Abstract
This study assesses the possibility of obtaining regenerated cellulose products (beads and films) from eucalyptus sawdust dissolving pulps produced by non-conventional processes, compared with a commercial dissolving pulp as a reference. Eucalyptus sawdust dissolving pulps were obtained by soda pulping followed by two TCF sequential bleaching processes OOpZ, and OOp, followed by a cold soda extraction. The characterization of dissolving pulps involved alpha-, beta- and gamma-cellulose content, alkali solubility with 10 wt% (S10), and 18 wt% NaOH (S18) aqueous solutions, and degree of polymerization. Fock´s method was used to measure cellulose reactivity and the alkali solubility in a 9 wt% NaOH aqueous solution at − 5 °C to evaluate the pulps dissolving capacity. Dissolving pulps presented high cellulose content (> 93%, expressed as α-cellulose) and good reactivity (almost 8%). The dissolving pulps were adequate raw materials for regenerated cellulose products (beads and films) from two cellulose dissolution methods: direct dissolution in NaOH/urea and cellulose carbamate solution. The sequence OOpE (where E is an alkaline extraction) was more economically feasible and straightforward to produce dissolving pulp than OOpZE. The experimental pulps showed the expected characteristics of the dissolving pulp to obtain regenerated cellulose products. However, it is necessary to deepen the study of producing regenerated cellulose films with enhanced mechanical properties from experimental dissolving pulps, solvents, coagulation, and regeneration conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulose and its derivatives are potential alternatives to petroleum-based polymers for flexible and rigid packaging, composites, and films, among others. The most critical segment in cellulose processing corresponds to regenerated cellulose products, primarily fibers (rayon), films, membranes, and sponges. These derivatives are obtained from the dissolving grade pulps (Gindl and Keckes 2005; Yousefi et al. 2011; Ghaderi et al. 2014; Singh et al. 2015).
The most relevant requirements of dissolving pulps are high content of α-cellulose (90–9%), low content of non-cellulosic compounds (lignin, hemicelluloses, extractives, and ash), good reactivity to derivatizing chemicals, and specified cellulose molar mass and its distribution (Gavrilescu 2013; Sayyed et al. 2019). Currently, agro-industrial and forestry lignocellulosic by-products are studied to produce biomaterials, bioproducts, bioenergy, and biofuels (Stafford et al. 2020; Kamusoko et al. 2021). Sawdust is a low-cost lignocellulosic residue from primary wood industrialization, abundant in regions with highly industrialized forestry activity (Simon et al. 2017). Around 1% sawdust is generated from each 1 m3 of the processed green log. Sawdust use as raw material for biorefineries promotes the integral use of wood in highly forested countries or regions (Clauser et al. 2018; Guigou et al. 2019; Abdou Alio et al. 2021).
Conventional dissolving pulp is obtained from cotton linter or wood by soda, kraft, or sulfite pulping. The main advantage of lignocellulosic waste (i.e., bagasse, straw, sawdust, others) as raw material instead of cotton linter or commercial wood pulp is water decrease and (or) land use. Besides, the use of eucalyptus sawdust, with relatively low hemicellulose content, is ideal as it produces a high yield and low xylans content dissolving pulp.
Dissolving pulps production in biorefineries has considerable potential (Kumar and Christopher 2017). Kraft, sulfite, and other conventional pulping processes have significant disadvantages for biorefineries, such as sulfur compounds use. Sulfur affects the recovery of dissolved wood components during the pulping process (hemicelluloses and lignin). Soda pulping is an alternative (sulfur-free) alkaline process, which can be applied on smaller scales and have environmental and operational advantages. Besides, kraft technologies for chips (batch and continuous Kamyr digesters) are not adequate also. Screw or rotary horizontal digesters are required because they provide an optimal solid-liquid contact in the case of small sizes lignocellulosic materials, such as sawdust, straw, bagasse, and others.
Dissolving pulp is mainly used to produce viscose. Besides, it is also a raw material for other cellulose derivatives, such as cellulose acetate, cellophane, carboxymethyl cellulose, and other high-value-added cellulose products (Chen et al. 2019). In particular, cellulose beads and their derivatives are used in many advanced applications, including chromatography over solid-supported synthesis and enzyme immobilization for retarded drugs release (Peška et al. 1976; Gemeiner et al. 1989). Besides their traditional uses, regenerated cellulose films have promising applications in food packaging (Huang and Wang 2022). In the last years, regenerated cellulose production significantly growth the dissolving pulps market (Sixta et al. 2013). Between 2016 and 2017, the global production of dissolving pulp was approximately 8 million tons (Kumar and Christopher 2017; Chen et al. 2019). Its price is affected by the bleached chemical pulp one, usually 150–300 USD more expensive per air dry ton than bleached chemical pulp. The United States, Canada, Brazil, and Europe produce around 60% of global dissolving pulp production from wood (Chen et al. 2019). Currently, Asia–Pacific regions are the principal producers of viscose (80% of the global production) due to lower labor costs and softer environmental regulations compared to Europe and the United States. China is the largest global viscose producer of that A-P region (62% of the total). It also imports other regenerated cellulosic fibers such as Lyocell and modal. Overcoming the cost and environmental burden of the transportation of dissolving pulps from wood sources could enhance the development of the local textile industry in various regions (Rana et al. 2014; Arce et al. 2020).
Economic and environmental reasons have driven the manufacturers of regenerated cellulose products to continuously search for alternative manufacturing practices more environmentally friendly processes (Liu et al. 2016). Cellulose carbamate is a cellulose derivative produced by the reaction of cellulose with urea (CO(NH2)2). The cellulose carbamate process is an environmentally friendly alternative to the viscose processes with great potential. However, it is in an early industrial phase, and requires more research and technological developments (Pinnow et al. 2008; Zhang et al. 2019). Currently, the Infinited Fiber Company produces 150 metric tons/year at a pre-commercial scale (Finland). The raw materials are post-consumer textile waste, cardboard waste, and pulp. The commercial-scale factory could be up and running in 2024. It is a significant step towards the cellulose carbamate industrial process and commercial production. However, these cellulosic wastes currently have preferred uses in the papermaking industry (recycled fibers for paper and packaging, paper money, others) (ANDRITZ 2021).
This study aims to assess the possibility of obtaining regenerated cellulose products (beads and films) from eucalyptus sawdust dissolving pulps (EPs) produced by non-conventional processes (direct dissolution using NaOH-urea and cellulose carbamate). The performance of the products was compared with a commercially available dissolving pulp. EPs were obtained by soda pulping followed by a sequential oxidative treatment. Two TCF bleaching short sequences were used (with and without ozone) with the target of achieving a dissolution degree from a eucalyptus sawdust pulp, with the least possible production and effluent treatment costs. Beads and films were produced prepared by coagulating and regenerating processes. Characterization included average diameter and porosity for the beads and tensile strength for films.
Materials and methods
Materials
Eucalyptus sp. sawdust was collected from local sawmill carpentry (Misiones, Argentina), which industrializes 12–50-years-old Eucalyptus (E. grandis, E. saligna, and E. rostrata) (Rangel et al. 2016). Commercial dissolving pulp Solucell® (CP) produced from a Eucalyptus globulus pre-hydrolyzed kraft dissolving pulp was provided by Bahia Specialty Cellulose S.A. (Camacari, Brazil).
Eucalyptus sawdust dissolving pulp production
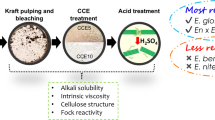
The chemical characterization of eucalyptus sawdust was performed and reported in previous work (Rangel et al. 2016). Briefly, the chemical composition was 41.8% of glucans, 10.7% of xylans, 0.38% of arabinans, 1.41% of acetyl groups, 31.3% of lignin, 7.86% of extractives, and 0.59% of ashes; % based on oven-dry sawdust. The processing scheme and conditions used to obtain dissolving pulp from eucalyptus sawdust are shown in Fig. 1.
The schematic process for dissolving pulps from eucalyptus sawdust obtaining (O: oxygen, Op: oxygen reinforced with hydrogen peroxide, Z: ozone, E: alkaline extraction). Dissolving pulp from Eucalyptus sawdust soda pulp: EP-1 bleached by OOp stages, and EP-2 bleached by OOpZ stages. (odp: on oven-dry pulp)
Pulps characterization
The characterization of the pulps involved the following analytical techniques: alpha-, beta-, and gamma-cellulose content (TAPPI T203 cm-99); alkali solubility with 10 wt% and 18 wt% NaOH aqueous solutions, called S10 and S18 respectively (TAPPI T235 cm-00); and intrinsic viscosity and viscosimetric average degree of polymerization (DPV) in cupriethylenediamine (CUEN) (Marx-Figini 1987). Fock´s method was used to measure the cellulose reactivity (Fock 1959), and the solubility in alkali allowed to determine the soluble material of pulp in 9 wt% NaOH at − 5 °C (Rahkamo et al. 1998).
Mechanical and enzymatic pretreatment of the dissolving pulps
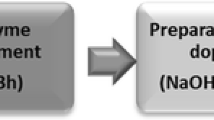
The refining mechanical pretreatment was carried out in a PFI mill under standard conditions (T 248 sp-00) with 6000 rpm as indicated in previous works (Olmos 2016). Subsequently, the enzymatic treatment was carried out with a cellulase preparation (Celluclast 1.5L – Novozymes A/S Denmark), at 5% consistency, 9% enzyme dosage on dry fiber, at 40 ºC and pH 5.4 for 60 min.
Direct dissolution of cellulose in NaOH/Urea
The direct dissolution of mechanical and enzymatically treated pulp was carried out in a cryo-bath at − 5 °C, in a mixture of 7 wt% NaOH and 12 wt% urea, under constant agitation for 30 minutes. Then the solution was centrifuged for 15 minutes at 12,000 rpm to remove the undissolved cellulose. The undissolved material was neutralized with 3% H2SO4 and washed with abundant distilled water. Finally, it was filtered using a porous glass filter and dried at 105 °C for 24 hours to quantify the dry residue. The cellulose solution was stored at low temperatures in closed containers. The processing steps and conditions used for direct dissolution are shown in Fig. 2.
Solubility was determined as the dissolved cellulose weight (the difference between the initial weight and the undissolved residue) concerning the initial weight of cellulose.
Production of cellulose carbamate (CC) solution
The cellulose carbamate was prepared from dissolving pulp suspension (4% consistency) by activation and impregnation treatments with 6 wt/v% NaOH and 30 w/v% urea solution at 25 °C under continuous stirring for 120 minutes. Then, the excess solution was removed by pressing to an impregnated cellulose/pulp ratio of 4.25:1. The impregnated cellulose was disintegrated and dried in an oven at 60 °C for 20 hours. The cellulose esterification reaction of the dried mixture was carried out by heating in a microwave oven at 160 W for 15 minutes. After the reaction, the CC was washed with abundant water until neutrality, separated from the by-products (urea and residual alkali), dried at 60 °C, and ground in a Wiley mill to a particle size of approximately 40 mesh.
The nitrogen content (N%) was determined by CHONS organic elemental content analysis of powered CC by dry combustion based on the Dumas principle using a CHONS Elemental Analyzer (LECO® CHN628). The degree of substitution (DS) of cellulose carbamate was calculated from its nitrogen content as described elsewhere (Klemm et al. 1998), corresponding to the number of carbamate groups for each anhydroglucose unit (UAG). The intrinsic viscosity and viscosimetric average degree of polymerization (DPV) in cupriethylenediamine were determined following the method described by Marx-Figini (Marx-Figini 1987).
The CC solution was prepared by dissolving the ground CC in 8 wt/v% NaOH and 0.6 wt/v% ZnO solution at 0 °C under stirring at 800 rpm for 15 minutes. The CC solution was centrifuged at 9,000 rpm at 0 °C for 15 minutes. The undissolved material was neutralized with 3% H2SO4 and washed with abundant distilled water. Finally, it was filtered using a porous glass filter and dried at 105 °C for 24 hours to quantify the dry residue. The cellulose solution was stored at low temperatures in a closed container.
CC solubility was determined as the dissolved cellulose weight (the difference between the initial weight and the undissolved residue) concerning the initial weight of cellulose carbamate. The processing scheme and conditions used to obtain CC are shown in Fig. 3.
Regenerated cellulose beads preparation
Cellulose solutions were taken and extruded through a syringe pump with a flow rate of 30 mL/h. The cellulose beads were prepared by coagulating and regenerating in a mixture of 5% H2SO4 and 5% anhydrous Na2SO4. Coagulated beads were washed and stored in a refrigerator for further use. The average size and shape of the beads were assessed from images of beads dried on tissue paper (wet weight). Then they were dried in an oven at 105 ºC overnight (dry weight). The particle diameter was measured from optical microphotographs, calculating the mean, standard deviation, and coefficient of variation. Total porosity was calculated from the wet and dry weights of the beads, using 1.5 g/cm3 for bulk cellulose (Ettenauer et al. 2011).
Cellulose regenerated films preparation and characterization
The cellulose solution was spread over a glass plate in the form of a gel sheet with a thickness of approximately 0.04 mm, and then immediately immersed in a coagulating and regenerating bath (5% H2SO4 and 5% anhydrous Na2SO4) for 10 minutes. The films were washed and stored in a refrigerator for further use.
The tensile strength (σ) and elongation at break (ε) of the films conditioned at 23 ºC and 5% relative humidity were measured with a universal testing machine (INSTRON 3344) according to ISO 527-3 at a speed of 2 mm/min.
Results and discussion
Eucalyptus sawdust dissolving pulp production
The yields, kappa number, and viscosity obtained in the sequential treatment of the eucalyptus sawdust by soda pulping and OOp bleaching stage are presented in Table 1.
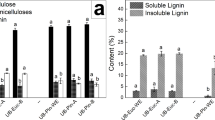
The alkaline extraction yield was 84.9%. Viscosities of Op + E and Z + E pulps were 487 and 559 mL/g, respectively. At low levels of lignin, the kappa number is not representative in eucalyptus pulps because of hexeneuronic acids. For example, the kappa number after the Op stage was 8.2. However, after an acid stage for hexeneuronic acids extraction, it was 2.3. So, in this case, insoluble lignin is a better indication of pulps quality. Insoluble lignin after the Op stage was 0.30%, and after the OpZ stage, it was 0.28%.
Both sequential treatments (soda pulping + TCF bleaching) applied to the eucalyptus sawdust followed by an alkaline extraction produced the required purity of cellulose to be considered a dissolving pulp (low hemicelluloses content (< 6%), and lignin and other impurities traces) (Sixta 2006). In the OOpE sequence, the OOp pulp contained 18.8% xylans, 0.3% residual lignin, and 1.1% ash, expressed as dry pulp base. The kappa number of the OOp pulp was 8.2, and the corrected Kappa number was 2.3 (on free hexenuronic acids pulp). The viscosity of the OOpE pulp was determined at 487 mL/g. In the OOpZE sequence, the Z bleaching stage (yield: 98.0%) allowed to extract 12.2% of residual lignin (kappa 7.9), and the viscosity of the OOpZE pulp was 559 mL/g. Both E stages allowed the hemicelluloses extraction with yields of 84.9 and 85.4% for EP-1 y EP-2, respectively.
Chemical characterization of the dissolving pulps
The chemical characterization of the commercial pulp (CP) and the eucalyptus sawdust pulps (EP-1 and EP-2) are shown in Table 2. The α-cellulose content represents the cellulose purity degree. The α−cellulose is the non-degraded fraction of the pulp with a high degree of polymerization, which is resistant to the sequential extraction by the 17.5 and 9.45 wt% NaOH solutions. The β-cellulose is the soluble fraction with a low degree of polymerization, which can be re-precipitated by acidification. Finally, γ-cellulose is the remaining material in the solution, consisting mainly of hemicelluloses. CP and EP pulps have high and similar cellulose content, high cellulose molecular weight (expressed as α-cellulose), and low β-cellulose and γ-cellulose contents.
Dissolving pulps are classified in different grades by their intrinsic viscosity. The intrinsic viscosity of CP and EP pulps is in the range of 250 and 600 mL/g, allowing its use for most applications (Manhaes and Ferreira Lima 2001). NaOH solutions extract low molecular weight carbohydrates, degraded cellulose, and hemicelluloses (S10 y S18). Solubility values of both CP and EP pulps are within the specified range for the different dissolving pulps grades. S18 also indicates the purity degree -the lower S18, the higher the pulp purity, while a higher S18 value is detrimental to derivatization yield and derivatives quality. Typical S18 ranges between 4 and 2% for cellophane, rayon, and other derivatives such as acetates. Table 2 shows low S10 and S18 values for both pulps, even though values are higher for EP-2 pulp due to its higher γ−cellulose content. Xylans content of EP-2 pulp was 4.4% odp.
Fock's reactivity is a parameter generally used to evaluate the reactivity of a dissolving pulp intended to obtain regenerated products. It is expressed as a percentage of regenerated cellulose yield (Fock 1959). Considering the pulps chemical characteristics in Table 2, EP-1 and CP showed similar reactivity (about 69% of Fock's reactivity). Solubility was higher in the commercial pulp, but EP-2 pulp presented the highest Fock's reactivity (83.9%), probably due to its higher γ−cellulose content (highly reactive hemicelluloses fraction). The solubility test in 9 wt% NaOH at − 5°C allows analyzing the dissolution capacity of a cellulose sample in mixtures of simple solvents at low temperatures (Rahkamo et al. 1998). The alkali solubility was significantly different for the experimental and commercial pulps. The CP pulp with intermediate content of α-cellulose showed a high solubility and lower average intrinsic viscosity.
This study shows that non-conventional sulfur-free processes can produce high-quality dissolving pulps from Eucalyptus sawdust. The dissolving pulps obtained from Eucalyptus globulus chips by kraft and soda pulping and O-CCE-Z-P bleaching (CCE: cold caustic extraction) achieved viscosity and S18 values comparable to those obtained in this work (bleached soda-AQ: 435 mL/g, 2.7%, bleached kraft: 450 mL/g, 2.3%, respectively) (Schild and Sixta 2011). Dissolving pulps from Eucalyptus bleached kraft pulp treated by CCE and dilute acid hydrolysis showed differences in alkali solubility, intrinsic viscosity, and Fock reactivity. It is attributable to inherent features of the native cellulose structure, cell wall arrangement, and molecular features of the wood components. The intrinsic viscosity of CCE treated kraft pulps was 110–450 mL/g, and, after acid hydrolysis, their Fock reactivity was 40.7–87.5%. E. globulus and the hybrid showed to be the most reactive pulps (Carrillo-Varela et al. 2019). After the alkali extraction, the enzymatically-treated (with xylanases and endoglucanases) Eucalyptus kraft pulp accomplished the cellulose reactivity and hemicellulose content requirements of commercial dissolving pulps, namely Fock reactivity 66.9%, viscosity 335 mL/g, and S18 4.5% (Köpcke et al. 2010). In brief, various Eucalyptus species and processes could lead to dissolving pulps with different features, which is of the highest importance for the final use of dissolving pulps.
Mechanical and enzymatic pretreatments
Cellulase treatment has been increasingly popular in the dissolving pulp industry in the last decade because it can adjust pulp viscosity and improve pulp reactivity in a greenway. (Gehmayr and Sixta 2012). A combination of mechanical refining and enzymatic pretreatments was optimized to increase pulp accessibility and reactivity and lower the intrinsic viscosity in previous work. It is necessary to achieve high levels of dissolution in NaOH-based solvents of commercial dissolving pulp (Olmos 2016). The solubility in alkali allows determining the soluble material in 9 wt% NaOH at − 5°C and evaluating the incidence of the pretreatments on the pulp accessibility (Rahkamo et al. 1998).
The mechanical and enzymatic pretreatments applied to the dissolving pulp improve the accessibility and, consequently, the reactivity of these pulps. Table 3 shows that the alkali solubility of the pretreated pulps considerably increased, maintaining the same trend as the pulps without pretreatments (Table 2). Additionally, the DPv was reduced to values lower than 700 (about 200–300 mL/g of intrinsic viscosity) to facilitate the solubility of cellulose in a simple solvent system (Lue and Zhang 2010). In general, pulps with lower DPv values present a higher cellulose dissolution (Isogai and Atalla 1998; Zhou et al. 2002; Wang et al. 2006, 2008; Wang 2008). The mechanical and enzymatic sequential pretreatment showed a favorable decrease in the degree of polymerization of the pulps, 50% for EP-1, 58% for EP-2, and 35% for CP (Table 3).
Cellulose dissolution
The supramolecular structure of cellulose causes it to be insoluble in most common organic solvents. The dissolution of cellulose involves the strong hydrogen bonding network disruption; both inter-and intramolecular and different dissolution alternatives exist. While direct cellulose dissolution using non-derivatizing solvents breaks the hydrogen bonds and avoids their re-conformation, derivatizing solvents modify the –OH groups avoiding the hydrogen bonding formation.
The conventional cellulose dissolution method involves the following stages: (i) cellulose activation, (ii) formation of intermediates, (iii) processing and shaping, and (iv) regeneration (Gericke et al. 2013; Hagman et al. 2017; Yang et al. 2017). The most commonly used solvents for the direct dissolution of cellulose are LiCl/DMAc, NMMO, NaOH, and BmimCl (Gindl and Keckes 2005; Shibata et al. 2013; Kosan et al. 2020). However, not all of these apply to industrial scales due to their low dissolution rates, toxicity, and recycling inability. The use of ionic liquids and deep eutectic solvents are also promising techniques, currently remaining at laboratory or pilot scales (Mendes et al. 2021).
The viscose process is the most used to dissolve and mold cellulose. In this process, the cellulosic pulp is treated with CS2 to obtain cellulose xanthate - a metastable intermediate - solubilized in dilute aqueous alkali to form the viscose solution. For the molded products, the substituents are removed from the viscose solution, and then it is regenerated into highly pure cellulose. This process is highly efficient and fully known but poses some environmental concerns. The use of CS2 causes sulfur products to be released during the cellulose regeneration, creating contamination at both atmospheric and aqueous levels, which are very expensive and difficult to remove (Vo et al. 2010; Chen et al. 2016; Arce et al. 2020). These economic and environmental reasons have driven the manufacturers of regenerated cellulose products to continuously search for alternative manufacturing practices more environmentally friendly processes (Liu et al. 2016).
Direct dissolution of cellulose (DD)
NaOH and urea mixtures at low temperatures have been heavily investigated in the last years, showing improved performance than in the case of dissolving cellulose using pure NaOH. This process causes the fast dissolution of native cellulose with the additional advantage of alkali recovery, avoiding contaminants generation (Chen et al. 2016; Liu et al. 2016). Several reasons complicate the dissolution of cellulose in NaOH-based solvents. One reason is the low maximum concentration of cellulose possible to dissolve in any NaOH-based solvent, with or without additives. Even if its theoretical maximum is around 8 wt%, it is about 5 wt% because of the quick gelation of "concentrated" solutions. In addition to the above, high molecular weight cellulose is difficult to dissolve (Budtova and Navard 2016).
NaOH/urea as a direct solvent effectively dissolved a high cellulose amount from all dissolving pulps (> 70%). EP-1 and EP-2 reached a solubility of 74 and 75% for EP-1 and EP-2, respectively, and 80% for CP (Table 4). The pretreated commercial pulp achieved the highest solubility with an intermediate viscosity value, as shown in Table 3 (consider that the intrinsic viscosity is an average molar mass distribution of the dissolved polysaccharides in the solvent). As mentioned, CP presented the highest soluble material amount in 9 wt% NaOH at − 5 °C, which shows high accessibility of CP attributable to the production process (Rahkamo et al. 1998). The molar mass distribution curves obtained by size-exclusion chromatography (SEC) before and after the combined treatment could clarify EP-1 and EP-2 behavior in the direct dissolution of cellulose.
Production of cellulose carbamate (CC) and its dissolution
Obtaining regenerated cellulose through the carbamate process involves several steps: (i) cellulose and urea react at high temperatures to form cellulose carbamate, (ii) cellulose carbamate is dissolved in NaOH solution, and (iii) the products are formed in a regenerating bath using diluted sulfuric acid and sodium sulfate. The preparation of cellulose carbamate solution is the critical stage of this process (Fu et al. 2014c; Xu et al. 2021; Kang et al. 2021) due to required conditions in the synthesis step (catalyst, solvents, reaction time, and temperature).
As well reported in the literature, the introduction of carbamate groups on the cellulose backbones increases its solubility (Fu et al. 2015). Previous reports revealed that a high degree of substitution (DS ≥ 0.25, N% ≥ 2%) is beneficial for the dissolution of CC in NaOH solution. However, Fu et al. found that CCs with N% as low as 0.5% can be dissolved in NaOH solution by adding a small amount of ZnO (0.4−2.0 wt%) (Fu et al. 2015). Cellulose carbamate (CC) was obtained from experimental and commercial dissolving pulps by activation and impregnation with NaOH/urea, based on previous studies (Lanieri 2017). The cellulose esterification reaction was carried out heating in a microwave at 160 W for 15 min. Table 4 shows that the commercial pulp has the highest N content (DS = 0.2), whereas the EP pulps have similar ones (DS = 0.17).
The CC dissolution was carried out in NaOH: ZnO aqueous solution (ratio 8:0.6 wt/v%) at a relatively low temperature (0 °C). The solubility of cellulose carbamates from EP-2 and CP were similar and higher (73 and 74%, respectively) than that of EP-1 (63%). However, the low levels of solubility achieved lead to regenerated products with low cellulose content. An optimization of solubility and CC dissolution temperature is planned for future work.
Regenerated cellulose products
Regenerated products were prepared by direct dissolution solution (DD) with NaOH/Urea and cellulose carbamate solution (CC). The products are formed in a regenerating bath using diluted sulfuric acid and sodium sulfate. Cellulose average DP value directly impacts the final regenerated cellulose product's mechanical properties. Then, the cellulose derivative intrinsic viscosity is a critical parameter of this process. According to the literature, lower DP values produce a higher dissolution rate of cellulose (Isogai and Atalla 1998; Zhou et al. 2002; Wang 2008).
The carbamate method is comparable to the viscose process because it has similar preparation steps and has the advantage of being a more environmentally friendly alternative (Fink et al. 2014). Likewise, the viscose process requires intrinsic viscosity values between 200–300 mL/g. Considering these characteristics, Table 4 shows that the intrinsic viscosity values of the carbamates prepared are within this range. Another study reported intrinsic viscosity values of CC between 100–150 mL/g with 1–2 N% (DS = 0.11–0.25) to produce regenerated cellulose products (Fu et al. 2014a, b). On the other hand, the intrinsic viscosity values of the pretreated pulps (Table 3) are also in the range of the viscose process taken as reference.
Regenerated cellulose beads
Table 5 shows the average diameters and porosity of the beads obtained (Fig. 4) by the drip technique (4.0% cellulose solution). CC bead diameters were slightly higher (2.53–2.78 mm) than DD beads (2.47–2.73 mm). Both methods produced CP beads with similar diameters and porosity. However, the porosity and diameters of EP-1 and EP-2 beads varied depending on the dissolution method. CC beads from CP, EP-1, and EP-2 presented similar porosity.
The coefficients of variation for the diameters of the beads were below 10%, indicating that the diameters distribution of the cellulose beads was homogeneous in both cases.
Regenerated cellulose films
The aspect feature of regenerated cellulose films from the cellulose carbamate method (CC) and direct dissolution method (DD) is shown in Fig. 5.
The stress (σ) - strain (ε) curves of the regenerated cellulose films from direct dissolution and cellulose carbamate dissolution from experimental and commercial pulps conditioned at 23 ºC and 5% relative humidity are shown in Fig. 6.
The different dissolution methods have a clear impact on the mechanical performance of the films, as observed in the stress-strain curves (Fig. 6). The tensile behavior of regenerated cellulose films obtained from EP-1 and CP pulps by DD was similar in the elastic region, with maximums of 35.7 and 48.5 MPa, respectively (Fig. 6a). However, CP pulp showed higher strength and ductility. On the other hand, regenerated cellulose films from EP-2 showed lower tensile strength with a maximum of 21.2 MPa and elongation capacity similar to the commercial pulp. This difference could be because the EP-2 film showed the lowest intrinsic viscosity of the series (Table 6).
Stress-strain curves of the regenerated cellulose films obtained from the eucalyptus sawdust (EP-1 and EP-2) and the commercial (CP) dissolved pulps by a direct dissolution method and b cellulose carbamate method (EP-1: eucalyptus sawdust soda pulps + OOp + E, EP-2: eucalyptus sawdust soda pulps + OOpZ + E)
Films produced using the CC solution from EP-2, and CP pulps, showed similar tensile strength, reaching maximums values of 41.3 and 40.4 MPa, respectively. On the other hand, the maximum tension value of the film prepared with EP-1 was 28.2 MPa (Fig. 6b). The elongation capacity was approximately 10% higher for EP-2 (2.5%) and CP (2.6%) compared to EP-1 (2.3%). The lower tension of the EP-1 film could be because the carbamate solution presented a low degree of substitution, which led to a lower percentage of dissolution (Table 4). On the other hand, the lower elongation observed could be attributed to the higher intrinsic viscosity of the series (Table 6).
The properties of the regenerated products are related to the cellulose solubility and DP of the sample. The films from CP by DD, with the highest solubility and intermediate DP, presented the highest tensile strength. Intrinsic viscosity and DP determined by viscometry are average values of the dissolved cellulose sample.
On the other hand, CC samples reached relatively lower solubility values under the conditions of this study. The results obtained in this study indicated that low freezing temperatures are vital to achieving optimal CC dissolution. Fu et al. showed that adding a small amount of ZnO (0.4−2.0 wt%) significantly improves the solubility of CC in the NaOH solution (Fu et al. 2014c). Furthermore, the solubility of CC rapidly increased as the temperature decreased from − 5 to − 23 °C.
In another study, Fu et al. analyzed the structure and properties of regenerated cellulose membranes produced from aqueous CC solution in NaOH/ZnO by coagulation in dilute sulfuric acid (Fu et al. 2014a). CC was obtained from cotton linters dissolving pulps with the content of α-cellulose of 93% by impregnation in urea solution and heating with microwaves at 425 W for 15 min. They obtained two cellulose carbamate samples with different N content (1.22 and 1.04 N%) and DP (540 and 400). The membranes coagulated in a 3 wt% H2SO4 solution at 10 °C for 10–15 min exhibited good tensile strength and elongation at break (120 MPa and 15% respectively). Fu et al. showed that both the coagulant concentration and the temperature influence their strength properties (Fu et al. 2014a).
Overall, the eucalyptus sawdust bleached dissolving pulps obtained by soda pulping and two different short TCF sequences followed by cold soda extraction steps showed the desired purity, a-cellulose, intrinsic viscosity, reactivity, and solubility of dissolving pulps to obtain regenerated cellulose products. However, the dissolution conditions in the mixing stage of simple solvents (NaOH/urea with or without ZnO addition) need optimization. Besides, the derivatization stage to cellulose carbamate with subsequent dissolution in NaOH/ZnO solution requires further study. To highlight is that dissolving pulp production (EP-2) bleached by a shorter TCF sequence (OOp) could reduce investment and production costs.
Conclusions
This study showed the feasibility of using sawdust as a source of high-quality dissolving pulps as evidenced by the experimental values of purity, α-cellulose, intrinsic viscosity, reactivity, and solubility. Eucalyptus sawdust bleached dissolving pulps (EP-2) obtained by soda pulping and two different short TCF sequences (OOp) followed by a cold soda extraction produces dissolving pulps and regenerated products by cellulose carbamate dissolution with quality comparable to a commercial one.
Both experimental dissolving pulps presented high and similar cellulose content and high molecular weights compared to CP. S10 and S18 values of CP and EP pulps were within the range specified for the different grades of dissolving pulps. Both pulps showed very similar reactivity, being slightly higher in CP. The sequential mechanical and enzymatic pretreatment applied to the pulps favored the decrease of the degree of polymerization.
The direct dissolution method produced a high dissolved cellulose amount from all dissolving pulps studied. The solubility of cellulose carbamate from experimental and commercial pulps was slightly lower than in the direct dissolution method. The solubility of cellulose carbamate from EP-2 was similar to commercial dissolving pulp, whereas EP-1 was lower than both pulps (almost 10%).
The beads obtained with the commercial pulp had higher average diameters and total porosity than those obtained from the sawdust pulp. The distribution of diameters of the cellulose beads was homogeneous in both cases.
The films obtained by both dissolution methods showed different stress-strain curves.
This study showed that dissolving pulps and regenerated cellulose products (beads and films) can be produced from eucalyptus sawdust by non-conventional processes. The OOpE sequence could be more economical and straightforward for dissolving pulp production than the OOpZE sequence by the cellulose carbamate method. However, if the objective is obtaining regenerated cellulose films from experimental dissolving pulps with high mechanical properties, the solvent, coagulation, and regeneration conditions need optimization.
References
Abdou Alio M, Marcati A, Pons A, Vial C (2021) Modeling and simulation of a sawdust mixture-based integrated biorefinery plant producing bioethanol. Bioresour Technol 325:124650. https://doi.org/10.1016/j.biortech.2020.124650
Arce C, Llano T, García P, Coz A (2020) Technical and environmental improvement of the bleaching sequence of dissolving pulp for fibre production. Cellulose 27:4079–4090. https://doi.org/10.1007/s10570-020-03065-1
Budtova T, Navard P (2016) Cellulose in NaOH–water based solvents: a review. Cellulose 23:5–55. https://doi.org/10.1007/s10570-015-0779-8
Carrillo-Varela I, Retamal R, Pereira M, Mendonça RT (2019) Structure and reactivity of cellulose from bleached kraft pulps of different Eucalyptus species upgraded to dissolving pulp. Cellulose 26:5731–5744. https://doi.org/10.1007/s10570-019-02491-0
Chen C, Duan C, Li J et al (2016) Cellulose (Dissolving Pulp) manufacturing processes and properties: a mini-review. BioResources 11:5553–5564
Chen Z, Zhang H, He Z, Zhang L (2019) Current and future markets of dissolving pulp in China and other countries. BioResources 14:7627–7629
Clauser NM, Gutiérrez S, Area MC et al (2018) Alternatives of small-scale biorefineries for the integrated production of xylitol from sugarcane bagasse. J Renew Mater. https://doi.org/10.7569/JRM.2017.634145
Ettenauer M, Loth F, Thümmler K et al (2011) Characterization and functionalization of cellulose microbeads for extracorporeal blood purification. Cellulose 18:1257–1263. https://doi.org/10.1007/s10570-011-9567-2
Fink H-P, Ganster J, Lehmann A (2014) Progress in cellulose shaping: 20 years industrial case studies at Fraunhofer IAP. Cellulose 21:31–51. https://doi.org/10.1007/s10570-013-0137-7
Fock W (1959) Eine modifizierte method zur bestimmung der reaktivitat von zellstoffen fur viskosherstellung. Das Pap 13:92–95
Fu F, Guo Y, Wang Y et al (2014a) Structure and properties of the regenerated cellulose membranes prepared from cellulose carbamate in NaOH/ZnO aqueous solution. Cellulose 21:2819–2830. https://doi.org/10.1007/s10570-014-0297-0
Fu F, Yang Q, Zhou J et al (2014) Structure and properties of regenerated cellulose filaments prepared from cellulose carbamate–NaOH/ZnO aqueous solution. ACS Sustain Chem Eng 2:2604–2612. https://doi.org/10.1021/sc500559g
Fu F, Zhou J, Zhou X et al (2014) Green method for production of cellulose multifilament from cellulose carbamate on a pilot scale. ACS Sustain Chem Eng 2:2363–2370. https://doi.org/10.1021/sc5003787
Fu F, Xu M, Wang H et al (2015) Improved synthesis of cellulose carbamates with minimum urea based on an easy scale-up method. ACS Sustain Chem Eng 3:1510–1517. https://doi.org/10.1021/acssuschemeng.5b00219
Gavrilescu D (2013) Pulping fundamentals and processing. In: Popa VI (ed) Pulp production and processing: from papermaking to high-tech products. Smithers Rapra Technology Ltd, pp 35–69
Gehmayr V, Sixta H (2012) Pulp properties and their influence on enzymatic degradability. Biomacromolecules 13:645–651. https://doi.org/10.1021/bm201784u
Gemeiner P, Beneš MJ, Štamberg J (1989) Bead cellulose and its use in biochemistry and biotechnology. Chem Pap 46:805–848
Gericke M, Trygg J, Fardim P (2013) Functional cellulose beads: preparation, characterization, and applications. Chem Rev 113:4812–4836. https://doi.org/10.1021/cr300242j
Ghaderi M, Mousavi M, Yousefi H, Labbafi M (2014) All-cellulose nanocomposite film made from bagasse cellulose nanofibers for food packaging application. Carbohydr Polym 104:59–65. https://doi.org/10.1016/j.carbpol.2014.01.013
Gindl W, Keckes J (2005) All-cellulose nanocomposite. Polym (Guildf) 46:10221–10225. https://doi.org/10.1016/j.polymer.2005.08.040
Guigou M, Cabrera MN, Vique M et al (2019) Combined pretreatments of eucalyptus sawdust for ethanol production within a biorefinery approach. Biomass Convers Biorefinery 9:293–304. https://doi.org/10.1007/s13399-018-0353-3
Hagman J, Gentile L, Jessen CM et al (2017) On the dissolution state of cellulose in cold alkali solutions. Cellulose 24:2003–2015. https://doi.org/10.1007/s10570-017-1272-3
Huang K, Wang Y (2022) Recent applications of regenerated cellulose films and hydrogels in food packaging. Curr Opin Food Sci 43:7–17. https://doi.org/10.1016/j.cofs.2021.09.003
Isogai A, Atalla RH (1998) Dissolution of cellulose in aqueous NaOH solutions. Cellulose 5:309–319. https://doi.org/10.1023/A:1009272632367
Kamusoko R, Jingura RM, Parawira W, Chikwambi Z (2021) Strategies for valorization of crop residues into biofuels and other value-added products. Biofuels Bioprod Biorefining. https://doi.org/10.1002/bbb.2282
Kang Y, Wang F, Zhang Z, Zhou J (2021) Dissolution and interaction of cellulose carbamate in NaOH/ZnO aqueous solutions. Polym (Basel) 13:1092. https://doi.org/10.3390/polym13071092
Köpcke V, Ibarra D, Larsson PT, Ek M (2010) Optimization of treatments for the conversion of eucalyptus kraft pulp to dissolving pulp. Polym from Renew Resour 1:17–34. https://doi.org/10.1177/204124791000100102
Kosan B, Römhild K, Meister F et al (2020) Enzymatic pulp modification: an excellent way to expand the raw material base for. Lyocell applications? Cellulose 27:6577–6590. https://doi.org/10.1007/s10570-020-03243-1
Kumar H, Christopher LP (2017) Recent trends and developments in dissolving pulp production and application. Cellulose 24:2347–2365. https://doi.org/10.1007/s10570-017-1285-y
Liu Y, Shi L, Cheng D, He Z (2016) Dissolving pulp market and technologies: Chinese prospective - a mini-review. BioResources 11:2016
Marx-Figini M (1987) The acid-catalized degradation of cellulose linters in distinct ranges of degree of polymerization. J Appl Polym Sci 33:2097–2105
Mendes ISF, Prates A, Evtuguin DV (2021) Production of rayon fibres from cellulosic pulps: State of the art and current developments. Carbohydr Polym 273:118466. https://doi.org/10.1016/j.carbpol.2021.118466
Peška J, Štamberg J, Hradil J, Ilavský M (1976) Cellulose in bead form. J Chromatogr A 125:455–469. https://doi.org/10.1016/S0021-9673(00)85709-X
Pinnow M, Fink H-P, Fanter C, Kunze J (2008) Characterization of highly porous materials from cellulose carbamate. Macromol Symp 262:129–139. https://doi.org/10.1002/masy.200850213
Rahkamo L, Siika-aho M, Viikari L et al (1998) Effects of cellulases and hemicellulase on the alkaline solubility of dissolving pulps. Holzforschung 52:630–634
Rangel J, Hornus M, Felissia FE, Area MC (2016) Hydrothermal treatment of eucalyptus sawdust for a forest biorefinery. Cellul Chem Technol 50(5–6):521–528
Sayyed AJ, Deshmukh NA, Pinjari DV (2019) A critical review of manufacturing processes used in regenerated cellulosic fibres: viscose, cellulose acetate, cuprammonium, LiCl/DMAc, ionic liquids, and NMMO based lyocell. Cellulose 26:2913–2940. https://doi.org/10.1007/s10570-019-02318-y
Schild G, Sixta H (2011) Sulfur-free dissolving pulps and their application for viscose and lyocell. Cellulose 18:1113–1128. https://doi.org/10.1007/s10570-011-9532-0
Shibata M, Teramoto N, Nakamura T, Saitoh Y (2013) All-cellulose and all-wood composites by partial dissolution of cotton fabric and wood in ionic liquid. Carbohydr Polym 98:1532–1539. https://doi.org/10.1016/j.carbpol.2013.07.062
Singh P, Duarte H, Alves L et al (2015) From cellulose dissolution and regeneration to added value applications—synergism between molecular understanding and material development. In: Poletto M, Ornaghi H Jr et al (eds) Cellulose-fundamental aspects and current trends. InTech, Rijeka, pp 1–44
Sixta H, Iakovlev M, Testova L et al (2013) Novel concepts of dissolving pulp production. Cellulose 20:1547–1561. https://doi.org/10.1007/s10570-013-9943-1
Stafford W, De Lange W, Nahman A et al (2020) Forestry biorefineries. Renew Energy 154:461–475. https://doi.org/10.1016/j.renene.2020.02.002
Vo LTT, Široká B, Manian AP, Bechtold T (2010) Functionalisation of cellulosic substrates by a facile solventless method of introducing carbamate groups. Carbohydr Polym 82:1191–1197. https://doi.org/10.1016/j.carbpol.2010.06.052
Wang L, Zhang Y, Gao P et al (2006) Changes in the structural properties and rate of hydrolysis of cotton fibers during extended enzymatic hydrolysis. Biotechnol Bioeng 93:443–456. https://doi.org/10.1002/bit.20730
Wang Y, Zhao Y, Deng Y (2008) Effect of enzymatic treatment on cotton fiber dissolution in NaOH/urea solution at cold temperature. Carbohydr Polym 72:178–184. https://doi.org/10.1016/j.carbpol.2007.08.003
Xu M, Li T, Zhang S et al (2021) Preparation and characterization of cellulose carbamate membrane with high strength and transparency. J Appl Polym Sci 138:50068. https://doi.org/10.1002/app.50068
Yang Y, Zhang Y, Dawelbeit A et al (2017) Structure and properties of regenerated cellulose fibers from aqueous NaOH/thiourea/urea solution. Cellulose 24:4123–4137. https://doi.org/10.1007/s10570-017-1418-3
Yousefi H, Nishino T, Faezipour M et al (2011) Direct fabrication of all-cellulose nanocomposite from cellulose microfibers using ionic liquid-based nanowelding. Biomacromolecules 12:4080–4085. https://doi.org/10.1021/bm201147a
Zhang S, Yu C, Liu N et al (2019) Preparation of transparent anti-pollution cellulose carbamate regenerated cellulose membrane with high separation ability. Int J Biol Macromol 139:332–341. https://doi.org/10.1016/j.ijbiomac.2019.07.146
Zhou J, Zhang L, Shu H, Chen F (2002) Regenerated cellulose films from NaOH/urea aqueous solution by coagulating with sulfuric acid. J Macromol Sci Part B 41:1–15. https://doi.org/10.1081/MB-120002342
ANDRITZ (2021) Development deal for commercializing textile fiber regeneration technology. In: https://www.andritz.com/spectrum-en/development-deal-for-commercializing-textile-fiber-regeneration-technology
Klemm D, Philipp B, Heinze T et al (1998) Appendix to volume 2: experimental procedures for the functionalization of cellulose. In: Klemm D, Philipp B, Heinze T (eds) Comprehensive cellulose chemistry. Wiley-VCH Verlag GmbH, pp 327–375
Lanieri DB (2017) Doctoral thesis dissertation: Obtención y caracterización de carbamato de celulosa como alternativa de disolución al proceso de viscosa. Facultad de Ingeniería Química, Universidad Nacional del Litoral, http://hdl.handle.net/11185/943
Lue A, Zhang L (2010) Advances in aqueous cellulose solvents. pp 67–89
Manhaes GF, Ferreira Lima A (2001) Solucell: a special dissolving pulp from eucalyptus. In: 7th Brazilian Symposium on the Chemistry of lignins and other wood components
Olmos GV (2016) Doctoral Thesis Dissertation: Alternativas de disolución de celulosa para la obtención de productos regenerados. Facultad de Ingeniería Química, Universidad Nacional del Litoral. http://hdl.handle.net/11185/945
Rana S, Pichandi S, Parveen S, Fangueiro R (2014) Regenerated cellulosic fibers and their implications on sustainability. pp 239–276
Simon M, Barrufaldi S, Clauser N et al (2017) Residuos de Industrialización Primaria de la Madera como materia prima para biorrefinerías. Estudio de localización en Misiones (Argentina). Rev. El Pap
Sixta H (2006) Handbook of Pulp Volumen I. Weinheim
Wang Y (2008) Cellulose fiber dissolution in sodium hydroxide solution at low temperature: dissolution kinetics and solubility improvement, PhD Thesis., Georgia Institute of Technology, Atlanta, US
Acknowledgments
The authors acknowledge financial support from Projects of Scientific and Technological Research (PICT-Raíces) of the AGENCIA-MINCYT. The USDA National Institute of Food and Agriculture, Hatch program (ALA013-17003) and McIntire-Stennis program (1022526) financial support to complete this work is much appreciated.
Funding
This work was supported by Projects of Scientific and Technological Research (PICT-Raíces, 2015-N° 2843) of the AGENCIA-MINCYT and the USDA National Institute of Food and Agriculture, Hatch program (ALA013-17003) and McIntire-Stennis program (1022526).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vallejos, M.E., Olmos, G.V., Taleb, M.C. et al. Dissolving pulp from eucalyptus sawdust for regenerated cellulose products. Cellulose 29, 4645–4659 (2022). https://doi.org/10.1007/s10570-022-04581-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-022-04581-y