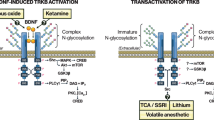
1. Emerging evidence indicates that brain-derived neurotrophic factor (BDNF) and its receptor TrkB play important roles in the mechanism of action of electroconvulsive shock (ECS) treatment. ECS produces a significant increase in brain BDNF synthesis together with a variety of neuroplastic changes including neurogenesis and axonal sprouting in the rodent brain, which is believed to be associated to the antidepressant effect of ECS. ERK1/2 (extracellular signal-regulated kinase-1/2) and Akt (protein kinase B), both intracellular signaling molecules being linked to neurotrophin signaling and synthesis, are important pathways triggered by TrkB autophosphorylation.
2. We have previously observed that chemical antidepressants induce a rapid activation of TrkB signaling in the rodent prefrontal cortex (PFC), which is likely a consequence of the stimulatory effect of antidepressants on BDNF synthesis. However, it is not known whether ECS triggers TrkB autophosphorylation and if any ECS-induced effect on TrkB function may be associated with the activation of the ERK1/2 and Akt pathways.
3. The present study assayed the phosphorylation levels of TrkB, ERK1/2, and Akt in the PFC of sham and ECS-treated rats. While the TrkB autophosphorylation (pTrkB) levels were decreased 30 min after both acute and chronic ECS, no change in pTrkB levels were observed at any other time points measured. In contrast, acute but not chronic ECS, transiently induced a very rapid and robust hyperphosphorylation of ERK1/2. Akt phosphorylation levels remained unchanged following acute or chronic ECS. Hence, although ECS effectively stimulates the ERK1/2 pathway in the PFC, this effect does not appear to involve upstream activation of TrkB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Electroconvulsive therapy (ECT) is considered to be the most potent antidepressant treatment. Many observations in rodent models of ECT, i.e., electroconvulsive shock (ECS), suggest that neurotrophic signaling is enhanced following the strong depolarization stimulus induced by ECS, and that this may contribute to the antidepressant action. Accordingly, ECS rapidly and robustly induces mRNA expression of brain-derived neurotrophic factor (BDNF) in the frontal cortex and hippocampus (Nibuya et al., 1995; Zetterstrom et al., 1998). Also, robust increase in BDNF protein levels in the rodent PFC (and hippocampus) is a consistent finding after repetitive, but not single, ECS treatment (Altar et al., 2003; Jacobsen and Mork, 2004).
Many intracellular neurotrophic signaling pathways are activated following ECS, most prominently by triggering sequential phosphorylation events via the ERK1/2 (extracellular signal-regulated kinase-1/2) and Akt pathways in the hippocampus and cortex (Kang et al., 1994; Bhat et al., 1998; Oh et al., 1999; Kang et al., 2004). Since the activation of the BDNF receptor, TrkB, induces phosphorylation of ERK1/2 and Akt (Huang and Reichardt, 2001), it is possible that stimulated TrkB function is associated with downstream activation of these signaling pathways following ECS. Indeed, positive modulation of the BDNF receptor, TrkB, is suggested by the finding of increased transcription levels of TrkB in the hippocampus and frontal cortex following chronic ECS (Nibuya et al., 1995; Lindefors et al., 1995). However, it is not known whether ECS-induced TrkB transcription translates into enhanced activity of TrkB, and it therefore, remains unclear whether TrkB might act as an activator of the ERK1/2 and Akt intracellular signaling pathways.
We have recently reported that chemical antidepressants rapidly activate TrkB in the rodent prefrontal cortex (PFC) by inducing phosphorylation at autocatalytic TrkB tyrosine residues, i.e., autophosphorylation (Saarelainen et al., 2003; Rantamaki et al., 2006; Rantamaki et al., 2007). The PFC is a key region implicated in the pathophysiology of depression (Manji et al., 2001), suggesting that TrkB activation in the PFC is an important mechanism for antidepressant action.
We, therefore, assessed whether ECS induces TrkB activation in the PFC, and whether changes in TrkB activity are temporally associated with the enhanced signaling via ERK1/2 and Akt pathways. To this end, we analyzed the levels of TrkB autophosphorylation, ERK1/2, and Akt phosphorylation in the PFC of rats exposed to either acute or chronic ECS.
MATERIALS AND METHODS
Adult male rats (240–260 g, Sprague-Dawley, Charles River Laboratories, Germany; or Wistar, Harlan, The Netherlands) were employed in all the experiments. All animal procedures were carried out in accordance with the European Communities Council Directive of November 24 1986 (86/609/EEC) and were approved by the Ethical Committee of the University of Helsinki. Animals were given a single or 14 daily ECS via ear clip electrodes (50 mA, 0.5 s, unidirectional square-wave pulses) and the experiment was terminated following a described lag-time period: 2, 5, or 30 min for the acute and 30 min for the chronic study. Sham animals were handled similarly but without passing the current. For comparison to a short-term effect on TrkB phosphorylation of a chemical antidepressant, male Wistar rats were subjected to acute administration of imipramine (30 mg/kg, i.p.) for 30 min.
The rats were decapitated, brains quickly removed, PFC dissected (<60 s following decapitation), snap-frozen in liquid nitrogen, and stored at −80°C until use. Samples were then homogenized in a lysis buffer (137 mM NaCl, 20 mM Tris, 1% NP-40, 10% glycerol, 48 mM NaF, H2O, 2 × Complete inhibitor cocktail (Roche) and 2 mM Na2VO3), incubated on ice (>15 min), centrifuged (16,100 × g, 15 min), and the supernatants collected for further processing. For the analysis of p-ERK1/2 and p-Akt levels, samples (20 μg) were separated in a 10% SDS-PAGE under reducing conditions and blotted onto a nitrocellulose membrane. For the analysis of p-TrkB levels, the samples (600 μg) were subjected to lectin (wheat germ agglutinin, WGA) precipitation as previously described (Rantamaki et al., 2006), and the fraction of glycosylated proteins were separated in a 7.5% SDS-PAGE under reducing conditions and blotted onto a PVDF membrane. Following blocking, the membranes were incubated (overnight, +4°C) with pY705/6-TrkB (1:500, see the following text and Rantamaki et al., (2006), p-ERK1/2 (1:2000; Cell Signaling Technology, CST), and p-Akt (1:1,000; CST) antibodies to visualize the phosphorylation levels of TrkB catalytic domain, ERK1/2 (p-ERK1/2), and Akt (p-Akt), respectively. Specifically bound antibodies were visualized using horse-radish peroxidase-labeled secondary antibodies (room temperature for 1 h) followed by electrochemiluminescence reaction (ECL) (Amersham Biosciences) and serial exposures on X-ray films. Protein levels of ERK1/2 and Akt were similarly analyzed using their respective nonphospho antibodies, anti-ERK1/2 (1:2000; CST), and anti-Akt (1:1000; CST). Finally, the membranes were stripped and TrkB or actin levels detected using pan-Trk (sc-11; 1:2000; Santa Cruz Biotechnology) or anti-actin (1:30000; Sigma, St. Louis, MI), respectively.
To assess the phosphospecificity of the pY705/6-TrkB antibody, the protein extraction, lectin precipitation, and antibody incubation conditions were applied to protein extracts (200 μg) of NIH3T3 cells transfected with rat full-length trkB cDNA. Briefly, cells were grown in serum-free DMEM (+4.5 g/L glucose and glutamax, 10% FCS, and 0.1 mg/mL gentamicin), and were 50–80% confluent prior to incubation (4 h, 37°C, 5% CO2) with transfection medium (2 μg plasmid in 12 μL lipofectamin supplemented with 8 μL plus Reagent and 250 μL OptiMem (Invitrogen, Carlsbad, CA)). Thereafter, the transfection mixture was replaced with DMEM, incubated overnight and treated with or without BDNF (100 ng/ml in DMEM, 5 min, 37°C). Next, the medium was removed and trypsin-EDTA (Cambrex, East Rutherford, NJ) added to facilitate cell detachment. Protein extraction was performed as described above. A set of lysates were incubated (2 h, +30°C) in a reaction mix (50 mM Tris-HCl, 100 mM NaCl, 2 mM dithiothreitol, 0.1 mM EGTA) containing lambda protein phosphatase (PPase) (1,600 units in 2 mM MnCl2; New England Biolabs, Beverly, MA). Western Blot was performed and pY705/6-TrkB immunoreactivity was assessed as described earlier. As controls, immunoreactivity for overall phosphotyrosine and TrkB were detected using p-Tyr (1:2000; Upstate, Lake Placid, NY) and TrkB (1:2000; BD Transduction, San Jose, CA) antibodies, respectively. Immunoblots were analyzed using NIH ImageJ 1.32 or QuantityOne v.4.5.2 (BioRad, Hercules, CA), and the optical densitometric data were expressed relative to the corresponding level of actin (for ERK1/2 and Akt) or Trk (for p-TrkB), respectively. Statistical analyses were performed using two-tailed Student’s t test. Significant p-value was set to <0.05. All the data are presented as mean ± SEM relative to control levels.
RESULTS AND DISCUSSION
In line with the previous observations (Bhat et al., 1998), a single ECS produced a rapid and transient phosphorylation of ERK1/2 in the PFC, which was triggered within 2 min, peaked at 5 min, and declined below baseline at 30 min (Fig. 1, left panel). In contrast, p-Akt levels remained unchanged at all time-points examined (Fig. 1, right panel). Interestingly, earlier reports have shown that both ERK1/2 and Akt are phosphorylated in the hippocampus within a few minutes following the ECS (Kang et al., 1994; Kang et al., 2004), signifying that the Akt pathway in the PFC does not respond to ECS. This would indicate that ERK1/2 and Akt are regulated differently in the PFC, at least at the time points investigated here.
The effects of ECS on the ratio of phosphorylation: total protein levels of ERK1/2 (left panel) and Akt (right panel) in the rat prefrontal cortex. Data are calculated as optical density (OD), expressed relative to the corresponding level of actin, normalized to the corresponding total ERK level, and presented as mean ± SEM percentage relative to sham levels (100%). *** p < 0.001, * p < 0.05, two-tailed Student’s t test.
To examine whether the ECS-induced rapid changes in p-ERK1/2 levels are associated with changes in TrkB receptor activity, autophosphorylation of TrkB catalytic domain (the initial step in TrkB activation) (Segal et al., 1996; Huang and Reichardt, 2001) was assayed at 2, 5, and 30 min after the ECS using a pY705/6-TrkB antibody (Rantamaki et al., 2006). Initial experiments were conducted using TrkB-transfected NIH3T3 cells to confirm the phosphospecificity of the antibody. As shown in Fig. 2, TrkB-transfected cells showed increased levels of rat full-length TrkB. The pY705/6-TrkB and phosphotyrosine antibodies recognized both basal and BDNF-stimulated levels of TrkB tyrosine phosphorylation. Importantly, these immunoreactivities were absent after PPase treatment.
Characterization of the phosphospecificity of pY705/6-TrkB (p-TrkB) antibody in trkB-transfected NIH3T3 cells. Cells were incubated with or without BDNF (100 nM, 5 min), proteins extracted and further separated in 10% SDS-PAGE for immunodetection of TrkB (upper panel), pY705/6-TrkB (middle panel), and phosphotyrosine (lower panel) levels. Anti-pY705/6-TrkB antibody detects basal and BDNF-induced autophosphorylation levels of full-length and partially unglycosylated TrkB. Similar immunoreactivity was observed using phospho-tyrosine antibody. Both pY705/6-TrkB and phosphotyrosine immunoreactivities were almost undetectable upon sample incubation with lambda protein phosphatase (PPase) after the protein extraction.
We did not detect any significant changes in pY705/6-TrkB levels at 2 and 5 min after acute ECS, thus, suggesting that the p-ERK1/2 response elicited by ECS was not mediated by TrkB at these time points. Interestingly, however, pY705/6-TrkB levels were slightly but significantly reduced 30 min after ECS as compared to the control samples (Fig. 3). This decrease is reminiscent of the reduction of p-ERK1/2 levels under the baseline at the same time point. Another set of rats were given a single ECS (70 mA, 0.5 s) and pY705/6-TrkB levels were analyzed 10 and 20 min post-ECS. These experiments revealed that the pY705/6-TrkB levels also remained at control levels at these time points (10 min: 101.6 ± 7.1% of sham; 20 min: 96.12 ± 11.2% of sham). Taken together, the ECS-induced increase in p-ERK1/2 levels is not preceded by the activation of TrkB receptors. In contrast, the phosphorylation status of TrkB and its signaling capacity are inhibited by the ECS by a yet unknown mechanism.
Next, we examined whether the attenuated response of TrkB and ERK1/2 phosphorylation following acute ECS is sensitized or modulated by a repetitive treatment. A set of rats were given ECS daily for 14 consecutive days and pY705/6-TrkB, p-ERK1/2, and p-Akt levels were assayed 30 min after the last ECS. While p-ERK1/2 and p-Akt levels remained within control values in this treatment paradigm, pY705/6-TrkB levels were reduced to a similar degree as seen after a single ECS (Fig. 4). Protein levels of TrkB, ERK1/2, and Akt were not altered by the ECS treatment. These findings further support the notion that phosphorylation (activation) of ERK1/2 and TrkB are not coupled molecular events following the exposure to ECS.
The effects of repetitive ECS treatments on the phosphorylation levels of TrkB, ERK1/2, Akt, and their corresponding total protein levels in the rat prefrontal cortex. Optical densitometric data on ERK and Akt levels are expressed relative to the corresponding level of actin. p-TrkB and TrkB levels are calculated as optical density (OD). Data are presented as mean ± SEM percentage relative to sham levels. ** p < 0.01, two-tailed Student’s t test.
The lack of p-ERK1/2 regulation following multiple ECS treatment contrasts with a recent finding (Kang et al., 2006), reporting that ECS in 5–10 consecutive days produced a 50% upregulation of p-ERK1/2 levels in the rat frontal cortex measured 24 h post-ECS. This may indicate a differential response to ECS on ERK1/2 activation in different parts of the cortex, or alternatively, the timing for ERK1/2 analysis may be critical due to the much longer recovery time used in the study.
ERK1/2 signaling has been observed to be essential for neurite outgrowth and neuronal survival (Encinas et al., 1999), responses which are typically induced by neurotrophic factors and which may be related to the cellular effects of ECS. However, the lack of correlation between ERK1/2 activation and TrkB autophosphorylation by ECT strongly suggests that this effect of ERK1/2 is not mediated by an upstream activation of TrkB. The ECS-induced activation of ERK1/2 is, therefore, likely a consequence of acute membrane depolarization in the PFC, which may trigger ERK1/2 phosphorylation via enhanced NMDA receptor activity. In support of this notion, administration of ECS or drugs producing seizures, both result in NMDA receptor-dependent increase of p-ERK1/2 levels (Ahn et al., 2000; Merlo et al., 2004; Chen et al., 2007).
In contrast to ECS, acute imipramine treatment evoked a robust increase in pY705/6-TrkB levels in the PFC while not altering the total levels of TrkB (Fig. 5), thus, signifying a specific increase in the autophosphorylation level of TrkB. These data are in agreement with the previously reported data on acute imipramine treatment of the mouse (Saarelainen et al., 2003; Rantamaki et al., 2007).
p-TrkB immunoreactivity in PFC samples from rats subjected to acute imipramine treatment (IMI, 30 mg/kg, i.p.) or saline (SAL) vehicle. Acute imipramine triggers TrkB autophosphorylation (left panel) without changing the levels of total TrkB (pantrk, right panel). Data are presented as mean ± SEM percentage relative to sham levels (100%). *** p < 0.001, two-tailed Student’s t test. WGA, wheat germ agglutinin (lectin).
Repetitive ECS treatment has consistently been shown to increase BDNF protein levels in the rodent PFC (and hippocampus) (Altar et al., 2003; Jacobsen and Mork, 2004). In support, BDNF protein levels were significantly upregulated in the PFC after the chronic ECS paradigm in the present study, as compared to the sham animals (data not shown). The present results suggest that the downregulation of p-TrkB levels elicited 30 min post-ECS is not compensated by a significant and simultaneous increase in BDNF levels.
Together, these data indicate that a single ECS does not produce significant changes in BDNF release and thus, does not lead to TrkB activation. Rather, a single ECS rapidly reduces the TrkB function in at least some specific neuronal connections in the rat PFC. Therefore, chemical antidepressants and ECS may produce immediate neurotrophic actions through different molecular pathways: antidepressants by directly stimulating the release of BDNF and rapidly activating TrkB signaling (Saarelainen et al., 2003; Rantamaki et al., 2006); and ECS by rapidly activating the ERK1/2 signaling independent of TrkB function.
REFERENCES
Ahn, Y. M., Oh, S. W., Kang, U. G., Park, J., and Kim, Y. S. (2000). An N-methyl-D-aspartate antagonist, MK-801, preferentially reduces electroconvulsive shock-induced phosphorylation of p38 mitogen-activated protein kinase in the rat hippocampus. Neurosci. Lett. 296:101–104.
Altar, C. A., Whitehead, R. E., Chen, R., Wortwein, G., and Madsen, T. M. (2003). Effects of electroconvulsive seizures and antidepressant drugs on brain-derived neurotrophic factor protein in rat brain. Biol. Psychiatry 54:703–709.
Bhat, R. V., Engber, T. M., Finn, J. P., Koury, E. J., Contreras, P. C., Miller, M. S., Dionne, C. A., and Walton, K. M. (1998). Region-specific targets of p42/p44MAPK signaling in rat brain. J. Neurochem. 70:558–571.
Chen, Q., He, S., Hu, X. L., Yu, J., Zhou, Y., Zheng, J., Zhang, S., Zhang, C., Duan, W. H., and Xiong, Z. Q. (2007). Differential roles of NR2A- and NR2B-containing NMDA receptors in activity-dependent brain-derived neurotrophic factor gene regulation and limbic epileptogenesis. J. Neurosci. 27:542–552.
Encinas, M., Iglesias, M., Llecha, N., and Comella, J. X. (1999). Extracellular-regulated kinases and phosphatidylinositol 3-kinase are involved in brain-derived neurotrophic factor-mediated survival and neuritogenesis of the neuroblastoma cell line SH-SY5Y. J. Neurochem. 73:1409–1421.
Huang, E. J., and Reichardt, L. F. (2001). Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 24:677–736.
Jacobsen, J. P., and Mork, A. (2004). The effect of escitalopram, desipramine, electroconvulsive seizures and lithium on brain-derived neurotrophic factor mRNA and protein expression in the rat brain and the correlation to 5-HT and 5-HIAA levels. Brain Res. 1024:183–192.
Kang, U. G., Hong, K. S., Jung, H. Y., Kim, Y. S., Seong, Y. S., Yang, Y. C., and Park, J. B. (1994). Activation and tyrosine phosphorylation of 44-kDa mitogen-activated protein kinase (MAPK) induced by electroconvulsive shock in rat hippocampus. J. Neurochem. 63:1979–1982.
Kang, U. G., Roh, M. S., Jung, J. R., Shin, S. Y., Lee, Y. H., Park, J. B., and Kim, Y. S. (2004). Activation of protein kinase B (Akt) signaling after electroconvulsive shock in the rat hippocampus. Prog. Neuropsychopharmacol. Biol. Psychiatry 28:41–44.
Kang, U. G., Koo, Y. J., Jeon, W. J., Park, D. B., Juhnn, Y. S., Park, J. B., and Kim, Y. S. (2006). Activation of extracellular signal-regulated kinase signaling by chronic electroconvulsive shock in the rat frontal cortex. Psychiatr. Res. 145:75–78.
Lindefors, N., Brodin, E., and Metsis, M. (1995). Spatiotemporal selective effects on brain-derived neurotrophic factor and TrkB messenger RNA in rat hippocampus by electroconvulsive shock. Neuroscience 65:661–670.
Manji, H. K., Drevets, W. C., and Charney, D. S. (2001). The cellular neurobiology of depression. Nat. Med. 7:541–547.
Merlo, D., Cifelli, P., Cicconi, S., Tancredi, V., and Avoli, M. (2004). 4-Aminopyridine-induced epileptogenesis depends on activation of mitogen-activated protein kinase ERK. J. Neurochem. 89:654–659.
Nibuya, M., Morinobu, S., and Duman, R. S. (1995). Regulation of BDNF and TrkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neurosci. 15:7539–7547.
Oh, S. W., Ahn, Y. M., Kang, U. G., Kim, Y. S., and Park, J. B. (1999). Differential activation of c-Jun N-terminal protein kinase and p38 in rat hippocampus and cerebellum after electroconvulsive shock. Neurosci. Lett. 271:101–104.
Rantamaki, T., Knuuttila, J. E., Hokkanen, M. E., and Castren, E. (2006). The effects of acute and long-term lithium treatments on TrkB neurotrophin receptor activation in the mouse hippocampus and anterior cingulate cortex. Neuropharmacology 50:421–427.
Rantamaki, T., Hendolin, P., Kankaanpaa, A., Mijatovic, J., Piepponen, P., Domenici, E., Chao, M. V., Mannisto, P. T., and Castren, E. (2007). Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-cgamma signaling pathways in mouse brain. Neuropsychopharmacology [Epub ahead of print].
Saarelainen, T., Hendolin, P., Lucas, G., Koponen, E., Sairanen, M., MacDonald, E., Agerman, K., Haapasalo, A., Nawa, H., Aloyz, R., Ernfors, P., and Castren, E. (2003). Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J. Neurosci. 23:349–357.
Segal, R. A., Bhattacharyya, A., Rua, L. A., Alberta, J. A., Stephens, R. M., Kaplan, D. R., and Stiles, C. D. (1996). Differential utilization of Trk autophosphorylation sites. J. Biol. Chem. 271:20175–20181.
Zetterstrom, T. S., Pei, Q., and Grahame-Smith, D. G. (1998). Repeated electroconvulsive shock extends the duration of enhanced gene expression for BDNF in rat brain compared with a single administration. Brain Res. Mol. Brain Res. 57:106–110.
ACKNOWLEDGMENTS
The rat full-length TrkB cDNA was provided by Dr. K. Kjaer, NeuroSearch A/S, Copenhagen, Denmark. The authors would like to thank M.-E. Hokkanen, V. Nousiainen, and O. Nikkilä for excellent technical assistance and O. F. O’Leary for critical reading of this manuscript. This study was supported by grants from the Sigrid Juselius Foundation, Finnish Cultural Foundation, Farmos Foundation, Friis Foundation, and Danish Medical Research Council (Project No. 64750) (DPDW).
Author information
Authors and Affiliations
Corresponding author
Additional information
Henrik H. Hansen and Tomi P. J. Rantamäki — Both authors contributed equally to this work
Rights and permissions
About this article
Cite this article
Hansen, H.H., Rantamäki, T.P.J., Larsen, M.H. et al. Rapid Activation of the Extracellular Signal-Regulated Kinase 1/2 (ERK1/2) Signaling Pathway by Electroconvulsive Shock in the Rat Prefrontal Cortex Is Not Associated with TrkB Neurotrophin Receptor Activation. Cell Mol Neurobiol 27, 585–594 (2007). https://doi.org/10.1007/s10571-007-9145-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-007-9145-1