial respiratory chain, reactions catalysed by NOS synthase, cyclo- and lipooxygenase, xanthine oxidase, autooxidation of various small molecules etc. (Nita et al. 2001; Fiskum et al. 2004; Kolesarova et al. 2006). The massive burst of free radicals seen during reperfusion may have a different subcellular origin than during ischaemia. This source has not yet been convincingly identified. However, activated microglia and inflammation are possible candidates (Shi and Liu 2007).
Although the direct initial insult to neurons may not be caused by free radicals, the effects mediated by increased free radical generation can cause secondary damage that is far greater and of more importance for the understanding of the various neurodegenerative disorders (Heiss 2002). Free radical generation in ischaemic and reperfused tissues causes oxidative damage to cellular macromolecules including membrane lipids, proteins and nucleic acids (Lehotsky et al. 2004; Urikova et al. 2006). The toxicity of the free radicals results from their modification of macromolecules, especially DNA, and from the resulting induction of apoptotic and necrotic pathways. The most commonly measured markers of oxidative damage to DNA are 8-oxo-7,8-dihydroguanine (8-oxoG) and 8-oxodeoxyguanosine (8-oxodG), which are products of oxidative modification of guanine or deoxyguanosine of nuclear or mitochondrial DNA (Collins 2005). The level of oxidative DNA damage and its repair can be measured by the modified comet assay using a restriction enzyme formamidopyrimidine-DNA glycosylase (Fpg), which recognizes and removes the oxidized purines and some alkylate DNA products. For instance, this endonuclease is involved in the first step of the base excision repair to remove specific modified bases from DNA to create apurinic or apyrimidinic sites (AP-site), which are subsequently cleaved by its AP lyase activity giving a gap in the DNA strand. These lesions are measured as additional strand breaks, which can be detected by the comet assay (Collins et al. 1997; Fracasso et al. 2006).
Endogenous antioxidants play an integral role in dealing with the massive increases in free radicals that are generated after I/R injury in the brain (Crack and Taylor 2005). This defence against free radical injury is provided by enzymatic (catalase, superoxide dismutase, glutathione peroxidase etc.), nonenzymatic (glutathione, vitamin A, C, E, coenzyme Q, uric acid etc.) free radical scavenging systems and metal chelators (Sies 1993; Green and Ashwood 2005; Margaill et al. 2005; Danielisova et al. 2006).
The purpose of this study was to investigate whether oxidative stress in the brain after ischaemia and reperfusion could be associated with peripheral oxidative damage to DNA (recorded by lymphocyte 8-oxoG levels) and changes in plasma total antioxidant capacity and uric acid levels in a rat model of global brain ischaemia.
Material and Methods
Chemicals
R-Phycoerythrin (R-PE); 6-OH-2,5,6,7-tetramethyl-2-carboxylic acid (Trolox) and 2,2′-azobis(2-methylpropionamide) dichloride (AAPH) were obtained from Aldrich Chem. Co. (USA). Histopaque 1083; Agarose Type VII A (low melting point); Agarose Type I-A (normal melting point); 4,6-diamidine-2-phenylindol (DAPI); formamidopyrimidine-DNA glycosylase (Fpg) and all other chemicals used in this study were purchased from Sigma Co. (USA). Glass microscope slides were used non-fully frosted.
Procedures for Global Forebrain Ischaemia/Reperfusion in Rat
Male Wistar rats (6 months) weighing 330–380 g were obtained from the breeding station of Slovak Academy of Sciences, Dobrá Voda, Slovakia and were divided into four groups: (a) C—sham-operated control animals; (b) I—animals with 15 min of ischaemia; (c) 3 h I/R—animals with 3 h of continuous reperfusion after 15 min of global brain ischaemia and (d) 72 h I/R—animals with 72 h of continuous reperfusion after 15 min of global brain ischaemia.
Animal care and treatment were conducted according to the guidelines of the National Institute of Health for the care and use of laboratory animals and this study was approved with Ethical Committee, State Veterinary Administration of the Slovak Republic. The animals were housed in wired-bottomed cages in a temperature-controlled room (22°C) with a 12-h light/dark cycle. Food and distilled water were provided ad libitum.
In animal model preparation, four-vessel occlusion was used to induce global brain ischaemia, described by Pulsinelli and Brierley (1979) with modifications described by Lehotsky et al. (2004). Briefly, under anaesthesia with halothane, carotid arteries were exposed and bilateral vertebral arteries were occluded permanently by electrocautery. On the next day, both carotid arteries were occluded with aneurysm clips for 15 min. At the end of 15 min, the carotid artery claps were released to allow re-circulation of the brain lasting for short (3 h) and prolonged (72 h) period. Sham-operated animals received the same surgical procedures except that carotid arteries were not occluded. Normothermic conditions were maintained in the range of 36.5–37.5°C using a homeothermic blanket and a temperature-regulated heating lamp. During the first 3 h reperfusion rats were placed on a warm container to maintain body temperature at 37°C. After ischaemic and/or reperfusion periods, rats were decapitated and blood was removed and collected into heparinized tubes. Aliquots of whole blood were immediately centrifuged at 12,000g for 3 min, kept on ice-bath and rapidly assayed for TRAP. A portion (0.3 ml) of whole blood was retained for comet assay. The rest of blood was used for preparation of plasma by centrifugation at 2,000g for 15 min.
Total Radical-Trapping Antioxidant Potential (TRAP Assay)
The total radical-trapping antioxidant potential (TRAP) was determined by a modified method of Ghiselli et al. (1995). This method is based on the protection afforded by plasma antioxidants against the decay of R-PE (R-Phycoerythrin) fluorescence emission during a controlled peroxidation reaction initiated by AAPH. Reaction mixture (2 ml) contained 7.5 μl R-PE (final concentration 1.5 × 10−8 mol/l), rat plasma (8 μl) in 75 mmol/l phosphate buffer, pH 7.0 and was pre-incubated at 37°C for 5 min in 10 mm fluorimeter cells. Adding AAPH to a final concentration of 4.0 mmol/l started the oxidation reaction. The decay of R-PE fluorescence at 575 nm (10 nm slit width) was monitored by excitation at 495 nm (10 nm slit width) every 5 min for 120 min on a spectrofluorometer RF-540 (Shimadzu), equipped with a thermostatically controlled cell-holder. The results were standardized using 30 μl of 120 μmol/l Trolox, a water-soluble analogue of α-tocopherol. TRAP values were calculated from the length of the lag-phase due to the sample compared with that of Trolox and were expressed as μmol of Trolox/l of plasma.
Contribution of Uric Acid to TRAP
Plasma uric acid levels were measured using the commercial Uric acid liquicolor kit (Human, Sigma, St. Louis, MO). The potential contribution of uric acid to TRAP values was determined in three independent groups: (a) in control plasma itself; (b) in uric acid dissolved in water with equivalent concentration as detected in control plasma (36 μmol/l), 2-fold and 3-fold as in control plasma (72 and 108 μmol/l) and (c) in control plasma with added uric acid at a concentration as found in plasma at 3 h reperfusion. TRAP values shown are the mean of two independent measurements.
Oxidative Damage to DNA—Single Cell Gel Electrophoresis (SCGE)-Comet Assay
Isolation of Lymphocytes
Three hundred microlitre of fresh blood was mixed with 1 ml of PBS (0.1 mol/l, pH 7.4) and incubated on ice for 30 min. After incubation, blood was layered onto 100 μl of Histopaque 1083 (on ice) and centrifuged at 200g, 4°C, for 5 min. The layer of lymphocytes was collected into 500 μl of PBS and centrifuged again in the same conditions. Sediment (lymphocytes) was used for comet assay. The yield of lymphocytes was sufficient for four gels (2 × 104 cells in each gel).
Comet Assay
DNA single strand breaks were measured using alkaline comet assay (Collins et al. 1997). Glass microscope slides were frosted with 1% normal melting point agarose (type I-A) prepared in deionized water. Lymphocytes (from 300 μl of blood) were resuspended in 400 μl of 0.8% low melting point agarose (type VII A) in PBS at 37°C and pipetted onto a frosted microscope slide precoated with 100 μl of 1% normal melting point agarose. Slides with layers of lymphocytes in agarose were incubated at 4°C for 10 min and then immersed in lysis solution (2.5 mol/l NaCl, 100 mmol/l Na2EDTA, 10 mmol/l Tris, 1% Triton, pH 10.0) for 1 h to remove cellular membranes. After washing in enzyme buffer (40 mmol/l HEPES, 0.1 mol/l KCl, 0.5 mmol/l EDTA and 0.2 mg/ml BSA, pH 8.0), the gels were incubated with formamidopyrimidine-DNA glycosylase (FPG) protein in enzyme buffer or in enzyme buffer at 37°C only. Then, slides were placed in a horizontal electrophoresis tank containing electrophoretic solution (1 mmol/l Na2EDTA, 300 mmol/l NaOH, pH 13.0) at 4°C for 40 min (DNA unwinding). Electrophoresis was performed in the same solution at 25 V, 300 mA, 4°C, for 30 min. The slides were washed thrice for 5 min at 4°C with neutralizing buffer (0.4 mmol/l Tris, pH 7.5) before staining with 20 μl 4′,6-diamidine-2-phenylindole dihydrochloride (DAPI) at 2 μg/ml.
Visual Scoring
Each slide was viewed by fluorescence microscopy (Olympus BX 41, United Kingdom) and the degree of damage in nucleotides was assessed visually. Each of 100 nucleotides or comets was assigned a score from 0 to 4, depending on the fraction of DNA pulled out into the tail under the influence of the electric field (Collins et al. 1996). Results were expressed as the total damage to DNA (TD). TD was calculated as sum = (class of damage × number of cells in this class). The levels of 8-oxoG were calculated from values of TD using calibration curve y = 134.97x + 7.0612 according to ESCODD (2005), where y means TD and x means breaks of DNA. From the breaks, the fraction of 8-oxoG per 106 guanine was calculated according to ESCODD (2005). Experiments were done in duplicate.
Statistics
The data are reported as mean ± standard error of the mean. The differences between ischaemia, ischaemia/reperfusion and control groups of rats were analysed with Student’s t-test. A P value of less than 0.05 was considered statistically significant.
Results
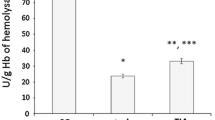
Global brain ischaemia initiated damage to DNA in peripheral lymphocytes, as the comet assay detected a statistically significant increase of lymphocyte 8-oxoG levels at the end of 15 min of ischaemia (0.56 ± 0.06 8-oxoG/106 G, P < 0.05) in comparison to values in lymphocytes from sham-operated rats (0.37 ± 0.04 8-oxoG/106 G). After ischaemia followed by early (3 h) and by late (72 h) reperfusion, DNA damage (8-oxoG levels) remained elevated (0.48 ± 0.1 and 0.43 ± 0.02 8-oxoG/106 G) in comparison to values in sham-operated rats, however, this difference was not statistically significant (Fig. 1).
The total radical-trapping potential and overall antioxidant potential of rat plasma during brain ischaemic insult was determined by the TRAP assay by using AAPH, a peroxyl radical generator. This assay measures antioxidants located in both the hydrophilic and lipophilic compartments of plasma. Time course of the total antioxidant capacities in blood plasma during global brain I/R is shown in Fig. 2. TRAP values were significantly increased at the end of 15 min of ischaemia (660.0 ± 17.8 μmol/l, P < 0.001), as well as at the end of early (3 h) reperfusion (652.6 ± 18.9 μmol/l, P < 0.001). Interestingly, TRAP values were significantly decreased at the end of late (72 h) reperfusion (410.2 ± 20.2 μmol/l, P < 0.05) and did not even reach levels seen in sham-operated animals (513.3 ± 41.5 μmol/l).
Time course of the plasma total radical-trapping antioxidant potential (TRAP) during global brain ischaemia, early (3 h) and late (72 h) reperfusion. The production of peroxyl radicals in plasma was initiated by adding AAPH at a final concentration of 4.0 mmol/l. Results are presented as mean ± SEM for n = 6. **P < 0.01; *P < 0.05 significantly different as compared to sham-operated rats
It is generally accepted that uric acid accounts for a greater part of total antioxidant plasma potential. In order to evaluate contribution of uric acid to TRAP, the plasma level of this antioxidant of metabolic origin was measured. As shown in Fig. 3, levels of plasma uric acid were altered in a time-dependent way after ischaemic insult. After 15 min of ischaemia it reached a value of 82.8 ± 17 μmol/l, (P < 0.001), remained high at early (3 h) reperfusion period (75.5 ± 10 μmol/l, P < 0.001), whereas at the late (72 h) reperfusion time the uric acid level (28.2 ± 2.6 μmol/l, P < 0.05) had recovered to levels of sham-operated animals (30.8 ± 7 μmol/l).
To support our view that increased TRAP values can be at least partially explained by antioxidant activity of uric acid, we measured TRAP in the reaction mixture containing plasma and increasing levels of exogenous uric acid (72 and 108 μmol/l). As shown in Fig. 4, uric acid itself expressed significant TRAP potential and accounts for 40–70% of TRAP values corresponding to control plasma (uric acid level = 36 μmol/l). The addition of uric acid to control plasma at levels observed at 3 h of reperfusion had an additive antioxidant effect proportional to the amount added. TRAP values of plasma supplemented with 72 μmol/l or 108 μmol/l uric acid were increased by respectively 22% (674 ± 11 μmol/l) and 60% (885 ± 22 μmol/l) above untreated plasma samples (553 ± 36.8 μmol/l). These experiments therefore suggest that uric acid contributes significantly to TRAP values in plasma and that it accounts for the greater part of total plasma antioxidant potential.
Effect of uric acid on the plasma (P) total radical-trapping antioxidant potential (TRAP). Uric acid was used at different concentrations—72 μmol/l (UA 72) and 108 μmol/l (UA 108). UA 72/UA 108 + P (exper) expresses the combination effect of uric acid with plasma on TRAP values obtained experimentally and UA 72/UA 108 + P (math) calculated mathematically as a sum of TRAP values of individuals agents. Values are mean of two independent experiments
Discussion
Previous studies of animal models of neuronal damage have demonstrated that during the post-ischaemic period, single-strand breaks and oxidized bases in brain DNA are rapidly formed (Chen et al. 1997; Nagayama et al. 2000; Giovannelli et al. 2002). DNA damage following brain ischaemia involves two distinct mechanisms: oxidative injury and endonuclease-mediated nuclear DNA fragmentation. There is evidence that oxidative DNA damage is an early and potentially reversible event in ischaemic brain injury. In contrast, DNA degradation occurs at a relatively later stage of neuronal apoptotic and necrotic cell death (Chen et al. 1997; Liu et al. 2004). In our experimental model, we have observed that the lymphocyte levels of 8-oxoG from animals with I/R of brain was significantly increased at the end of ischaemia and remained elevated at the first measurement after starting reperfusion (3 h). However, after a longer (72 h) reperfusion time, this parameter was not significantly increased as compared to preoperated levels. To the best of our knowledge, this is the first indication for increased total DNA damage to lymphocyte during global brain ischaemia. Dysbalance in production/scavenging of very reactive oxygen (hydroxyl radical, superoxide) and nitrogen species leads to spatial and temporal injury after brain ischaemia (Lehotsky et al. 2004; Burda et al. 2006). The hydroxyl radical is known to react with all components of the DNA molecule, damaging both the purine and pyrimidine bases and also the deoxyribose backbone. The hydroxyl radical is generated from superoxide or hydrogen peroxide in the presence of ferrous iron (Repine et al. 1981), or from the degradation of peroxynitrite, a product of the reaction between superoxide and nitric oxide (Beckman et al. 1990). It is known that H2O2 is not polar and that it readily crosses membrane. We assume that an increase in lymphocyte 8-oxoG levels during global brain ischaemia could be due to crossing of hydrogen peroxide from brain cells into the blood, generating highly reactive hydroxyl radicals by Fenton reactions with massive attack on lymphocyte DNA. Liu et al. (2004) have shown that in rats with middle cerebral artery occlusion, the increase in 8-oxodG-containing cells in the cortical region is associated with its immediate movement into the blood and with an increase of 8-oxodG in plasma. Accumulation of oxidative DNA adducts with I/R could increase the level of spontaneous mutagenesis.
The amount of reactive species produced during I/R of brain is influenced by antioxidant defence mechanisms. The total antioxidant capacity of body fluid is the result of a cooperative interaction between various antioxidants and it is crucial for the maximum suppression of free radical reactions in extracellular compartments (Valko et al. 2007; Margaill et al. 2005). The relative contribution of the peripheral TRAP to brain ischaemia-related oxidative stress is not well defined, and its time course has not been described. We found that the TRAP values in plasma were significantly increased after 15 min of ischaemia, remained high at early (3 h) reperfusion and decreased significantly at the end of later (72 h) reperfusion. Indeed, recent studies have provided evidence that under pathologic conditions such as cerebral I/R, the antioxidant systems of plasma (Frassetto et al. 1999) and brain have been changed (Hosseinzadeh and Sadeghnia 2005). Other studies demonstrated in different brain areas significantly decreased activities and levels of both non-enzymatic antioxidants like reduced glutathione (Nita et al. 2001) and enzymatic antioxidants like superoxide dismutase (Aabdallah and Eid 2004), catalase (Homi et al. 2002), glutathione reductase, glutathione-S-transferase and glutathione peroxidase (Ahmad et al. 2006).
Uric acid accounts for the greater part of the total reducing power in blood plasma (Ryan et al. 1997). This compound protects against excitotoxic and Fe+2 insults in cell culture and against damage from focal ischaemia in vivo (Yu et al. 1998). Uric acid is particularly effective in detoxifying hydroxyl radical and peroxynitrite and therefore it is thought to prevent lipid peroxidation and the consequent damage to membranes (Becker 1993). An adaptation mechanism which includes a decrease in the overall reducing power of low molecular weight antioxidants and subsequent mobilization to extracellular compartments, including plasma, has been described in several injurious experimental paradigms such as head trauma (Moor et al. 2001), intestinal ischaemia (Slavikova et al. 1998), morphine administration (Enrico et al. 1997) and focal ischaemia (Yu et al. 1998). In addition, ischemic brain preconditioning is known to upregulate antioxidants in brain and in peripheral organs especially by uric acid elevation (Glantz et al. 2005). In experimental studies, uric acid administration showed a protective effect against I/R injury in a rat model of transient middle cerebral artery occlusion (Yu et al. 1998). Reduction of brain damage by uric acid has been indicated also in a recent study on tromboembolic stroke (Romanos et al. 2007). In humans, elevated plasma uric acid is thought to represent an independent cardiovascular and stroke risk factor. However, in a recent study Gerber et al. (2006) found that in addition to the well-known increased mortality among hyperuricemic subjects, there is also an association between low levels of uric acid and fatal stroke, a link deserves further exploration. Likewise, systemic uric acid administration increases serum antioxidant capacity in healthy volunteers (Waring et al. 2001).
Importantly, the present data provide evidence that during global brain I/R the organism is trying to cope with the deleterious effects of free radicals by increasing the products of endogenous antioxidants (e.g. uric acid, ascorbic acid). In addition, a close association between the plasma antioxidant status and the uric acid content has been confirmed by findings that changes in TRAP values positively correlate with uric acid concentration in rat plasma after ischaemic injury. Moreover, results of experiments with extra uric acid addition to control plasma have shown that uric acid contributes for a greater part to TRAP values. We assume that ischaemia/early reperfusion accumulation of uric acid is the result of purine catabolism by xantine oxidase which can metabolize hypoxanthine to xanthine and uric acid (Glantzounis et al. 2005). Although this reaction leads to the release of free radicals (Nihei et al. 1989), our results and results from other laboratories (Yu et al. 1998; Romanos et al. 2007) suggest that uric acid formation by xanthine oxidase may provide a significant antioxidant defence against free radicals.
Several studies have provided conflicting results about the clinical significance of elevated uric acid in patients with cerebrovascular diseases. The antioxidant capacity of uric acid has been shown to correlate with the clinical prognosis of patients with acute ischaemic stroke (Cherubini et al. 2000; Chamorro et al. 2002; Romanos et al. 2007). On the other hand, increased levels of uric acid did not prevent the decrease in total antioxidant capacity in stroke patients (Gariballa et al. 2002). Our study suggests a close correlation between ischaemic oxidative stress of brain and the time course of peripheral antioxidant defence status and/or oxidative stress in an animal model. It is suggested that the antioxidant capacity and endogenous production of uric acid might be important factors providing adaptation to neurological damage caused by I/R-associated oxidative stress. These parameters may be useful as independent clinical outcome predictors in stroke patients.
References
Aabdallah DM, Eid NI (2004) Possible neuroprotective effects of lecithin and alpha-tocopherol alone or in combination against ischemia/reperfusion insult in rat brain. J Biochem Mol Toxicol 18:273–278
Ahmad S, Yousuf S, Ishrat T, Khan MB, Bhatia K, Fazli IS, Khan JS, Ansari NH, Islam F (2006) Effect of dietary sesame oil as antioxidant on brain hippocampus of rat in focal cerebral ischemia. Life Sci 79:921–1928
Becker BF (1993) Towards the physiological function of uric acid. Free Radic Biol Med 14:615–631
Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA (1990) Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA 87:1620–1624
Burda J, Danielisova V, Nemethova M, Gottlieb M, Matiasova M, Domorakova I, Mechirova E, Ferikova M, Salinas M, Burda R (2006) Delayed postconditioning initiates additive mechanism necessary for survival of selectively vulnerable neurons after transient ischemia in rat brain. Cell Mol Neurobiol 26:1141–1151
Collins AR (2005) Assays for oxidative stress and antioxidant status: applications to research into the biological effectiveness of polyphenols. Am J Clin Nutr 81:261–267
Collins AR, Dusinska M, Franklin M, Somorovska M, Petrovska H, Duthie S, Fillion L, Panayiotidis M, Raslova K, Vaughan N (1997) Comet assay in human biomonitoring studies: reliability, validation, and applications. Environ Mol Mutagen 30:139–146
Collins AR, Dusinska M, Gedik CM, Stetina R (1996) Oxidative damage to DNA: do we have a reliable biomarker? Environ Health Perspect 104:465–469
Chamorro A, Obach V, Cervera A, Revilla M, Deulofeu R, Aponte JH (2002) Prognostic significance of uric acid serum concentration in patients with acute ischemic stroke. Stroke 33:1048–1052
Chen J, Jin K, Chen M, Pei W, Kawaguchi K, Greenberg DA, Simon RP (1997) Early detection of DNA strand breaks in the brain after transient focal ischemia: implications for the role of DNA damage in apoptosis and neuronal cell death. J Neurochem 69:232–245
Cherubini A, Polidori MC, Bregnocchi M, Pezzuto S, Cecchetti R, Ingegni T, di Iorio A, Senin U, Mecocci P (2000) Antioxidant profile and early outcome in stroke patients. Stroke 31:2295–2300
Crack PJ, Taylor JM (2005) Reactive oxygen species and the modulation of stroke. Free Radic Biol Med 38:1433–1444
Danielisova V, Nemethova M, Gottlieb M, Burda J (2006) The changes in endogenous antioxidant enzyme activity after postconditioning. Cell Mol Neurobiol 26:1181–1191
Enrico P, Esposito G, Mura MA, Migheli R, Serra PA, Desole MS, Miele E, De Natale G, Miele M (1997) Effects of allopurinol on striatal dopamine, ascorbate and uric acid during an acute morphine challenge: ex vivo and in vivo studies. Pharmacol Res 35:577–585
ESCODD (2005) Establishing the background level of base oxidation in human lymphocyte DNA: results of an interlaboratory validation study. FASEB J 19:82–84
Fiskum G, Rosenthal RE, Vereczki V, Martin E, Hoffman GE, Chinopoulos C, Kowaltowski A (2004) Protection against ischemic brain injury by inhibition of mitochondrial oxidative stress. J Bioenerg Biomembr 36:347–352
Fracasso ME, Doria D, Franceschetti P, Perbellini L, Romeo L (2006) DNA damage and repair capacity by comet assay in lymphocytes of white-collar active smokers and passive smokers (non- and ex-smokers) at workplace. Toxicol Lett 167:131–141
Frassetto SS, Schetinger MR, Webber A, Sarkis JJ, Netto CA (1999) Ischemic preconditioning reduces peripheral oxidative damage associated with brain ischemia in rats. Braz J Med Biol Res 32:1295–1302
Gariballa SE, Hutchin TP, Sinclair AJ (2002) Antioxidant capacity after acute ischaemic stroke. QJM 95:685–690
Gerber Y, Tanne D, Medalie JH, Goldbourt U (2006) Serum uric acid and long-term mortality from stroke, coronary heart disease and all causes. Eur J Cardiovasc Prev Rehabil 13:193–198
Ghiselli A, Serafini M, Maiani G, Azzini E, Ferro-Luzzi A (1995) A fluorescence-based method for measuring total plasma antioxidant capability. Free Radic Biol Med 18:29–36
Giovannelli L, Cozzi A, Guarnieri I, Dolara P, Moroni F (2002) Comet assay as a novel approach for studying DNA damage in focal cerebral ischemia: differential effects of NMDA receptor antagonists and poly(ADP-ribose) polymerase inhibitors. J Cereb Blood Flow Metab 22:697–704
Glantz L, Avramovich A, Trembovler V, Gurvitz V, Kohen R, Eidelman LA, Shohami E (2005) Ischemic preconditioning increases antioxidants in the brain and peripheral organs after cerebral ischemia. Exp Neurol 192:117–124
Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA (2005) Uric acid and oxidative stress. Curr Pharm Des 11:4145–4151
Green AR, Ashwood TF (2005) Free radical trapping as a therapeutic approach to neuroprotection in stroke: experimental and clinical studies with NXY-059 and free radical scavengers. Curr Drug Targets CNS Neurol Disord 4:109–118
Heiss WD (2002) Stroke-acute interventions. J Neural Transm 63:37–57
Homi HM, Freitas JJ, Curi R, Velasco IT, Junior BA (2002) Changes in superoxide dismutase and catalase activities of rat brain regions during early global transient ischemia/reperfusion. Neurosci Lett 333:37–40
Hosseinzadeh H, Sadeghnia HR (2005) Safranal, a constituent of Crocus sativus (saffron), attenuated cerebral ischemia induced oxidative damage in rat hippocampus. J Pharm Pharm Sci 8:394–399
Kolesarova M, Pavel J, Lukacova N, Kolesar D, Marsala J (2006) Effect of ischemia in vivo and oxygen-glucose deprivation in vitro on NOS pools in the spinal cord: comparative study. Cell Mol Neurobiol 26:1281–1294
Lehotsky J, Murin R, Strapkova A, Urikova A, Tatarkova Z, Kaplan P (2004) Time course of ischemia/reperfusion-induced oxidative modification of neural proteins in rat forebrain. Gen Physiol Biophys 23:401–415
Liu H, Uno M, Kitazato KT, Suzue A, Manabe S, Yamasaki H, Shono M, Nagahiro S (2004) Peripheral oxidative biomarkers constitute a valuable indicator of the severity of oxidative brain damage in acute cerebral infarction. Brain Res 1025:43–50
Margaill I, Plotkine M, Lerouet D (2005) Antioxidant strategies in the treatment of stroke. Free Radic Biol Med 39:429–443
Moor E, Kohen R, Reiter RJ, Shohami E (2001) Closed head injury increases extracellular levels of antioxidants in rat hippocampus in vivo: an adaptive mechanism? Neurosci Lett 316:169–172
Nagayama T, Lan J, Henshall DC, Chen D, O’Horo C, Simon RP, Chen J (2000) Induction of oxidative DNA damage in the peri-infarct region after permanent focal cerebral ischemia. J Neurochem 75:1716–1728
Nihei H, Kanemitsu H, Tamura A, Oka H, Sano K (1989) Cerebral uric acid, xanthine, and hypoxanthine after ischemia: the effect of allopurinol. Neurosurgery 25:6173–6177
Nita DA, Nita V, Spulber S, Moldovan M, Popa DP, Zagrean AM, Zagrean L (2001) Oxidative damage following cerebral ischemia depends on reperfusion-a biochemical study in rat. J Cell Mol Med 5:163–170
Pulsinelli WA, Brierley JB (1979) A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke 10:268–272
Repine JE, Fox RB, Berger EM (1981) Hydrogen peroxide kills Staphylococcus aureus by reacting with staphylococcal iron to form hydroxyl radical. J Biol Chem 256:7094–7096
Ryan M, Grayson L, Clarke DJ (1997) The total antioxidant capacity of human serum measured using enhanced chemiluminescence is almost completely accounted for by urate. Ann Clin Biochem 34:688–689
Romanos E, Planas AM, Amaro S, Chamorro A (2007) Uric acid reduces brain damage and improves the benefits of rt-PA in a rat model of thromboembolic stroke. J Cereb Blood Flow Metab 27:14–20
Shi H, Liu KJ (2007) Cerebral tissue oxygenation and oxidative brain injury during ischemia and reperfusion. Front Biosci 12:1318–1328
Sies H (1993) Strategies of antioxidant defense. Eur J Biochem 215:213–219
Slavikova H, Lojek A, Hamar J, Duskova M, Kubala L, Vondracek J, Ciz M (1998) Total antioxidant capacity of serum increased in early but not late period after intestinal ischemia in rats. Free Radic Biol Med 25:9–18
Urikova A, Babusikova E, Dobrota D, Drgova A, Kaplan P, Tatarkova Z, Lehotsky J (2006) Impact of Ginkgo biloba extract EGb 761 on ischemia/reperfusion—induced oxidative stress products formation in rat forebrain. Cell Mol Neurobiol 26:1343–1353
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84
Waring WS, Webb DJ, Maxwell SR (2001) Systemic uric acid administration increases serum antioxidant capacity in healthy volunteers. J Cardiovasc Pharmacol 38:365–371
Yu ZF, Bruce-Keller AJ, Goodman Y, Mattson MP (1998) Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J Neurosci Res 53:613–625
Acknowledgements
This work was supported by Grants VEGA 3380/06, UK/38/2005, MVTS 39, MVTS-COST B30, APVV 51-027404, from the Ministry of Education and Science of the Slovak Republic.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sivoňová, M., Kaplán, P., Ďuračková, Z. et al. Time Course of Peripheral Oxidative Stress as Consequence of Global Ischaemic Brain Injury in Rats. Cell Mol Neurobiol 28, 431–441 (2008). https://doi.org/10.1007/s10571-007-9246-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-007-9246-x