Abstract
The thermal decomposition or even explosion of nitrocellulose during long-term storage is prevented by adding stabilizers to nitrocellulose-based propellants. A series of novel arylmalonamide[70]fullerocyclopropane (3a–c) were synthesized through Bingel reaction. The molecular structures of 3a–c were verified through 1H NMR, 13C NMR, Fourier transform infrared spectroscopy (FT-IR), UV–visible spectroscopy, and mass spectrum. The thermal stability of 3a–c to nitrocellulose was studied by methyl violet paper test and iso-thermogravimetry method, and the results showed that the stability of 3a–c to nitrocellulose was significantly better than that of the [60]fullerene-based stabilizers. The thermal stability of 3a–c to nitrocellulose improved as the increase of the carbon chain length on the p-position of the benzene ring. The effects of 3a–c on the thermal decomposition of nitrocellulose were obtained by differential thermal analysis, and the results showed that the critical temperature of the thermal explosion of nitrocellulose can be increased by 0.1–2.8 °C by 3a–c. The thermal stability of 3a–c to nitrocellulose in adiabatic environment was confirmed by accelerating rate calorimetry. In addition, the stabilization mechanism was studied through ESR and FT-IR, and the results showed that 3a–c can react with nitrogen oxide radicals released by nitrocellulose. These arylmalonamide[70]fullerocyclopropane with excellent thermal stability and strong radical scavenging ability can be used as a promising stabilizer for single and double based propellants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
High nitrogen content nitrocellulose, which is a kind of flammable polymer has energy characteristics and can provide power for propellant combustion (Tarchoun et al. 2021, 2022a, b, 2022c). Nitrocellulose-based propellants are widely used in missile launchers to provide energy for rocket systems (Srinivas et al. 2016; Agrawal et al. 2000). However, owing to the special chemical structure of nitrate, an O–NO2 bond with weak bond energy is prone to mild thermal decomposition in long-term storage and transportation (Luo et al. 2019; Trache et al. 2019; Wang et al. 2017). The decomposition products of propellant further accelerate the thermal decomposition of nitrate and eventually induce propellant to emit large amounts of heat that lead to performance failure and even cause combustion explosion accidents (Sun et al. 2017; Shehata et al. 2003; Naud et al. 1992). Some traditional chemical stabilizers, mainly aniline and phenylurea compounds, are added to propellants to inhibit the autocatalytic reactions of nitrate esters and extend the service lives of propellants (Trache et al. 2013; Drzyzga et al. 2003; Tong et al. 2001). In addition, some novel biological macromolecules stabilizers have been studied by many researches but they are not received any industrial applications like traditional aromatic amine stabilizers (Cherif et al. 2020a, b, 2021). However, the most common traditional aromatic amine stabilizers have poor thermal stability, high toxicity, and poor stability effect under extreme conditions (De Klerk et al. 2015; Fryš et al. 2011; Lussier et al. 2006; Lindblom et al. 2002). The autocatalytic decomposition of nitrate can be inhibited by traditional aromatic amines stabilizers given that the alkaline compounds of aromatic amines can quickly combine with nitrogen oxides, but their strong alkalinity promotes the saponification of nitrate and reduces the chemical stability of the propellant, greatly limiting the application of weapon systems (Katoh et al. 2007). In addition, the stability of traditional stabilizers decreases at high temperature. The traditional stabilizer cannot meet the requirements and development of modern weapons. Therefore, the development of novel stabilizers are urgently essential.
Electron-withdrawing carbonyl group was introduced into the amino group inn the aromatic amine to form phenylurea derivative, reducing its basicity and the possibility of saponification. However, as a weakly basic compound, although the phenylurea derivatives also have the ability to absorb nitrogen oxides, their thermal stability to nitrocellulose is weak (Tang et al. 2017; Krumlinde et al. 2016). Bergmann and Junk test showed that the malonamide derivatives could effectively improve the thermal stability of nitrocellulose, and the stability effect was stronger than the traditional stabilizer C1. The charge effect of the substituent group affected the thermal stability of the malonamide derivatives (Zayed et al. 2010; Hassan et al. 2001; Zayed et al. 2000). Moreover, fullerene has the advantages of good thermal stability, strong acid resistance, and oxidation resistance owing to its good radical scavenging ability of various radicals, and has good compatibility with the main components of propellants (Dogru et al. 2012; Krishna et al. 2010; Dennler et al. 2009; Markovic et al. 2008; Jia et al. 2005; Butts et al. 2003). Therefore, fullerene-phenylurea derivatives are considered potential fullerene-based stabilizers (Li et al. 2020). Compared with other fullerene-based stabilizers, fullerocyclopropane derivatives have some advantages, such as weak alkalinity, high yield and no large amounts of by-products (Liao et al. 2021; Zhao et al. 2020a, b; Chai et al. 2020). The effect of C70-phenylurea derivatives, which have stronger electron absorption and π electron conjugation effects than that of C60-phenylurea derivatives on the thermal stability of nitrocellulose, have not been studied (Dresselhaus et al. 1996; Xie et al. 1992).
A series of C70 derivatives, arylmalonamide[70]fullerocyclopropane, were successfully synthesized using the Bingel reaction for the first time. The thermal stability of arylmalonamide[70]fullerocyclopropane on nitrocellulose was determined with isothermal experiments, and the stabilization mechanism and thermal behavior of arylmalonamide[70]fullerocyclopropane in the thermal decomposition of nitrocellulose were examined through non-isothermal thermal analysis. The thermal stability of arylmalonamide[70]fullerocyclopropane with different functional groups to nitrocellulose was evaluated.
Experimental sections
Synthesis procedures
All the reagents for organic synthesis were purchased from Aladdin Industrial Corporation, and organic solvents were purchased from Kelong Chemical Reagents Corporation. Fullerene C70 was obtained from Puyang Yongxin Fullerene Technology Corporation.
Synthesis of p-aryl malonamide derivatives: 4 mmol p-methylaniline (1a, 428 mg) or p-methoxyaniline (1b, 492 mg) or p-ethoxyaniline (1c, 548 mg) was dissolved in 40 mL of resteamed dichloromethane, and triethylamine (1 mL, 4 mmol) was added as the acid-binding agent. In an ice bath, the dichloromethane diluent (0.08 M) of malonyl chloride was added slowly using a constant pressure drop funnel, and the mixture was stirred for 0.5 h. Then, the reaction was continued at room temperature for 6 h. After the reaction, the solution was washed several times with distilled water, and the excess dichloromethane solvent was removed. The obtained light yellow solid products were recrystallized with ethyl acetate, and white solid malonamide derivatives (2a–c) were obtained through vacuum desiccation in yields of 86, 88 and 89%, respectively. The characterization results were shown in Figs. S1 − 9. p-toluene malonamide (2a): 1H NMR (600 MHz, d-DMSO) δ/ppm 10.10(s, 2H); 7.49(d, 4H, J = 8.41), 7.11(d, 4H, J = 8.27), 3.44(s, 2H), 2.25(s, 6H); 13C NMR (150 MHz, d-DMSO) δ/ppm 165.68, 136.96, 132.72, 129.58, 119.53, 56.31, 20.91; FTIR ν/cm–1 (KBr) 3136 (Ar–H), 2924 (–CH3), 1741 (C = O), 1650, 1531, 1407 (Ar), 1250 (Ar–N–), 818 (C–H). p-methoxyphenyl malonamide (2b): 1H NMR (600 MHz, d-DMSO) δ/ppm 10.03(s, 2H), 7.52(d, 4H, J = 9.01), 6.89(d, 4H, J = 9.06), 3.72(s, 6H), 3.41(s, 2H); 13C NMR (150 MHz, d-DMSO) δ/ppm 164.89, 155.15, 132.05, 120.49, 113.75, 55.04, 45.51; FTIR ν/cm–1 (KBr) 3053 (Ar–H), 2929 (–CH3), 1740 (C = O), 1642, 1549, 1512, 1411 (Ar), 1244 (Ar–N–), 1032 (C–O–C), 823 (C–H). p-ethoxyphenyl malonamide (2c): 1H NMR (600 MHz, d-DMSO) δ/ppm 10.30(s, 2H), 7.54(d, 4H, J = 9.01), 6.86(d, 4H, J = 9.04), 3.98(q, 4H, J = 6.96), 3.44(s, 2H), 1.30(t, 6H, J = 6.96); 13C NMR (150 MHz, d-DMSO) δ/ppm 165.37, 154.91, 132.69, 120.94, 114.80, 63.52, 46.27, 15.16; FTIR ν/cm–1 (KBr) 3048 (Ar–H), 2976, 2943 (–CH2–, –CH3), 1646, 1549, 1513, 1396 (Ar), 1243 (Ar–N–), 1052 (C–O–C), 823 (C–H).
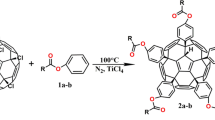
Synthesis of arylmalonamide[70]fullerocyclopropane: arylmalonamide[70]fullerocyclopropane were synthesized using the Prato reaction. C70 (84 mg, 0.1 mmol), malonamide derivatives (2a/2b/2c, 28 mg/32 mg/34 mg, 0.1 mmol), DBU (23 μL, 0.15 mmol), and CBr4 (50 mg, 0.15 mmol) were completely dissolved in 50 mL of toluene. The reaction was stirred at 5 ℃ under nitrogen protection for 0.5 h. After the reaction, impurities were removed through filtration, and the solvent was removed through rotary evaporation. The crude products were separated and purified through silica gel column chromatography. First, unreacted C70 was eluted with CS2, and then dichloromethane/tetrahydrofuran was used as the eluent. Dark brown solid arylmalonamide[70]fullerocyclopropanes (3a–c) were obtained in yields of 30%, 36% and 34%. The process is shown in Fig. 1.
Characterization
1H NMR and 13C NMR spectra were obtained using a Bruker Avance 600 spectrometer. Residual CHCl3 or DMSO was detected at 7.26 or 2.49 ppm in the 1H NMR spectra, respectively. and residual CHCl3 or DMSO was detected at 77.16 or 39.52 ppm in the 13C NMR spectra, respectively. Fourier transform infrared (FT-IR) spectra were recorded with a Nicolet 380 FT-IR spectrophotometer using spectrograph KBr in a range of 4000 − 400 cm–1. The UV–vis spectrophotometer with a double-beam light source of 190 − 1100 nm was used. The mass spectrum analysis was conducted using an Varian 1200 LC/MS.
Nitrocellulose samples preparation
Nitrocellulose with nitrogen content of 12.76% was supplied by the China Academy of Engineering Physics.
Nitrocellulose samples were prepared with the solvent evaporation method, and the preparation process was showed in Fig. S10. First, stabilizers (9 mg) were dissolved in 1 mL of CS2 to form a uniform solution. Then, 291 mg of nitrocellulose was added, and the solution was stirred for 1 h. The solvent was evaporated. The resulting uniform mixture was dried in a vacuum oven at 45 ℃ for 48 h. The composition of each nitrocellulose sample is listed in Table 1.
The compatibility between stabilizer and nitrocellulose was obtained by differential thermal analysis. The test results were shown in Table S1, which shows that the 3a–c has good compatibility with nitrocellulose.
Thermal stability analysis
Methyl violet test was performed with the GJB 770B-2005 method. The nitrocellulose sample was heated in a test tube at 134.5 °C with a sample mass of 300 mg in the presence of methyl violet paper. Subsequently, time was measured until the color of the paper changes from purple to salmon pink. Differential thermal analysis (DTA) was performed using a WCR-1B differential thermal analyzer at a heating rate of 10 °C·min−1. Thermogravimetric (TG) analysis was performed at constant temperatures of 135, 145 and 155 °C with a WRT-1D thermal analyzer. Differential scanning calorimetry (DSC) analysis was carried out with a Q200 (USA) TA instrument at a heating rate of 10 °C·min−1. The “interruption and re-scanning” method described in previous studies was used (Wang et al. 2015). The accelerating rate calorimetry (ARC) was carried out on a NETZSCH instrument, and a titanium bomb with a thermocouple clip at the bottom was used. The heating–searching–waiting (H–W–S) mode procedure was carried out at a heating rate of 10 °C·min−1 and a searching time of 30 min, and the sample masses was 40.0 ± 0.1 mg.
Stability mechanism analysis
ESR spectrometry was performed using a Bruker EMXmicro instrument. The settings were as follows: microwave power, 2 mW; modulation frequency, 100 kHz; field center, 3440 G; sweep width, 100 G; conversion time, 40.04 ms; time constant, 40.96 s; number of scans, 3; and sweep time, 60.06 s.
Results and discussion
Structural characterization
The structures of arylmalonamide[70]fullerocyclopropanes (3a–c) were characterized through 1H NMR, 13C NMR, FT-IR, UV–vis, and LC–MS spectra. The 1H NMR spectrum of 3c is shown in Fig. 2(a). Two doublets at δ 7.42 and 6.83 ppm appeared in the hydrogen proton region of the benzene ring, and the hydrogen area integral ratio was 2:2. One quartet at δ 4.00 ppm and one triplet at δ 4.18 ppm were obtained in a hydrogen area integral ratio of 2:3, which were attributed to the methylene and methyl groups in ethoxy, respectively. One singlet appears at δ 9.00 ppm, which was attributed to amino proton. The 13C NMR spectrum of 3c (Fig. 2(b)) displayed signals at δ of 155.26 − 131.18 ppm for the carbon atoms on C70. The signals at δ 122.09 − 114.73, 156.11, and 130.28 ppm were phenyl carbons connected to hydrogen, ethoxyl, and amino, respectively. The signals at δ 99.97 − 77.52 and 44.25 ppm were attributed to the methylene carbon on the cyclopropane and the sp3 carbon of C70 connected with cyclopropane, respectively. The signals at δ 63.67 and 14.83 ppm were attributed to the ethoxyl carbon. The signal at δ 165.42 ppm was attributed to the carboxyl carbon. The structures of 3c was a completely symmetric p-ethoxyphenyl malonamide[70]fullerocyclopropane. The results were consistent with the 1H NMR and 13C NMR spectra (Figs. S11–14) of 3a–b. Subsequently, LC–MS spectra results further indicated that 3a-c were monoaddition C70 derivatives (Figs. S15 − 17).
The structures of 3a–c were confirmed using FT-IR spectra, as shown in Fig. 3. The stretching vibration absorption peak at 3430 cm−1 was attributed to the N − H bond. The absorption peaks at 2920 − 2845 cm−1 were attributed to C − H bond. The stretching vibration absorption peak at 1737 cm−1 was attributed to carbonyl C = O bond. The absorption peaks at 1640 − 1510 cm−1 were attributed to the benzene ring skeleton. The characteristic absorption peaks of C70 were detected at 1377 and 526 cm−1. The characterization results were consistent with the target functional groups.
The absorption peak at 441.5 nm in the UV–Vis spectra of 3a–c (Fig. 4) was attributed to the C1 − C2 bond of C70 derivatives (Wang et al. 2010). It indicated that the structure of 3a–c conformed to expectations.
Stability experiment
The thermal stability of nitrocellulose was evaluated through isothermal thermal analysis, and the thermal decomposition reactions of different nitrocellulose samples at a constant temperature were measured. The common methods included volumetric gas and gravimetric methods. First, the methyl purple paper method was used to evaluate the stability of 3a–c to nitrocellulose, and the temperature was 134.5 °C. The discoloration times of S-1–S-4 in the methyl purple paper test were shown in Table 2. 3a–c extended the discoloration time of methyl purple paper test of nitrocellulose by 70, 74, and 81 min, respectively. Nitrocellulose samples S-1–S-4 did not burn or deignite after heating for 5 h, and the discoloration time of S-1–S-4 was longer than the previous results (Li et al. 2020), indicating that the stability of 3a–c were superior to that of the previously reported arylmalonamide[60]fullerocyclopropane.
In the thermogravimetric method, weight loss of nitrocellulose samples at constant temperature was used as a standard for evaluating the thermal stability of nitrocellulose samples. Isothermal thermogravimetry was conducted at 135, 145 and 155 °C, which were all higher than the temperature for the methyl violet paper test. The results are shown in Fig. 5(a–c), the weight loss of nitrocellulose samples S-1–S-4 increased with temperature. Weight loss of the nitrocellulose samples S-2–S-4 after the addition of 3a–c was obviously weaker than that of nitrocellulose S-1, indicating that 3a–c can effectively alleviate the thermal decomposition of nitrocellulose. The order by degree of influence was consistent with that of the methyl violet paper test.
The safe storage life of nitrocellulose samples were obtained through isothermal kinetics at different temperatures. The weight loss of nitrocellulose was 1.6% when the methyl violet test paper changed color. Then, the time required when the weight loss of nitrocellulose samples reached 1.6% was taken as the evaluation standard. The time required for different nitrocellulose samples S-1–S-4 to reach 1.6% weight loss was shown in Table S2. The safe storage life can be calculated according to Semenov’s equation:
where T is the constant temperature, K; tT is the time required for the weight loss to reach 1.6% at temperature T, min; a and b are coefficients.
The fitting curves of Semenov’s equation for the nitrocellulose samples S-1–S-4 were shown in Fig. 5(d), and the calculation results were shown in Table 3. The safe storage life of the nitrocellulose samples increased gradually from S-1 to S-4, indicating that 3a–c can effectively prolong the safe storage life of nitrocellulose. The sequence of 3a–c was consistent with that of the thermal stability of nitrocellulose samples S-1–S-4.
Thermal interaction test
The non-isothermal thermodynamics reflected the ability of 3a–c to interact with nitrocellulose. The activation energy of thermal decomposition generally reflects the thermal decomposition capacities of samples, and the autocatalytic decomposition products released by nitrocellulose during thermal decomposition can catalyze the thermal decomposition of nitrocellulose. Therefore, the thermal decomposition activation energy of nitrocellulose can be affected theoretically after the heating process. The nitrocellulose samples S-1–S-4 before and after the methyl violet paper test were subjected to DTA tests at different heating rates (5, 10, 15 and 20 C min–1). The DTA curves obtained were showed in Fig. 6 and Fig. S18.
According to the thermal decomposition temperature of nitrocellulose samples S-1–S-4, the thermal decomposition parameters were calculated by using Kissinger equation Eqs. 2, 3, and 4.
where β is the gas constant (8.314 J·mol−1·K−1); Tp is the peak temperature of the DTA curve; Ea is the value of the activation energy; A is the pre-exponential factor; Tpi is the peak temperature of the DTA curve at coefficients of βi, b, c, and d (Table S3); and Tbp0 is the critical temperature of the thermal explosion. The results obtained using these methods were listed in Table 4 and Table S4.
Compared with nitrocellulose S-1, the Tp0 of nitrocellulose samples S-2–S-4 increased by 1.5 °C, 3.4 and 4.3 °C, and the Tbp0 increased by 0.1, 1.8 and 2.8 °C, respectively. In addition, the activation energy of S-2–S-4 was significantly lower than that of nitrocellulose S-1.
The process of generating autocatalytic products at a certain temperature is called the thermal history of nitrocellulose. The influence of thermal history on the thermal decomposition of nitrocellulose samples was investigated with the DSC interruption and re-scanning method. The obtained DSC curves were shown in Fig. 7, and the thermal decomposition parameters were shown in Table 5. The release of nitrocellulose thermal decomposition products increased with interrupt temperature, the initial and peak temperatures moved to low temperatures, and the peak width gradually widened. After three times of heating, the peak temperature of the thermal decomposition of nitrocellulose S-1 decreased by 5.1 °C. However, 3a–c reduced the influence of thermal history on the thermal decomposition of nitrocellulose, and the peak temperatures of S-2, S-3, and S-4 only decreases by 3.4, 3.4 and 3.0 °C after three times of heating.
The linear fitting curves of the peak temperature gradient (γ) of nitrocellulose samples S-1–S-4 at different initial conversion rates (α0) were shown in Fig. 8. The results showed that the slope and intercept of the fitting curve of S-1 was the largest, indicating that the thermal decomposition of nitrocellulose S-1 was the most seriously affected by the thermal history. These results showed that 3a–c interacted with the thermal decomposition products of nitrocellulose and inhibited the autocatalytic thermal decomposition of nitrocellulose.
The central region of a large accumulation of nitrocellulose is mostly adiabatic. Therefore, in the evaluation of the thermal stability of the nitrocellulose samples S-1–S-4, thermal decomposition parameters under adiabatic conditions should be considered. The thermal decomposition parameters of samples under adiabatic conditions can be measured through ARC method. The adiabatic decomposition curves of S-1–S-4 with an initial temperature of 100 °C were shown in Fig. 9. After 9, 11, 11, and 12 heating procedures, the nitrocellulose samples were self-exothermic. The initial temperature of the exothermic reaction of nitrocellulose was increased by 3a–c. In addition, the heat rate-temperature curves of S-1–S-4 were shown in Fig. S19.
The experimental conditions of nitrocellulose at the self-accelerating decomposition stage were shown in Table 6. The maximum temperature rise rate of S-1 was 1.08 °C min−1, whereas the rates of S-2, S-3, and S-4 were 0.30, 0.82, 0.74, and 0.59 °C min−1. The results showed that 3a–c can increase the initial decomposition temperature and decomposition pressure of nitrocellulose.
Sample S-1–S-4 were graded with the reaction risk assessment method, and the thermal reaction risk was divided into four grades: I, II, III, and IV (Saraf et al. 2003), the reaction risk decreased gradually with increasing grade. The hazard classification for nitrocellulose samples S-1–S-4 were shown in Fig. 10. The results showed that 3a–c can effectively reduce the thermal risk of nitrocellulose, the initial decomposition temperature of nitrocellulose gradually increased, and the amount of heat released gradually decreased.
Stabilization mechanism
The NO· scavenging capacities of 3a–c were measured through ESR, and the results were shown in Figs. 11(a–c). After the addition of 3a–c, the signal of NO· was weakened, and the signal of NO· decreased with increasing 3a–c concentration, indicating that 3a–c had excellent scavenging ability for NO·.
The nitrogen oxide radical scavenging rates of 3a–c were shown in Fig. 11(d). The fitting curves can be expressed as follows:
where C denotes the concentrations of 3a–c and η represents the nitrogen oxide radical scavenging rates. The corresponding parameters were shown in Table 7.
The values of 50% inhibitory concentration (IC50) and 95% confidence interval (CI) of 3a–c were shown in Table 8, and only 0.247, 0.215 and 0.211 mM of 3a–c can achieve the semi-inhibition of nitrogen oxide free radical. The results showed that 3a–c have an extremely high scavenging effect on NO· radical, and only a small amount of 3a–c can satisfy the 50% inhibitory of the NO· radical.
The infrared spectra of the secondary derivatives of 3a–c reaction with NOx were shown in Fig. 12, which was extracted by CS2. The NO2 group was obvious in the infrared spectra of the intermediate after the reaction of 3a–c with nitrocellulose. The results showed that 3a–c can react with the nitrogen oxides generated by the thermal decomposition of nitrocellulose, and finally form nitro-substituted fullerene derivatives.
Finally, the thermal stability of nitrocellulose samples is affected not only by the functional groups but also by the number of carbon atoms in fullerenes. Based on previously reported data (Li et al. 2020), the thermal stability of the fullerene-based stabilizers with different fullerene was compared. Discoloration time in methyl violet paper, semi-inhibitory concentration (IC50) of NO· and the NO· scavenging rate at 1.2 mM stabilizer concentration were showed in Fig. 13 and Table S5. The results showed that the higher the number of carbon atoms of fullerenes, the better the thermal stability of the fullerene-based stabilizer on nitrocellulose and the scavenging activity of nitrogen oxide radicals. In addition, we compared the stability of typical traditional stabilizer (DPA) with that of fullerene-based stabilizer, and the results were showed in Table S5. The results showed that the unique stabilization mechanism of fullerene-based stabilizers make its stability to nitrocellulose significantly higher than that of DPA.
Conclusion
A serious of arylmalonamide[70]fullerocyclopropanes (3a–c) were synthesized by Prato reaction and their structures were characterized with FT-IR, UV–Vis, LC–MS, and NMR. The reaction has the advantages of convenient raw material, mild reaction conditions, high product yield, and easy amplification, providing a means for the subsequent large-scale synthesis of [70]fullerene-based stabilizers. Isothermal thermal analysis confirmed the superior thermal stability of 3a–c to nitrocellulose even at high temperature, the safe storage life of nitrocellulose at 50 °C can be increased by 31.94 years at most. Non-isothermal thermal analysis showed that the critical temperature of thermal explosion of nitrocellulose could be increased by 2.8 by 3a–c. At the initial stage of the thermal decomposition of nitrocellulose, 3a–c can react with nitrogen oxides and other autocatalytic products, thus inhibiting the autocatalytic thermal decomposition of nitrocellulose. In addition, 3a–c maintains good thermal stability under adiabatic conditions. This finding is of great significance to the safe storage of nitrocellulose under practical conditions. This work provides a novel idea for the design and large-scale preparation of [70]fullerene-based stabilizers.
References
Agrawal JP, Surve RN, Mehilal M, Sonawane SH (2000) Some aromatic nitrate esters: synthesis, structural aspects, thermal and explosive properties. J Hazard Mater 77:11–31
Butts CP, Jazdzyk M (2003) The preparation and structures of non-hydrocarbon functionalised fullerene-diamine adducts. Chem Commun 34:1530–1531
Chai ZH, Luo LQ, Jin B, Zhao Y, Xiao LPC, Li G, Zhang QC, Peng RF (2020) Fullerene stabilizer 4,11,15,30-tetraarylamino fullerenoarylaziridine: regioselective synthesis, crystallographic characterization derivatives, and potential application as propellant stabilizer. ACS Appl Energy Mater 3:3005–3014
Cherif MF, Trache D, Benaliouche F, Chelouche S, Tarchoun AF, Mezroua A (2020a) Effect of Kraft lignins on the stability and thermal decomposition kinetics of nitrocellulose. Thermochim Acta 692:178732
Cherif MF, Trache D, Benaliouche F, Chelouche S, Tarchoun AF, Chelouche S, Mezroua A (2020b) Organosolv lignins as new stabilizers for cellulose nitrate: thermal behavior and stability assessment. Int J Biol Macromol 164:794–807
Cherif MF, Trache D, Benaliouche F, Chelouche S, Tarchoun AF, Kesraoui M, Abdelaziz A (2021) Mordenite zeolite for scavenging nitroxide radicals and its effect on the thermal decomposition of nitrocellulose. J Energ Mater. https://doi.org/10.1080/07370652.2021.1998250
De Klerk WPC (2015) Assessment of stability of propellants and safe lifetimes. Propell Explos Pyrot 40(3):388–393
Dennler G, Scharber MC, Brabec CJ (2009) Polymer-fullerene bulk-heterojunction solar cells. Adv Mater 21(13):1323–1338
Dogru M, Handloser M, Auras F, Kunz T, Medina D, Hartschuh A, Knochel P, Bein T (2012) A photoconductive thienothiophene-based covalent organic framework showing charge transfer towards included fullerene. Angew Chem Int Edit 52:2920–2924
Dresselhaus MS, Dresselhaus G, Eklund PC (1996) Science of fullerenes and carbon nanotubes. Academic Press, Cambridge, pp 437–438
Drzyzga O (2003) Diphenylamine and derivatives in the environment: a review. Chemosphere 53(8):809–818
Fryš O, Bajerová P, Eisner A, Skládal J, Ventura K (2011) Utilization of new non-toxic substances as stabilizers for nitrocellulose-based propellants. Propell Explos Pyrot 36(4):347–355
Hassan MA (2001) Effect of malonyl malonanilide dimmers on the thermal stability of nitrocellulose. J Hazard Mater 88(1):33–49
Jia G, Wang HF, Yan L, Wang X, Pei RJ, Yan T, Zhao YL, Guo XB (2005) Cyto-toxicity of carbon nanomaterials: single-wall nanotube, multi-wall nanotube, and fullerene. Environ Sci and Technol 39(5):1378–1383
Katoh K, Yoshino S, Kubota S, Wada Y, Ogata Y, Nakahama M, Kawaguchi S, Arai M (2007) The effects of conventional stabilizers and phenol compounds used as antioxidants on the stabilization of nitrocellulose. Propell Explos Pyrot 32(4):314–321
Krishna V, Stevens N, Koopman B, Moudgil B (2010) Optical heating and rapid transformation of functionalized fullerenes. Nature Nanotechnol 5(5):330–334
Krumlinde P, Ek S, Tunestål E, Hafstrand A (2016) Synthesis and characterization of novel stabilizers for nitrocellulose-based propellants. Propell Explos Pyrot 42(1):78–83
Li G, Jin B, Chai ZH, Liao L, Chu SJ, Peng RF (2020) Synthesis and stabilization mechanism of novel stabilizers for fullerene-malonamide derivatives in nitrocellulose-based propellants. Polym Test 86:106493
Liao L, Jin B, Guo ZC, Xian F, Hou CJ, Peng RF (2021) Fullerene bisadduct stabilizers: the effect of different addition positions on inhibiting the autocatalytic decomposition of nitrocellulose absorbed nitroglycerin. Def Tech 17(6):1944–1953
Lindblom T (2002) Reactions in stabilizer and between stabilizer and nitrocellulose in propellants. Propell Explos Pyrot 27(4):197–208
Luo LQ, Jin B, Xiao YY, Zhang QC, Chai ZH, Huang Q, Chu SJ, Peng RF (2019) Study on the isothermal decomposition kinetics and mechanism of nitrocellulose. Polym Test 75:337–343
Lussier LS, Bergeron E, Gagnon H (2006) Study of the daughter products of Akardite-II. Propell Explos Pyrot 31(4):253–262
Markovic Z, Trajkovic V (2008) Biomedical potential of the reactive oxygen species generation and quenching by fullerenes. Biomater 29:3561–3573
Naud DL, Brower KR (1992) Pressure effects on the thermal decomposition of nitramines, nitrosamines, and nitrate esters. J Org Chem 57:3303–3308
Saraf SR, Rogers WJ, Mannan WS (2003) Using screening test data to recognize reactive chemical hazards. J Hazard Mater 104:255–267
Shehata AB, Hassan MA, Nour MA (2003) Effect of new poly 2-acryloyl-N, N’-bis (4-nitrophenyl) propandiamide and poly 2-acryloyl-N, N’-bis (4-methylphenyl) propandiamide and their synergistic action on the stability of nitrocellulose. J Hazard Mater 102(2/3):121–136
Srinivas D, Ghule VD (2016) Synthesis of nitrate ester and nitramine derivatives of polyfluoro alkyl compounds for high energy materials. RSC Adv 6(10):7712–7716
Sun ZD, Fu XL, Yu HJ, Fan XZ, Ju XH (2017) Theoretical study on stabilization mechanisms of nitrate esters using aromatic amines as stabilizers. J Hazard Mater 339:401–408
Tang QF, Fan XZ, Li JZ, Bi FQ, Fu XL, Zhai LJ (2017) Experimental and theoretical studies on stability of new stabilizers for N-methyl-P-nitroaniline derivative in CMDB propellants. J Hazard Mater 327:187–196
Tarchoun AF, Trache D, Krumm B, Derradji M, Bessa W (2021) Design and characterization of new advanced energetic biopolymers based on surface functionalized cellulosic materials. Cellulose 28:6107–6123
Tarchoun AF, Trache D, Klapötke TM, Abdelaziz A, Bekhouche S, Boukeciat H, Sahnoun N (2022a) Making progress towards promising energetic cellulosic microcrystals developed from alternative lignocellulosic biomasses. J Energ Mater. https://doi.org/10.1080/07370652.2022.2032484
Tarchoun AF, Trache D, Klapötke TM, Slimani K, Abdelaziz BB, A, Bekhouche S, Bessa W, (2022b) Valorization of esparto grass cellulosic derivatives for the development of promising energetic azidodeoxy biopolymers: synthesis, characterization and isoconversional thermal kinetic analysis. Propell Explos Pyrot 47(3):e202100293
Tarchoun AF, Sayah ZBD, Trache D, Klapötke TM, Belmerabt M, Abdelaziz A, Bekhouche S (2022c) Towards investigating the characteristics and thermal kinetic behavior of emergent nanostructured nitrocellulose prepared using various sulfonitric media. J Nanostruct Chem. https://doi.org/10.1007/s40097-021-00466-x
Tong Y, Wu ZP, Yang C, Yu J, Zhang X, Yang S, Deng X, Xu Y, Wen Y (2001) Determination of diphenylamine stabilizer and its nitrated derivatives in smokeless gunpowder using a tandem MS method. Analyst 126(4):480–484
Trache D, Khimeche K (2013) Study on the influence of ageing on chemical and mechanical properties of N, N’-dimethyl-N, N’-diphenylcarbamide stabilized propellants. J Therm Anal Calorim 111:305–312
Trache D, Tarchoun AF (2019) Analytical methods for stability assessment of nitrate esters-based propellants. Crit Rev Anal Chem 49:415–438
Wang GW, Yang HT, Wu P, Wang CZ (2010) Reaction of [70]Fullerene with tetraethyl methylenediphosphonate or diethyl (cyanomethyl)phosphonate revisited. Eur J Org Chem 29:5714–5721
Wang K, Liu DB, Xu S, Cai GW (2015) Research on the thermal history’s influence on the thermal stability of EHN and NC. Thermochim Acta 610:23–28
Wang B, Xin L, Wang Z, Deluca LT, Liu Z, You F (2017) Preparation and properties of a nRDX-based propellant. Propell Explos Pyrot 42:649–658
Xie QS, Eduardo PC, Luis E (1992) Electrochemical detection of C606– and C706–: enhanced stability of fullerides in solution. J Am Chem Soc 114(10):3978–3980
Zayed MA, Soliman AAW, Hassan MA (2000) Evaluation of malonanilides as new stabilizers for doubleBase propellants(I). J Hazard Mater 73(3):237–244
Zayed MA, Mohamed AA, Hassan MAM (2010) Stability studies of double-base propellants with centralite and malonanilide stabilizers using MO calculations in comparison to thermal studies. J Hazard Mater 179(1–3):453–461
Zhao Y, Jin B, Peng RF, Ding L, Zheng T (2020a) Novel fullerene-based stabilizer for scavenging nitroxide radicals and its behavior during thermal decomposition of nitrocellulose. J Hazard Mater 191:121857–121863
Zhao Y, Jin B, Peng RF, Zheng T (2020b) Interaction of nitrocellulose with pentaacyloxyphenyl fullerene derivatives: autocatalytic inhibition in thermal decomposition of nitrocellulose. Cellulose 27:3611–3622
Acknowledgments
This work was supported by the financial support received from National Natural Science Foundation of China (51972278), Associated Foundation of Xi’an Modern Chemistry Research Institute (No. 204-J-2020-2634), and Open Project of State Key Laboratory of Environment-friendly Energy Materials (Southwest University of Science and Technology, No. 21fksy19).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, Y., Jin, B., Zheng, T. et al. Structure–activity relationship of thermal interaction between arylmalonamide[70]fullerocyclopropane stabilizer and nitrocellulose. Cellulose 29, 6579–6593 (2022). https://doi.org/10.1007/s10570-022-04669-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-022-04669-5