Summary
1. Emerging clinical studies of treating brain and spinal cord injury (SCI) led us to examine the effect of autologous adult stem cell transplantation as well as the use of polymer scaffolds in spinal cord regeneration. We compared an intravenous injection of mesenchymal stem cells (MSCs) or the injection of a freshly prepared mononuclear fraction of bone marrow cells (BMCs) on the treatment of an acute or chronic balloon-induced spinal cord compression lesion in rats. Based on our experimental studies, autologous BMC implantation has been used in a Phase I/II clinical trial in patients (n=20) with a transversal spinal cord lesion.
2. MSCs were isolated from rat bone marrow by their adherence to plastic, labeled with iron-oxide nanoparticles and expanded in vitro. Macroporous hydrogels based on derivatives of 2-hydroxyethyl methacrylate (HEMA) or 2-hydroxypropyl methacrylamide (HPMA) were prepared, then modified by their copolymerization with a hydrolytically degradable crosslinker, N,O-dimethacryloylhydroxylamine, or by different surface electric charges. Hydrogels or hydrogels seeded with MSCs were implanted into rats with hemisected spinal cords.
3. Lesioned animals grafted with MSCs or BMCs had smaller lesions 35 days postgrafting and higher scores in BBB testing than did control animals and also showed a faster recovery of sensitivity in their hind limbs using the plantar test. The functional improvement was more pronounced in MSC-treated rats. In MR images, the lesion populated by grafted cells appeared as a dark hypointense area and was considerably smaller than in control animals. Morphometric measurements showed an increase in the volume of spared white matter in cell-treated animals. In the clinical trial, we compared intraarterial (via a. vertebralis, n=6) versus intravenous administration of BMCs (n=14) in a group of subacute (10–33 days post-SCI, n=8) and chronic patients (2–18 months, n=12). For patient follow-up we used MEP, SEP, MRI, and the ASIA score. Our clinical study revealed that the implantation of BMCs into patients is safe, as there were no complications following cell administration. Partial improvement in the ASIA score and partial recovery of MEP or SEP have been observed in all subacute patients who received cells via a. vertebralis (n=4) and in one out of four subacute patients who received cells intravenously. Improvement was also found in one chronic patient who received cells via a. vertebralis. A much larger population of patients is needed before any conclusions can be drawn. The implantation of hydrogels into hemisected rat spinal cords showed that cellular ingrowth was most pronounced in copolymers of HEMA with a positive surface electric charge. Although most of the cells had the morphological properties of connective tissue elements, we found NF-160-positive axons invading all the implanted hydrogels from both the proximal and distal stumps. The biodegradable hydrogels degraded from the border that was in direct contact with the spinal cord tissue. They were resorbed by macrophages and replaced by newly formed tissue containing connective tissue elements, blood vessels, GFAP-positive astrocytic processes, and NF-160-positive neurofilaments. Additionally, we implanted hydrogels seeded with nanoparticle-labeled MSCs into hemisected rat spinal cords. Hydrogels seeded with MSCs were visible on MR images as hypointense areas, and subsequent Prussian blue histological staining confirmed positively stained cells within the hydrogels.
4. We conclude that treatment with different bone marrow cell populations had a positive effect on behavioral outcome and histopathological assessment after SCI in rats; this positive effect was most pronounced following MSC treatment. Our clinical study suggests a possible positive effect in patients with SCI. Bridging the lesion cavity can be an approach for further improving regeneration. Our preclinical studies showed that macroporous polymer hydrogels based on derivatives of HEMA or HPMA are suitable materials for bridging cavities after SCI; their chemical and physical properties can be modified to a specific use, and 3D implants seeded with different cell types may facilitate the ingrowth of axons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
STRATEGIES FOR SPINAL CORD INJURY TREATMENT
Spinal cord injury (SCI) invariably results in the loss of neurons and axonal degeneration at the lesion site, leading to severe functional impairment, paraplegia, or tetraplegia. Currently, there is no effective treatment for SCI. Standard treatment consists of stabilization and a conservative therapy using high doses of methylprednisolone (Hugenholtz et al., 2002), although there have been clinical studies with naloxone (Bracken et al., 1990), tirilazade (Bracken et al., 1998), GM-1 ganglioside (Geisler et al., 2001), TRH (Pitts et al., 1995), and nimodipine (Pointillart et al., 2000). Long-term therapy after spinal cord injury focuses on rehabilitation, pain relief, spasticity treatment, and the prevention of complications. Supportive techniques come to the fore, e.g., functional electrical stimulation, tendon replantation, and neuroprosthetics. However, the regenerative capacity of the central nervous system (CNS) is highly limited and, in the region of a trauma, a glial scar develops rather than normal tissue (Fawcett and Asher, 1999). The scar contains various substances inhibiting axonal growth and forms a mechanical barrier that separates the injured tissue from the rest of the CNS. The restoration of tissue function in the injured region requires the development of methods that allow for the reconstruction of grey and white matter composed of elements of central nervous system tissue (neurons, astrocytes, oligodendrocytes, blood vessels, extracellular matrix components, and myelinated nerve fibres).
The development of spinal cord injury treatment is focused on new methods of regenerative medicine such as the administration of growth factors, e.g., PDGF and NT3, or antagonists of inhibiting molecules (NOGO), preventing the deposition of extracellular matrix molecules (chondroitin sulphate), and stimulating the growth of damaged axons as well as protecting them from further damage (Bixby and Harris, 1991; Venstrom and Reichardt, 1993; Lee et al., 2003). An emerging strategy for replacing and/or regenerating damaged tissue is the implantation of stem cells and/or artificial biomaterials such as scaffolds to form tissue bridges between damaged spinal cord stumps. Recent preclinical studies include the implantation of foetal nervous tissue (Bregman, 1987), embryonic stem cells (Brustle et al., 1999), bone marrow mesenchymal stem cells (Prockop, 1997; Akiyama et al., 2002a; Jendelová et al., 2003, 2004b), Schwann cells (Kuhlengel et al., 1990), peripheral nervous tissue (Wrathall et al., 1982; Horvat, 1991), collagen-based matrices containing cells or neuroactive substances (Houweling et al., 1998; Liu et al., 1998), nitrocellulose membranes (Houle and Ziegler, 1994), tubes made from polymeric materials (Houle and Ziegler, 1994; Xu et al., 1997; Oudega et al., 2001), polymer hydrogels (Woerly et al., 1998, 2001a,b; Lesný et al., 2002; Jendelová et al., 2004a), and biodegradable polylactide implants (Maquet et al., 2001).
TRACKING OF IMPLANTED CELLS IN VIVO
Cell transplantation represents a potentially powerful treatment method for spinal cord injury; however, current experiments with stem cells do not give us information about the behavior of the transplanted cells in the host organism in vivo, especially about their migration and fate within the target structures and their potential neoplastic growth. The lack of these data can create a serious obstacle for the therapeutic use of cell therapy. Human medicine would benefit from the labeling of implanted stem cells and the use of noninvasive methods to image the labeled cells postimplantation. It has been shown that MSCs or ESCs can be labelled with superparamagnetic iron-oxide nanoparticles. Nanoparticle-labeled cells, when implanted into rats with a cortical or spinal cord lesion, are visible on MR images as a sharply bounded hypointense area at the injection site, and subsequently a hypointense signal is also seen at the lesion site. The cells can migrate along the corpus callosum and, if injected intravenously, can cross the BBB and home to a cortical or spinal cord lesion. The hypointense signal on MR images persists in brain or spinal cord for more than 50 days (Jendelová et al., 2003, 2004b; Syková and Jendelová, 2005). Nanoparticles, based on dextran-coated iron oxide, e.g., Endorem (Guerbet, France), can therefore be used as a stem cell marker for noninvasive MR tracking of cell migration and fate following transplantation. The use of MRI to monitor transplanted cells will be directly applicable to human medicine to monitor the course, results and potential risks of cell therapy.
BONE MARROW CELLS AS A TOOL FOR SPINAL CORD INJURY REPAIR
The use of bone marrow stromal cells (MSCs) in cell therapies may have some advantages over the use of other sources of cells: they are relatively easy to isolate from bone marrow, they grow well in tissue cultures, they may be used in autologous transplantation protocols and bone marrow as a source of cells has been already approved for the treatment of hematopoietic diseases. Preclinical studies have been performed on rats with a spinal cord injury and have shown that transplanted cells in the injured spinal cord survive, migrate into the host tissue and lead to axonal regeneration and motor function recovery (Sasaki et al., 2001; Akiyama et al., 2002a,b; Hofstetter et al., 2002; Chopp and Li, 2002; Inoue et al., 2003; Jendelová et al., 2004b). This has been achieved not only through the implantation of bone marrow stromal cells (MSCs), but also through the implantation of freshly collected bone marrow containing nucleated cells (BMCs) with a relatively small percentage of mesenchymal cells (Sasaki et al., 2001; Akiyama et al., 2002b; Inoue et al., 2003; Syková et al., 2005b; Urdzíková et al., in press). Two clinical studies so far have shown the safety of such an approach and the partial improvement of function in acute patients (Park et al., 2005; Syková et al., 2005b).
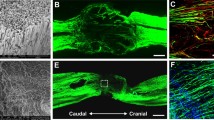
We have shown that the intravenous injection of MSCs, BMCs, or G-CSF significantly improves the recovery of hindlimb motor function in rats with a spinal cord compression lesion (Syková et al., 2005a; Urdzíková et al., in press). The fate of transplanted MSCs labeled with iron-oxide nanoparticles was followed by ex vivo MRI (Syková and Jendelová, 2005). Locomotor function was assessed weekly for 5 weeks after SCI by the BBB test (Basso et al., 1996); hindlimb sensitivity was tested over the same time period using the plantar test. Lesioned animals grafted with MSCs or BMCs had higher scores in BBB testing than did control animals injected with saline (Fig. 1(A)). All cell-treated animals showed a recovery of hind limb sensitivity using the plantar test; recovery was most apparent and most rapid in animals grafted with MSCs (Fig. 1(B)). Similar results were seen after the injection of G-CSF. Locomotor function and hindlimb sensitivity significantly improved 35 days after treatment. MR images of transversal and longitudinal spinal cord sections from animals grafted with magnetically labeled MSCs showed the entire lesion as a dark hypointense area (Fig. 2(A and B)). Staining for iron (Prussian blue) revealed many cells labelled with nanoparticles in the lesion site, and the lesion cavities were significantly smaller than in control animals. (Fig. 2(C and D)). Morphometric measurements of the spared white and grey matter were performed in the centre of the lesions. The spared cross-sectional area of the white matter, as well as the volume of spared white matter, was significantly greater in all cell-treated animals. The spared cross-sectional area of the grey matter was significantly larger only in MSC treated animals, while the volume of spared grey matter was not significantly influenced by cell treatment (Fig. 2(E)).
Behavioural testing after treatment with MSCs and BMCs. A: Behavioural open field BBB motor scores of MSC- and BMC-treated rats were significantly higher than those of control animals 14, 21, 28, and 35 days after SCI (p < 0.05). B: Time course of the animals' response to radiant heat measured by the plantar test in treated and control rats. In all treated rats, latency times decreased as their recovery progressed. The most prominent effect was seen in MSC-treated rats. The latency time in saline-injected (control) rats did not change during the 35-day survival period. Data are averaged between right and left hind limbs and expressed as mean ± SEM. * p < 0.05 compared to control group.
MSCs labeled with nanoparticles implanted into rats with a spinal cord compression lesion. A,B: Transversal and longitudinal images of a spinal cord compression lesion populated by intravenously injected nanoparticle-labeled MSCs, four weeks after implantation. The lesion with nanoparticle-labeled cells is visible as a dark hypointensive area. C: Prussian blue staining of a spinal cord compression lesion (control animal). D: Prussian blue staining of a spinal cord lesion with intravenously injected nanoparticle-labeled MSCs. Note the smaller lesion size in the implanted animal than in the control. Insert: The lesion is populated with Pussian blue-positive cells. A–D Modified from (Jendelová et al., 2004b). E: Analysis of the spared white and grey matter volume in the centre of the lesion. The total volume of the white matter (WM) and grey matter (GM) in 11 mm long segments of the spinal lesion in treated and saline injected animals. No statistically significant differences in spared grey matter volume were observed between the groups. Data are represented as group means ± SEM. * p < 0.05 compared to control group.
Based on these studies in which the cells were implanted 1 week after spinal cord injury, we studied the effect of an intravenous injection of MSCs on functional outcome in chronically injured rats (Urdzíková et al., 2005). The rats underwent a spinal cord compression lesion and 4 months later were intravenously injected with MSCs. Rats were tested behaviourally using the locomotor open field BBB test, and the plantar test was used to assess hindlimb sensitivity. Morphometric analysis was performed to evaluate the extent of spared white and grey matter in 11 mm long lesion-centered spinal cord segments. The volume of the spared tissue in these 11 mm long segments was calculated as the sum of cross-sectional areas multiplied by the distance between them. Our results demonstrate that the intravenous transplantation of MSCs 4 months after spinal cord compression injury had no effect on motor function expressed as the BBB score but it significantly improved sensitivity as measured by the plantar test. The total volume of the spared grey and white matter in the lesion epicentre was not significantly different between MSC-treated animals and controls, but morphometric analysis showed a significant increase in the cross-sectional area of spared white and grey matter caudally to the lesion centre. The effect of MSCs implanted into rats with SCI is therefore more pronounced when the cells are grafted 7 days after lesioning rather than 4 months postinjury.
HYDROGELS AND CELL–POLYMER CONSTRUCTS
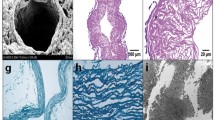
The importance of biomaterials has steadily increased in recent years, and the number of polymer applications in tissue engineering continues to grow. Since CNS injury (particularly spinal cord injury) is accompanied by cell death, pseudocyst formation and glial scarring, which compromise regeneration in the injured region, extensive research is currently focused on the development of treatment approaches that prevent scarring and bridge the lesion cavities. Macroporous biocompatible hydrogels can be used to eliminate scarring, bridge cavities and facilitate regeneration (Woerly et al., 1998, 2001b). These hydrogels are highly biocompatible, and when implanted into nervous tissue they are known to be chemically inert and nontoxic (Přádný et al., 2002). They have a high water content (70–90%) and a very large surface area, and they are macroporous with pore sizes of 10–50 μm (Fig. 3(A)) (Lesný et al., 2002; Přádný et al., 2002).
The use of hydrogels in spinal cord injury repair. A: The structure of a HEMA hydrogel observed using a scanning electron microscope. B: Four weeks after implantation, a HEMA hydrogel firmly adheres to the spinal cord tissue. The dotted line marks the interface between spinal cord tissue and the hydrogel, which is fully integrated into the spinal cord. C: A biodegragable hydrogel (HPMA +10% EOEMA) degraded from the interface with the spinal cord towards the central part of the hydrogel. The size (volume) of the peripheral zone of degraded hydrogel is marked by the black line. The central part of the hydrogel (marked with an asterisk) consisted of amorphous matter, where mostly the ingrowth of connective tissue and capillaries was observed. D: The detail of the peripheral zone of a degraded hydrogel. The gel was resorbed by macrophages and replaced by newly formed tissue. E: The detail of the peripheral zone of a degraded hydrogel. NF160-positive neurofilaments are entering the gel from the spinal cord border. F: Interface between the spinal cord stump and a hydrogel acutely implanted into a complete spinal cord transection. G: Interface between the spinal cord stump and a hydrogel implanted seven days after complete spinal cord transection. Note the smaller cavity with delayed implantation. H: Immunostaining with anti-neurofilament antibody (anti-NF160) showing the presence of NF160-positive axons in the centre of a hydrogel inserted 7days after complete spinal cord transection. Images C–H were taken four weeks postimplantation. I: Endorem-labeled cells seeded into a hydrogel. J: A hydrogel seeded with MSCs was visible on MR images 6 weeks after implantation as a hypointensive area (arrow). K: Ingrowth of NF 160-positive neurofilaments into a hydrogel seeded with MSCs. I–J Modified from (Syková and Jendelová, 2005).
Spinal cord tissue regeneration was studied following the implantation of polymer hydrogels with a macroporous structure, based on derivatives of 2-hydroxyethyl methacrylate (HEMA), 2-hydroxypropyl methacrylamide (HPMA) or copolymers of HPMA and ethoxyethyl methacrilate (EOEMA) (Woerly et al., 1998; Přádný et al., 2002, in press). The hydrogels were modified either by different surface electric charges (HEMA-MA negative charge; HEMA-MOETACl positive charge) or by their copolymerization with a hydrolytically degradable crosslinker, N,O-dimethacryloylhydroxylamine (Přádný et al., in press). Blocks of hydrogel were implanted into hemisected rat spinal cords; the animals were sacrificed 28 days after implantation. All of the hydrogels were biocompatible and adhered well to the host tissue, bridging the whole spinal cord lesion (Fig. 3(B)); cellular ingrowth was observed in all the implanted hydrogels, with the most pronounced ingrowth seen in copolymers of HEMA with a positive electric charge. Although most of the cells had the morphological properties of connective tissue elements, NF-160-positive axons were found invading all the implanted hydrogels from both the proximal and distal stumps. No astrocytic processes inside the gels were observed; however, the gels were permissive for Schwann cells (p75-positive) (Jendelová et al., 2004a).
The biodegradable hydrogels degraded from the borders that were in direct contact with the spinal cord tissue (Fig. 3(C)). They were resorbed by macrophages and replaced by newly formed tissue containing connective tissue elements, blood vessels, GFAP-positive astrocytic processes, and NF-160-positive neurofilaments (Fig. 3(D and E)). The size of the degraded zone was dependent on the degradation rate of the hydrogel (Přádný et al., in press); The largest degraded zone was found in hydrogels biodegradable within 7 days, comprising approximately one-half of the implant volume. The central part of the hydrogels consisted of amorphous matter, where only the ingrowth of connective tissue was observed. This is most likely the result of the hindered clearance of degradation products from the centre of the gel (Přádný et al., in press).
The formation of lesion cavities in chronic injuries is one of the factors inhibiting neuronal regeneration. HEMA hydrogels were tested for their ability to bridge lesions in rats with a complete spinal cord transection at the Th9 level (Hejčl et al., 2005). The lesions were bridged using hydrogels implanted either immediately or after a one week delay. Histological assessment was performed 3 months after transection. Histological staining showed that the hydrogels were filled with connective tissue elements, blood vessels, and neurofilaments (Fig. 3(H)). Morphometric analysis of the spinal cord transection lesions showed that the volume of the pseudocysts formed in the spinal cords with delayed hydrogel implantation was significantly smaller than the volume of the cavities in rats treated by immediate hydrogel implantation (Fig. 3(F and G)). The results indicate that delayed implantation may be even more effective than immediate reconstructive surgery.
Hydrogel implantation can be combined with stem cell grafting: Before transplantation into a lesion, the hydrogels are seeded with MSCs. In this case the hydrogels form an inert environment, allowing for the free diffusion of intrinsic growth factors, in which the cells start to differentiate and migrate. The inert environment of the hydrogels also provides an adequate standard background for MR imaging of the cells (Jendelová et al., 2004a). We employed cell–polymer constructs in order to facilitate the regeneration of injured spinal cord (Lesný et al., in press). In macroporous polymer hydrogels based on HEMA with an average porosity of 50 μm, the cellular growth was distinctly influenced by the surface electric charge. In negatively charged hydrogels (HEMA-MA), the cells grew in clusters uniformly scattered within the hydrogel volume; these clusters had minimal contact with the hydrogel surface. In hydrogels with a positive surface charge (HEMA-MOETACl), the cells adhered well to the hydrogel surfaces and grew to higher culture densities.
To evaluate the ability of cell–polymer constructs to bridge a lesion, the right half of a spinal cord segment was removed by hemisection and a block of HEMA hydrogel seeded with Endorem-labeled MSCs was inserted (Fig. 3(I); Syková and Jendelová, 2005). Six weeks after implantation, the hydrogel had formed a continuous bridge between the hemisected spinal segments, re-establishing the anatomical continuity of the tissue. The hydrogel was visible on MRI as a hypointense area (Fig. 3(J)) and Prussian blue staining confirmed positively stained cells within the hydrogel. Staining for neurofilaments (NF160 Sigma, St. Louis, USA) showed axonal ingrowth into the hydrogel (Fig. 3(K); (Jendelová et al., 2004a). Hydrogels seeded with stem cells may therefore serve as an alternative to the conventional grafting of dissociated cells, benefiting from advances in surface chemistry and the cell–cell or cell–matrix interactions that occur during development or regeneration.
ONGOING CLINICAL STUDY
Based on recent experimental studies, autologous BMC implantation is being used in a Phase I clinical trial in patients (n=20) with a transversal spinal cord lesion at Motol Hospital in Prague, Czech Republic. Ethical approval for this study was obtained from the Ministry of Health of the Czech Republic and the Ethical Committee of Motol Hospital in Prague. Informed consent was obtained from each patient who was enrolled in the study; all patients had suffered traumatic SCI and complete motor and sensory dysfunction. The patients were divided into two groups. The first group was a group of “subacute” patients (n=8) who received BMCs between 10 and 33 days after SCI. The second group comprised “chronic” patients who received BMCs between 2 and 18 months after SCI (n=12). In the eight subacute patients, four received BMCs via catheterization of a. vertebralis and four patients were infused intravenously. Of the 12 chronic patients, 2 received BMCs via catherization of a. vertebralis and the rest intravenously. Spinal cord lesions were evaluated using MRI. At both initial and follow-up examinations (at 3, 6 and 12 months after BMC transplantation), standard neurological classification of the SCI was performed according to the American Spinal Injury Association [ASIA] protocol and the Frankel score, which provides for a standardised assessment of neurological deficits in patients with SCI. To assess the functional integrity of the corticospinal tract and dorsal columns, electrophysiological recordings of motor and somatosensory potentials (MEP and SEP) were performed prior to and at 3, 6, and 12 months after BMC transplantation. Partial improvement in the ASIA score, along with a partial recovery of MEP or SEP, has been observed in all subacute patients who received cells via a. vertebralis (n=4) and in one out of four subacute patients who received cells intravenously. Improvement was also found in one out of two chronic patients who received cells via a. vertebralis. The improved ASIA outcome was mostly from a score of A to B, in one case from B to D. A much larger population of patients is needed before any conclusions can be drawn (Syková et al., 2005a,b). At present, it can be concluded from this clinical study that the implantation of autologous BMCs is safe, as there were no complications following intravenous or intraarterial cell administration. We can also conclude that it appears important that implantation is done during the first 3–4 weeks after injury and that administering the cells closer to the injury site, such as through the catherization of a. vertebralis, might be justified by a better outcome.
DISCUSSION AND FURTHER PERSPECTIVES
Postnatal bone marrow has traditionally been seen as an organ composed of two main systems rooted in distinct lineages: the hematopoietic tissue proper and the associated supporting stroma—marrow stromal cells. Unlike hematopoietic stem cells, whose role in the treatment of hematopoietic diseases has been known for a long time, MSCs were originally examined only because of their critical role in the formation of the hematopoietic microenvironment. More recent data came with the recognition that MSCs are stem/progenitor cells of ectodermal, mesodermal and endodermal tissues. Their potential to differentiate into nonhematopoietic organ cells granted them membership in the family of somatic stem cells. There is little doubt that they represent one of the most accessible sources of stem cells for therapeutic use. The ease with which they are harvested and the simplicity of the procedures required for their extensive growth in culture, together with easy expansion in vitro, may make them ideal candidates.
The question of which cell type is most beneficial for SCI treatment is still unresolved. One possible effect of cell therapy is “repair,” meaning that the grafted cells integrate into the host tissue and replace damaged or lost cells. Several studies have been performed using in vitro expanded neural stem/progenitor cells that were then implanted into injured rat or marmoset spinal cord. The cells survived and differentiated into neurons, astrocytes and oligodendrocytes and had a positive effect on functional outcome (Ogawa et al., 2002, Iwanami et al., 2005, Okada et al., 2005). Similarly, MSCs can also differentiate into neuron-like cells and glia (Prockop, 1997; Azizi et al., 1998; Eglitis et al., 1999; Brazelton et al., 2000; Mezey et al., 2000; Woodbury et al., 2000; Jendelová et al., 2003). In our previous experiments (Jendelová et al., 2003), we injected MSCs into rats with a cortical photochemical lesion and studied the differentiation of the grafted cells. We found that only a few (<5%) BrdU-labeled MSCs expressed the neuronal marker NeuN, and we did not find any BrdU-labeled MSCs expressing the astrocytic marker GFAP.
To date, preclinical studies have revealed several reasons why MSCs may be useful in spinal cord injury treatment. A number of studies have described the use of MSCs as cells that express factors beneficial to the nervous tissue or that activate compensatory mechanisms and endogenous stem cells within the tissue following their migration into an injured environment (for review see Chopp and Li, 2002). Studies of MSCs transplanted into different models of CNS injury (Chopp et al., 2000; Lu et al., 2001; Akiyama et al., 2002a; Hofstetter et al., 2002; Urdzíková et al., in press) have provided considerable information about their potential to improve functional outcome. MSCs secrete cytokines such as colony stimulating factor (CSF), interleukins, stem cell factor (SCF) (Eaves et al., 1991; Majunder et al., 1998), nerve growth factor (NGF), brain derived neurotrophic factor (BDNF), hepatocyte growth factor (HGF), and vascular endothelial cell growth factor (VEGF) (Bjorklund and Lindvall, 2000). It has also been reported that MSCs stimulate glial cells to produce neurotrophic factors such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) (Majunder et al., 1998; Mahmood et al., 2002; Wang et al., 2002). MSCs can promote axonal regeneration by guiding nerve fibres (Hofstetter et al., 2002). Wu showed that transplanted MSCs promote compensatory mechanisms to reorganise neural networks and activate endogenous stem cells (Wu et al., 2003). It was also shown that BMCs and MSCs improve neurologic deficits by generating either neural cells or myelin-producing cells (Chopp et al., 2000; Sasaki et al., 2001). However, understanding the actual differentiation spectrum of stromal cells requires further investigation.
Although our and other studies indicate that MSCs are more effective in the treatment of SCI, there are several good reasons supporting the use of BMCs in SCI therapies. BMCs include hematopoietic stem cells, macrophages, lymphocytes, as well as marrow stromal cells. One reason is that the identities of the subpopulations responsible for neuronal differentiation remain unknown. Second, the neuronal protective roles of not only MSCs, but also of hematopoietic stem cells, are well known (Chen et al., 2002; Chong et al., 2002). Hematopoietic stem cells secrete many cytokines, including trombopoietin and interleukin 11 (Mehler et al., 1993; Dame et al., 2003). These cytokines are also known to be essential factors for the survival and differentiation of neuronal progenitor cells.
A recent clinical study was performed by Park et al. (2005) on six patients with SCI. A combination of autologous BMCs implanted as early as 7 days after SCI and subsequent repetitive mobilization of bone marrow cells with granulocyte macrophage-colony stimulating factor (GM-CSF) resulted in five out of six patients showing improved motor and/or sensory function. In our clinical study with BMCs, we also found partial functional improvement in subacute patients, which corresponds well to preclinical studies in rats and nonhuman primates (Sasaki et al., 2001; Akiyama et al., 2002b; Iwanami et al., 2005). Even when we observed an improvement in the ASIA score, accompanied by enhanced MEP and SEP during electrophysiological tests, the improvement was generally only from the A to B score and in one case from B to D. This clinical study shows that the implantation of autologous BMCs is safe, but we cannot conclude that the observed effects were due to cell therapy. However, the outcome in one chronic patient, who was in stable condition for several months prior to cell implantation, is promising. Nevertheless, there seems to be a similar therapeutic window as in animal studies, which is between 3 days and 3 weeks after SCI. We suggest that administering the cells closer to the injury site, such as through the catherization of a. vertebralis, might be important for a better outcome. Clinical studies are necessary for transferring preclinical findings from animal experiments to humans. The therapeutic window, the implantation strategy, the method of administration, the number of cells and the possible side-effects can only be tested in human clinical trials.
However, in the case of large lesions, cells alone are not able to repair the tissue. It is necessary to fill the gap left by the lost cell population in order to provide support for tissue restoration, reduce the glial scar, and create a permissive environment for cellular ingrowth. Biocompatible polymer hydrogels, based on pHEMA, or pHPMA, have viscoelastic and adhesive properties that promote their rapid integration at the host–tissue boundary. Their macromolecular network provides mechanical cues that stimulate the ingrowth of cells. Water present within the network provides free space for the diffusion of host tissue extracellular fluids containing trophic and growth factors released by neighboring cells. Surprisingly, in a model of delayed tissue restoration (the implantation of hydrogels one week after complete spinal cord transection), much reduced pseudocyst and cavity formation was observed (Fig. 3(F and G)). These results may be due to the early removal of myelin debris and lesion “clearance” by activated microglia and macrophages. Similarly, Bregman's group reported the better ingrowth of axons into fetal spinal cord transplants after a delay of 2–4 weeks following spinal cord transection (Coumans et al., 2001). It is evident that finding the optimal therapeutic window will play an important role in any type of SCI treatment.
The chemical and physical properties of hydrogels can be tailored to a specific use, and the gels can be seeded with different types of stem cells to create cell–polymer constructs. These constructs may serve as stem cell carriers, delivering the cells into the lesion cavities and facilitating axonal regeneration. It was shown that the implantation of scaffold-neural stem cell constructs into an adult rat hemisection model of SCI (Teng et al., 2002) promoted long-term improvement in function (persistent for 1 year in some animals) relative to a lesioned control group. At 70 days postinjury, animals implanted with a scaffold seeded with cells exhibited coordinated, weight-bearing hindlimb stepping. Histology and immunocytochemical analysis suggested that this recovery might be attributable partly to a reduction in tissue loss from secondary injury processes as well as to diminished glial scarring. Tract tracing demonstrated corticospinal tract fibres passing through the injury epicentre to the caudal cord, a phenomenon not present in untreated groups. Different types of cell populations have various properties and regenerative capabilities, therefore more studies with cell-polymer constructs seeded with different cell populations may be promising and useful.
Satisfactory outcomes have not been achieved to date in treating SCI by means of a single approach. Spinal cord injury represents a complex event, and therefore effective therapeutic strategies will consist of a series of interventions. First, secondary tissue loss should be prevented through early neuroprotective, antiinflammatory, or immunomodulatory interventions. Subsequently, strategies to promote the regrowth of axons and the restoration of function will involve multiple approaches: reducing scar formation, overcoming additional inhibitory molecules, stimulating damaged nerve cells to regenerate axons, facilitating axonal growth across the site of injury, and enabling the formation of new connections. Our overview describing the use of bone marrow cells and polymer hydrogels as tools for SCI repair is only one contribution towards a multifaceted approach to SCI treatment. The concept of polymer scaffolds seeded with stem cells may provide a prototype for other multidisciplinary strategies for addressing complex neurological injuries.
REFERENCES
Akiyama, Y., Radtke, C., Honmou, O., and Kocsis, J. D. (2002b). Remyelination of the spinal cord following intravenous delivery of bone marrow cells. Glia 39:229–236.
Akiyama, Y., Radtke, C., and Kocsis, J. D. (2002a). Remyelination of the rat spinal cord by transplantation of identified bone marrow stromal cells. J. Neurosci. 22:6623–6630.
Azizi, S. A., Stokes, D., Augelli, B. J., DiGirolamo, C., and Prockop, D. J. (1998). Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats–similarities to astrocyte grafts. Proc. Natl. Acad. Sci. U.S.A. 95:3908–3913.
Basso, D. M., Beattie, M. S., Bresnahan, J. C., Anderson, D. K., Faden, A. I., Gruner, J. A., et al. (1996). MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J. Neurotrauma 13:343–359.
Bixby, J. L., and Harris, W. A. (1991). Molecular mechanisms of axon growth and guidance. Annu. Rev. Cell. Biol. 7:117–159.
Bjorklund, A., and Lindvall (2000). Cell replacement therapies for central nervous system disorders. Nat. Neurosci. 3:537–544.
Bracken, M. B., Shepard, M. J., Collins, W. F., Holford, T. R., Young, W., Baskin, D. S., et al. (1990). A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N. Engl. J. Med. 322:1405–1411.
Bracken, M. B., Shepard, M. J., Holford, T. R., Leo-Summers, L., Aldrich, E. F., Fazl, M., et al. (1998). Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow up. Results of the third National Acute Spinal Cord Injury randomized controlled trial. J. Neurosurg. 89:699–706.
Brazelton, T. R., Rossi, F. M., Keshet, G. I., and Blau, H. M. (2000). From marrow to brain: Expression of neuronal phenotypes in adult mice. Science 290:1775–1779.
Bregman, B. S. (1987). Spinal cord transplants permit the growth of serotonergic axons across the site of neonatal spinal cord transection. Brain Res. 431:265–279.
Brustle, O., Jones, K. N., Learish, R. D., Karram, K., Choudhary, K., Wiestler, O. D., et al. (1999). Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science 285:754–756.
Chen, X., Katakowski, M., Li, Y., Lu, D., Wang, L., Zhang, L., et al. (2002). Human bone marrow stromal cells cultures conditioned by traumatic brain tissue extracts: Growth factor production. J. Neurosci. Res. 69:687–691.
Chong, Z. Z., Kang, J. Q., and Maiese, K. (2002). Hematopoietic factor erythropoietin fosters neuroprotectionthrough novel signal transduction cascades. J. Cereb. Blood Flow Metab. 22:503–514.
Chopp, M., and Li, Y. (2002). Treatment of neural injury with marrow stromal cells. Lancet Neurol. 1:92–100.
Chopp, M., Zhang, X. H., Li, Y., Wang, L., Chen, J., Lu, D., et al. (2000). Spinal cord injury in rat: Treatment with bone marrow stromal cell transplantation. Neuroreport 11:3001–3005.
Coumans, J. V., Lin, T. T., MacArthur, L., McAtee, M., Nash, C., and Bregman, B. S. (2001). Axonal regeneration and functional recovery after complete spinal cord transection in rats by delazed treatment with transplants and neurotrophins. J. Neurosci. 21:9334–9344.
Dame, C., Wolber, E. M., Freitag, P., Hofmann, D., Bartmann, P., and Fandrey, J. (2003). Trombopoietingene expression in the developing human central nervous system. Brain Res. Dev. Brain Res. 143:217–223.
Eaves, C. J., Cashman, J. D., Kay, R. J., Dougherty, G. J., Otsuka, T., Gaboury, L. A., et al. (1991). Mechanisms that regulate the cell cycle status of very primitive hematopoietic cells in long-term human marrow cultures. II. Analysis of positive and negative regulators produced by stromal cells within the adherent layer. Blood 78:110–117.
Eglitis, M. A., Dawson, D., Park, K. W., and Mouradian, M. M. (1999). Targeting of marrow-derived astrocytes to the ischemic brain. Neuroreport 10:1289–1292.
Fawcett, J. W., and Asher, R. A. (1999). The glial scar and central nervous system repair. Brain Res. Bull. 49:377–391.
Geisler, F. H., Coleman, W. P., Grieco, G., and Poonian, D. (2001). The Sygen multicenter acute spinal cord injury study. Spine 26:S87–98.
Hejčl, A., Urdzíková, L., Přádný, M., Michálek, J., Jendelová, P., and Syková, E. (2005). Positively charged HEMA-based hydrogels implanted immediately and one week after spinal cord injury in rat. Abstract, Fifth Czech Neuroscience Conference, Prague, Czech Republic.
Hofstetter, C. P., Schwarz, E. J., Hess, D., Widenfalk, J., El Manira, A., Prockop, J. D., and Olson, L. (2002). Marrow stromal cellsform guiding strands in the injured spinal cord and promote recovery. Proc. Natl. Acad. Sci. U.S.A. 96:2199–2204.
Horvat, J. C. (1991). Transplants of fetal neural tissue and autologous peripheral nerves in an attempt to repair spinal cord injuries in the adult rat. An overall view. Paraplegia 29:299–308.
Houle, J. D., and Ziegler, M. K. (1994). Bridging a complete transection lesion of adult rat spinal cord with growth factor-treated nitrocellulose implants. J. Neural Transpl. Plast. 5:115–124.
Houweling, D. A., Lankhorst, A. J., Gispen, W. H., Bar, P. R., and Joosten, E. A. (1998). Collagen containing neurotrophin-3 (NT-3) attracts regrowing injured corticospinal axons in the adult rat spinal cord and promotes partial functional recovery. Exp. Neurol. 153:49–59.
Hugenholtz, H., Cass, D. E., Dvorak, M. F., Fewer, D. H., Fox, R. J., Izukawa, D. M., et al. (2002). High-dose methylprednisolone for acute closed spinal cord injury–only a treatment option. Can. J. Neurol. Sci. 29:227–235.
Inoue, M., Honmou, O., Oka, S., Houkin, K., Hashi, K., and Kocsis, J. D. (2003). Comparative analysis of remyelinating potential of focal and intravenous administration of autologous bone marrow cells into the rat demyelinated spinal cord. Glia 44:111–118.
Iwanami, A., Kaneko, S., Nakamura, M., Kanemura, Y., Mori, H., Kobayashi, S., et al. (2005). Transplantation of human neural stem cells for spinal cord injury in primates. J. Neurosci. Res. 80:182–190.
Jendelová, P., Herynek, V., De Croos, J., Glogarová, K., Andersson, B., Hájek, M., and Syková, E. (2003). Imaging the fate of implanted bone marrow stromal cells labeled with superparamagnetic nanoparticles. Magn. Reson. Med. 50:767–776.
Jendelová, P., Lesný, P., Přádný, M., Hejčl, A., Michálek, J., and Syková, E. (2004a). Hydrogel implantation into a spinal cord lesion— an alternative to conventional cell grafting. Program No. 106.13.2004. Abstract Viewer/Itinerary Planner: Online.
Jendelová, P., Herynek, V., Urdzíková, L., Glogarová, K., Kroupová, J., Bryja, V., et al. (2004b). MR tracking of transplanted bone marrow and embryonic stem cells labeled by iron oxide nanoparticles in rat brain and spinal cord. J. Neurosci. Res.:232–243.
Kuhlengel, K. R., Bunge, M. B., Bunge, R. P., and Burton, H. (1990). Implantation of cultured sensory neurons and Schwann cells into lesioned neonatal rat spinal cord. II. Implant characteristics and examination of corticospinal tract growth. J. Comp. Neurol. 293:74–91.
Lee, D. H., Strittmatter, S. M., and Sah, D. W. (2003). Targeting the Nogo receptor to treat central nervous system injuries. Nat. Rev. Drug Discov. 2:872–878.
Lesný, P., De Croos, J., Přádný, M., Vacik, J., Michálek, J., Woerly, S., and Syková, E. (2002). Polymer hydrogels usable for nervous tissue repair. J. Chem. Neuroanat. 23:243–247.
Lesný, P., Přádný, M., Jendelová, P., Michálek, J., Vacik, J., and Syková, E. (in press). Macroporous hydrogels based on 2-hydroxyethyl methacrylate. Part 4: Growth of rat bone marrow stromal cells in three-dimensional hydrogels with positive and negative surface charges and in polyelectrolyte complexes. J. Mater. Sci. Mater. Med.
Liu, S., Bodjarian, N., Langlois, O., Bonnard, A. S., Boisset, N., Peulve, P., et al. (1998). Axonal regrowth through a collagen guidance channel bridging spinal cord to the avulsed C6 roots: Functional recovery in primates with brachial plexus injury. J. Neurosci. Res. 51:723–734.
Lu, D., Mahmood, A., Wang, L., Li, Y., Lu, M., and Chopp, M. (2001). Adult bone marrow stromal cells administered intravenously to rats after traumatic brain injury migrate into brain and improve neurological outcome. Neuroreport 12:559–563.
Mahmood, A., Lu, D., Wang, L., and Chopp, M. (2002). Intracerebral transplantation of marrow stromal cells cultured with neurotrophic factors promotes functional recovery in adult rats subjected to traumatic brain injury. J. Neurotrauma. 19:1609–1617.
Majunder, M., Thiede, M., and Mosca, J. (1998). Phenotype and funtional comparison of cultured of marrow derived mesenchymal stem cells and stomal cells. J. Cell. Physiol. 176:57–66.
Maquet, V., Martin, D., Scholtes, F., Franzen, R., Schoenen, J., Moonen, G., and Jer me, R. (2001). Poly(D,L-lactide) foams modified by poly(ethylene oxide)-block-poly(D,L-lactide) copolymers and a-FGF: in vitro and in vivo evaluation for spinal cord regeneration. Biomaterials 22:1137–1146.
Mehler, M. F., Rozental, R., Dougherty, M., Spray, D. C., and Kessler, J. A. (1993). Cytokine regulation of neuronal differentiation of hippocampal progenitor cells. Nature 362:62–65.
Mezey, E., Chandross, K. J., Harta, G., Maki, R. A., and McKercher, S. R. (2000). Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science 290:1779–1782.
Ogawa, Y., Sawamoto, K., Miyta, T., Watanabe, M., Nakamura, M, Bregman, B., et al. (2002). Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J. Neurosci. Res. 69:925–933.
Okada, S., Ishii, K., Yamane, J., Iwanami, A., Ikegami, T., Katoh, H., et al. (2005). In vivo imaging of engrafted neural stem cells: its application in evaluating the optimal timing of transplantation for spinal cord injury. FASEB J. 19:1839–1841.
Oudega, M., Gautier, S. E., Chapon, P., Fragoso, M., Bates, M. L., Parel, J. M., and Bunge, M. B. (2001). Axonal regeneration into Schwann cell grafts within resorbable poly(alpha-hydroxyacid) guidance channels in the adult rat spinal cord. Biomaterials 22:1125–1136.
Park, H. C., Shims, Y. S., Ha, Y., Yoon, S. H., Park, S. R., Choi, B. H., and Park, H. S. (2005). Treatment of complete spinal cord injury patients by autologous bone marrow cell transplantation and administration of granulocyte-macrophage colony stimulating factor. Tissue Eng. 11:913–922.
Pitts, L. H., Ross, A., Chase, G. A., and Faden, A. I. (1995). Treatment with thyrotropin-releasing hormone (TRH) in patients with traumatic spinal cord injuries. J. Neurotrauma 12:235–243.
Pointillart, V., Petitjean, M. E., Wiart, L., Vital, J. M., Lassie, P., Thicoipe, M., and Dabadie, P. (2000). Pharmacological therapy of spinal cord injury during the acute phase. Spinal Cord. 38:71–76.
Přádný, M., Lesný, P., Fiala, J., Vacik, J., Slouf, M., Michálek, J., and Syková, E. (2002). Macroporous hydrogels based on 2-hydroxyethylmethacrylate. Part 1. Copolymers of 2-hydroxyethylmethacrylate with methacrylic acid. Collection Czech Chem. Commun. 68:812–822
Přádný, M., Michálek, J., Lesný, P., Hejčl, A., Vacík, J., Slouf, M., and Syková, E. (in press). Macroporous hydrogels based on 2-hydroxyethyl methacrylate. Part 5: Hydrolytically degradable materials. J. Mater. Sci. Mater. Med.
Prockop, D. J. (1997). Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276:71–74.
Sasaki, M., Honmou, O., Akiyama, Y., Uede, T., Hashi, K., and Kocsis, J. D. (2001). Transplantation of an acutely isolated bone marrow fraction repairs demyelinated adult rat spinal cord axons. Glia 35:26–34.
Syková, E., and Jendelová, P. (2005). Magnetic resonance tracking of implanted adult and embryonic stem cells in injured brain and spinal cord. Ann. N. Y. Acad. Sci. 1049:146–160.
Syková, E., Jendelová, P., Glogarová, K., Urdzíková, L., Burian, M., and Hájek, M. (2005a). Bone marrow cells as tool for the therapy of spinal cord injury. Program No 819.7 2005. Abstract viewer/Itinerary Planner, Society for Neuroscience, Washington, DC.
Syková, E., Urdzíková, L., Jendelová, P., Burian, M., Glogarová, K., and Hájek, M. (2005b). Bone marrow cells—a tool for spinal cord injury repair. Exp. Neurol. 193:261–262.
Teng, Y. D., Lavik, E. B., Qu, X., Park, K. I., Ourednik, J., Zurakowski, D., et al. (2002). Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. PNAS 99:3024–3029.
Urdzíková, L., Jendelová, P., Glogarová, K., Burian, M., Hájek, M., and Syková, E. (in press). Transplantation of bone marrow stem cells as well as mobilization by granulocyte— colony stimulating factor promote recovery after spinal cord injury in rat. J. Neurotrauma
Urdzíková, L., Jendelová, P., Glogarová, K., and Syková, E. (2005). The intravenous treatment with mesenchymal stromal cells promotes functional recovery of chronic spinal cord injuries. In Cassoviensia, F. M. (ed.), 5th International Symposium on Experimental and Clinical Neurobiology. Stara Lesna—The High Tatras, Slovak Republic, Institute of Neurobiology, Slovak Academy of Sciences, Faculty of Medicine, P.J. Šafárik University in Košice, pp. 124.
Venstrom, K. A., and Reichardt, L. F. (1993). Extracellular matrix. 2: Role of extracellular matrix molecules and their receptors in the nervous system. Faseb J. 7:996–1003.
Wang, L., Li, Y., Chen, J., Gautam, S. C., Zhang, Z., Lu, M., and Chopp, M. (2002). Ischemic cerebral tissue and MCP-1 enhance rat bone marrow stromal cell migration in interface culture. Exp. Hematol. 30:831–836.
Woerly, S., Doan, V. D., Sosa, N., de Vellis, J., and Espinosa, A. (2001b). Reconstruction of the transected cat spinal cord following NeuroGel implantation: axonal tracing, immunohistochemical and ultrastructural studies. Int. J. Dev. Neurosci. 19:63–83.
Woerly, S., Pinet, E., de Robertis, L., Van Diep, D., and Bousmina, M. (2001a). Spinal cord repair with PHPMA hydrogel containing RGD peptides (NeuroGel). Biomaterials 22:1095–1111.
Woerly, S., Pinet, E., De Robertis, L., Bousmina, M., Laroche, G., Roitback, T., et al. (1998). Heterogeneous PHPMA hydrogels for tissue repair and axonal regeneration in the injured spinal cord. J. Biomater. Sci. Polym. Ed. 9:681–711.
Woodbury, D., Schwarz, E. J., Prockop, D. J., and Black, I. B. (2000). Adult rat and human bone marrow stromal cells differentiate into neurons. J. Neurosci. Res. 61:364–370.
Wrathall, J. R., Rigamonti, D. D., Braford, M. R., and Kao, C. C. (1982). Reconstruction of the contused cat spinal cord by the delayed nerve graft technique and cultured peripheral non-neuronal cells. Acta Neuropathol. (Berl.) 57:59–69.
Wu, S., Suzuki, Y., Ejiri, Y., Noda, T., Bai, H., Kitada, M., et al. (2003). Bone marrow stromal cells enhance differentiation of cocultured neurospheres cells and promote regeneration of injured spinal cord. J. Neurosci. Res. 72:343–351.
Xu, X. M., Chen, A., Guenard, V., Kleitman, N., and Bunge, M. B. (1997). Bridging Schwann cell transplants promote axonal regeneration from both the rostral and caudal stumps of transected adult rat spinal cord. J. Neurocytol. 26:1–16.
ACKNOWLEDGMENTS
Supported by ASCR50390512, GACR309/06/1594, 309/06/1246, 1A8697-5/2005, NR8339-3, and MSMT 1M0021620803, LC554.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Syková, E., Jendelová, P., Urdzíková, L. et al. Bone Marrow Stem Cells and Polymer Hydrogels—Two Strategies for Spinal Cord Injury Repair. Cell Mol Neurobiol 26, 1111–1127 (2006). https://doi.org/10.1007/s10571-006-9007-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-006-9007-2