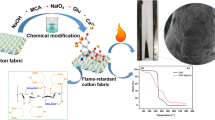
Abstract
Eco-friendly biomass-based coating was designed to endow cotton fabrics with durable flame retardancy through a dip-pad-dry-cure process. The coating involved phosphorylated chitosan (PCS) as intumescent flame retardant that is fixed onto the fiber surface by the co-action of electrostatic interaction and chemical grafting. Consequently, the obtained fabrics exhibit good flame retardancy and improved durability. The resultant cotton fabrics treated by 7.5 wt% of PCS solution show limiting oxygen index (LOI) of 25.7% and self-extinguished immediately after removing the igniter in the vertical flame test. Additionally, the total heat release value and heat release rate are significantly decreased by 59.4% and 88.2%, respectively, in the cone calorimeter test compared with pure cotton. A combined condensed-phase flame-retardant mechanism by forming a phosphorus-rich intumescent and gas-phase mechanism by diluting effect of the non-flammable volatiles is proposed. Moreover, enhanced washing durability is achieved even after 10 detergent laundering cycles with a LOI value of 23% due to the partial chemical grafting of PCS on cotton fibers. Meanwhile, the tensile property remained almost unchanged. Herein, this work provides a promising approach for fabricating durable flame-retardant cotton fabrics with satisfactory tensile performance and for applying to other fabric substrates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cotton fiber, a kind of natural fiber, attracts great attention increasingly recently in textile markets due to the increasing concerns regarding the effect of non-biodegradable synthetic polymer fibers on the environment, especially for these from nonrenewable sources. However, cotton fiber is cellulose-rich and flammable in nature with limiting oxygen index (LOI) of 18–19%, and it burns quickly accompanied by smoldering after being ignited (Lu et al. 2018; Rosace et al. 2018). Statistically, most of the fire accidents occurring in houses involve various cotton materials (Gao et al. 2015; Rehan et al. 2018; Zhang et al. 2019). Consequently, it is urgent to impart high flame retardancy to cotton fibers both in scientific research significance and application value.
Unlike synthetic fibers that have enhanced fire resistance by the inclusion of flame retardants into their matrices (Rehman et al. 2021; Shi et al. 2022a, b), surface treatment is the most effective and appropriate method for cotton fabrics. Some commercial treatment methods such as Pyrovatex® and Proban® are phosphorus-containing and endow fabrics with excellent fire-resistance, laundry-resistant and soft handle (Abdelrahman and Khattab 2019; Lewis et al. 2020). One of the major disadvantages is the release of formaldehyde in the process of using, which would bring severe physical discomfort, particularly for eyes and respiratory system (Zhang et al. 2021). Therefore, several studies have been devoted to exploit formaldehyde-free and flame-retardant cotton fabric finishing strategies such as layer-by-layer (LBL) assembly, sol–gel reaction, grafting and so on (Liu et al. 2017a, b; Chen et al. 2020b, a; Wang et al. 2020a, b; Xu et al. 2020; Zhao et al. 2017). LBL assembly technique is a sort of versatile and tunable strategy to construct flame-retardant coating on diverse substrates. However, the assembly process is always time-consuming and needs several bilayers to achieve satisfying flame retardancy (Lazar et al. 2018; Xue et al. 2020; Yang et al. 2013). However, the washing durability of LBL coating is always not mentioned. Sol–gel technique is regarded as a facile method to reduce flammability with only two steps, while changed morphology and reduced mechanical strength for cotton fabrics would occur (Yuan et al. 2017). There are different methods to graft flame-retardant groups into cotton fibers such as plasma or photo induced, chemical grafting, ultrasonic radiation to improve flame retardancy and durability. However, structural damage would inevitably occur due to the harsh grafting process (Liu et al., 2021a, b, c; Liang et al. 2017; Lin et al. 2019). Therefore, it is necessary to develop some eco-friendly and high-efficiency methods to fabricate durable flame-retardant cotton fabrics without damaging the mechanical properties and fibrous structure.
Environmental concerns encourage the development of high-efficiency and renewable flame retardants. As a consequence, biomass derived flame retardants draw great interest in improving the flame retardancy of cotton fabrics (Costes et al. 2017; Fang et al. 2019; Li et al. 2019a, b; Gao et al. 2021). Zhang et al. (2021) developed an eco-friendly biomass-based coating containing biomass tannin (TA), tartar emetic (TE) and Fe2+ on cotton fabrics. The fabrics showed great flame retardancy with excellent laundering and friction durability. Even after 100 laundering or friction cycles, the LOI value was hardly changed. Chitosan (CS) is a kind of biomass and possesses abundant deacetylated-glucosamine, serving as promising green charring agent and foaming agent (Leistner et al. 2015; Shi et al. 2018a, b). Nevertheless, it is reported that self-extinguishing cannot be achieved by employing chitosan alone. It is always applied in combination with acid source of phosphorus-containing such as ammonium polyphosphate and phytic acid to form intumescent flame retardants (IFRs) (He et al., 2022; Shi et al. 2018a, b; Shi et al. 2021a, b; Liu et al., 2021a, b, c). More specifically, the cellulose units in cotton fabrics can serve as carbon source. In this case, IFRs can generate some reactive phosphorus-containing acid directly in situ at high temperature, which is able to dehydrate and generate water vapor, favoring the formation of thermally stable char structure and enhancing the fire-resistance of cotton fabrics (Shi et al. 2018a, b). Moreover, chitosan can offer cotton fabric the additional benefit of antibacterial action. That action occurs by CS destroying the cell wall and infiltrating the cell to disturb the normal physiological activity of the bacterium with the positive charge of -NH3+ in CS (Li et al. 2020).
In this work, enlightened by the aforementioned, we synthesized a phosphorylated chitosan (PCS) by Mannich condensation as IFR to improve flame retardancy of cotton fabrics and functional PCS coating was constructed on the surface of fibers through dip-pad-dry-cure process. During the heating and curing process, the PCS flame retardant can construct a network within the fibers and even form covalent bonds with the hydroxyl groups of the cellulosic units. Therefore, it is expected that the obtained cotton fabrics would exhibit promising flame retardancy, washing fastness and mechanical strength. The morphologies and compositions of the flame-retardant cotton fabrics were characterized thoroughly. The flame retardancy, combustion behavior and flame-retardant mechanism were investigated in detail. Finally, the washing fastness and mechanical strength of the fabrics were also discussed.
Experimental
Materials
Chitosan (CS) is purchased from Damas-beta (99%, deacetylation 90%). Formaldehyde (37–40%), urea (99%) and phosphorous acid (99%) are provided by Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. Dicyandiamide (DCDA) is obtained from Yien Chemical Technology Co., Ltd Shanghai. All reagents are not purified before used. Deionized water is prepared by our laboratory. Pure cotton fabrics (100%; 110 g/m2) are purchased from Chaotianmen Market (Chongqing, China) and are washed by 2 wt% of sodium hydroxide solution at 100 °C for 3 h to remove the impurities and sizing agent and dried at 80 °C overnight in a vacuum oven.
Preparation of phosphorylated chitosan
The phosphorylated chitosan (denoted as PCS) was prepared through a Mannich condensation reaction according to our previous study (Shi et al. 2022a, b). As depicted in Fig. 1a. In brief, 10 g of chitosan was added into three-necked flask containing 200 mL deionized water and acetic acid was added dropwise until CS dissolved entirely and 4.6 g of phosphoric acid was added. Then 4.58 g of aqueous formaldehyde solution was added and followed by heating at 80 °C for 6 h under vigorous stirring. Afterwards, appropriate urea was added to the solution and heated to 110 °C for 2 h. Finally, the product was precipitated after cooling down to room temperature and washed by anhydrous ethanol. The yellowish product was dried at 80 °C for 12 h.
Preparation of flame-retardant cotton fabrics
The preparation of flame-retardant cotton fabrics is illustrated in Fig. 1b. In detail, 5 wt% dicyandiamide was added as catalyst in different concentrations (5 wt%, 7.5 wt%, 10 wt%) of PCS solution and stirred vigorously at 80 °C until dissolved completely. The cotton fabrics were soaked into the solution for 40 min followed by padding to achieve a wet pickup of 150%. Subsequently, the cotton fabrics were cured at 180 °C for 10 min and washed with deionized water to remove the excess PCS, and then dried at 80 °C overnight in a vacuum oven. The resultant flame-retardant cotton fabrics treated by 5 wt%, 7.5 wt% and 10 wt% of PCS solution were marked as FR1, FR2 and FR3, respectively. The untreated cotton fabric was marked as control.
The weight gain (WG) of treated cotton fabrics was calculated according to the following equation:
where \(WG{\text{\% }}\) represents the weight gain of treated cotton fabrics by PCS, \(W_{1}\) and \(W_{0}\) represent the weight of cotton fabrics before and after treatment with PCS, respectively.
Characterization
Fourier transform infrared spectroscopy (FTIR) spectra and attenuated total reflection Fourier infrared spectra (ATR-FTIR) spectra were recorded on Nicolet 380 FTIR instrument scanning from 4000 to 600 cm−1.
Scanning electron microscope (SEM) equipped with X-Ray spectrometry (EDX) was observed by Carl Zeiss EVO MA15. All the samples were cut and fixed with conductive adhesive and coated with gold dust.
X-ray photoelectron spectroscopy (XPS) was conducted by Kratas AXIS SUPRA fitted with an X-ray source (Al Kα with photon energies 1486.6 eV).
X-ray diffraction (XRD) data were obtained by using a TD-3500 device (Dandong Tongda science and Technology Co., Ltd) at the scanning rate of 4°/min with the step length of 0.02° in the range of 5–60°. The flame retardant was ground using agate and then tested. Cotton fabric was tested without any treatment. The XRD data were collected in reflection mode.
Thermogravimetric (TG) tests were performed on a TGA 550 instrument from 40 °C to 700 °C at a flow rate of 20 mL/min and a heating rate of 10 °C/min under nitrogen and air atmospheres.
Limiting Oxide Index (LOI) was determined with a HC-2C oxygen index apparatus (Nanjing Shangyuan Analytical Instruments Co., Ltd. China). The samples were tailored into 150 mm × 50 mm strips according to the ASTM D2863-2000 standard.
Vertical flame test (VFT) was conducted with a CZF-4 vertical flame test (Nanjing Shangyuan Analytical Instrument Co., Ltd). The samples were prepared into 300 mm × 80 mm according to the GB/T5455-2014 standard and placed in a vertical flame of 40 mm ± 2 mm for 12 s.
FTIR spectrometer connected with a TG analyzer (PerkinElmer Co., USA) was used for the thermogravimetry-Fourier transform infrared spectrometry (TG-FTIR) to analyze the gaseous components with a heating rate of 10 °C min−1 and a flow rate of 20 mL/min under nitrogen atmosphere from 40 to 700 °C, and the corresponding spectral range was from 4000 to 500 cm−1.
Cone Calorimeter test was conducted to assess the fire hazards of samples by Fire Testing Technology (UK) according to ISO5660-1 standard under a 25 kW/m2 heat flux with size of 100 mm × 100 mm. A wire grid was used to prevent expanding samples in the test.
The washing fastness of the flame-retardant cotton fabrics was tested according to ATCC Test Method 61–2006. The samples were repeatedly washed at 49 °C for 45 min in water and using 0.15% sodium dodecylbenzene sulfonate solution as the detergent. Each washing cycle was equivalent to five domestic laundering cycles.
The tensile property of cotton fabrics was conducted on the electronic universal testing machine (Shenzhen SUNS Technology Stock Co., Ltd.) according to GB/T 3923.1–2013. In the test, five replicates with a dimension of 200 mm × 50 mm were prepared. In the test, the drawing speed was 5 mm/min at a clamping distance of 100 mm.
Result and discussion
Characterization of PCS
The chemical structure of PCS was certified by FTIR and XPS as presented in Fig. 2. It is clearly observed that there are two characteristic peaks at 1618 cm−1 (asymmetric deformation) and 1513 cm−1 (symmetric deformation) for PCS compared with the spectrum of CS in Fig. 2a, which is caused by the deformation of amino modified by phosphorous acid and formaldehyde (Liu et al. 2017a, b). A weak absorption peak appears at 798 cm−1 and it is assigned to P-OH of PCS. Particularly, the amino group of CS is covered by the broad and strong peak of hydroxyl in the range from 3100 cm−1 to 3500 cm−1. To further demonstrate the chemical state and composition of PCS, XPS was carried out and the result was shown in Fig. 2b. In detail, PCS exhibits P-containing group in XPS and FTIR spectrum compared with that of CS due to reaction between P-OH of phosphorous acid and -NH2 of CS, so the high-resolution P 2p and N 1 s spectra are checked in Fig. 2c–d. The spectra of P 2p exhibit the fitted peaks at 132.91 eV (ascribed to P–C) and 133.67 eV (ascribed to P = O) (Shi et al. 2018a, b; Wang et al. 2021), which is agreement with the structure of PCS. The N 1 s could be deconvoluted to two peaks at 399.66 eV and 401.73 eV, which is attributed to N–C and typically protonated amine, respectively (Gou et al. 2021; Shi et al. 2020). Therefore, FTIR and XPS results confirmed that PCS is successfully prepared.
Characterization of the flame-retardant fabrics
SEM was utilized to distinguish the morphology of cotton fabrics as presented in Fig. 3. All fabrics have a typical morphology of natural fabrics and maintain threadlike cellulose. The control cotton fabrics (Fig. 3a) present a clean but a little rough surface with distinct fiber boundary. When it comes to 5 wt% of PCS treated fabrics, there is almost no visible difference from the control cotton, which is attributed to the padding technology with no damage to the morphology of fabrics. With the increase of the concentration of PCS, the boundary of fibers is increasingly indistinct and the surface is increasingly smooth as shown in Fig. 3b–d, indicating that most of the PCS is squeezed into the interface of cellulose and a continuous thin film is built on the surface of cotton fabrics. Furthermore, EDX mapping was carried out to analyze the element composition and distribution of the flame-retardant cotton fabrics as exhibited in Fig. 3e. Obviously, the specific elements such as phosphorus and nitrogen derive from PCS are detected and these elements are well-distributed in the thin film of the flame-retardant fabrics, further confirming that the functional PCS coating containing phosphorus and nitrogen is constructed on the surface of the fabrics.
In order to demonstrate the chemical composition and state of PCS on flame-retardant cotton, ATR-FTIR and XPS spectra were obtained as shown in Fig. 4a–b. The typical functional groups of cotton fabrics appear around 3332 cm−1, 3272 cm−1, 2894 cm−1, 1053 cm−1 and 1427 cm−1 are assigned to the stretching vibration of C–OH, C–H, C–O–C and bending vibration of C–H, respectively. Notably, the absorption intensities of these characteristic peaks for cotton fabrics are all decreased. Meanwhile, the two characteristic absorption peaks at 1650 cm−1 and 1550 cm−1 are observed due to the deformation to low frequency caused by the introduction of PCS as shown in Fig. 4a. Some new absorption peaks at 1241 cm−1, 798 cm−1 and 764 cm−1 are also detected, which are attributed to P = O, P–OH and P–C, respectively. Furthermore, the absolute values of the absorbance for these new peaks are increased with the increasing concentration of PCS, indicating the uniform growth and successful formation of homogeneous PCS functional layer on the surface of cotton fabrics. Analogously, P and N-containing groups are detected in FR3 compared with control fabric as seen in Fig. 4b, indicating the coating containing P and N is successfully constructed on the surface of cotton fabrics. Additionally, the flame-retardant fabric is fabricated by simulating the industrial technology of padding and baking to impregnate the fibers with flame retardant. Thus, XRD was utilized to analyze whether damage occurred to the structure of the treated fabrics. The structures of PCS and CS were firstly detected as seen in Fig. 4c. Broad and weak intensity diffraction peaks are observed in XRD pattern for both CS and PCS. The diffraction peaks center at 12° and 20° are assigned to the chain segment and hydrated conformation of CS (Affes et al. 2022), respectively. When it comes to PCS, the diffraction peak centered at 20° is shifted to 22.5° due to the mutual interference of phosphorous and other elements. For cotton fabrics as seen in Fig. 4d, the characteristic peaks centered at 2θ ≈ 15.06°, 16.46° and 22.76° are detected in XRD patterns in both control and FR3, which are attributed to the (1–10), (110), (200) crystal planes of cellulose, respectively (Ling and Guo 2020). The observation indicates that the structure of cellulose is not destroyed during the squeeze process. Notably, the diffraction peak of PCS is not observed in the spectra XRD of FR3 due to the coverage by peak of cellulose. The results of FTIR, XPS and XRD intensively demonstrate that PCS is squeezed into the surface of cellulose successfully and a continuous functional film is coated on the surface of cotton fabrics.
Thermal stability
The thermal-oxidative stability of cottons was analyzed by TGA. The decomposed curves are displayed in Fig. 5, and corresponding data are displayed in Table S1. All cotton fabrics display a main decomposition process and the decomposition peaks are found around from 240 to 270 °C in both N2 and air atmospheres. The initial decomposition temperature (T5%, defined as temperature of 5% weight loss) and Tmax (the temperature where the first maximum weight loss occurred) of flame-retardant fabrics under N2 atmosphere are decreased as listed in Table S1. This phenomenon is caused by the preceding decomposition of PCS meanwhile phosphoric acid compounds are generated at a relative lower temperature to promote the dehydration and carbonization of cotton fabrics (Jian et al., 2019). The formed char layer acts as a barrier to prevent the matrix from degradation. As a consequence, the residue significantly increases from 3.1 wt% of control to 36.3 wt% of FR3. As seen in Fig. 5c–d under air atmosphere, though the Tmax of flame-retardant fabrics still slightly decrease compared with that of control, the T5% of FR3 is almost the same with that of control and even increased for FR1 and FR2. Notably, the control sample nearly decomposes completely at 324 °C with only 1.2 wt% of residue at 700 °C. The degradation rate of control fabric is obviously higher than that of the flame-retardant fabrics, suggesting that the PCS coating can improve the stability of cotton fabrics and lead to an increased residue at 700 °C from only 1.2 wt% of control to 6.9 wt% of FR3. TGA results indicate that the flame-retardant samples perform improved thermal oxidative stability and carbonization ability, which is beneficial to enhance the flame retardancy of cotton fabrics.
Flame retardancy and combustion behavior
Vertical flame test and limiting oxygen index are carried out to evaluate the resistance of fire spreading under high heat feedback and ignitability, respectively (Li et al. 2018). The digital photos were recorded during VFT as shown in Fig. 6 and the corresponding data are illustrated in Table 1. As seen in Fig. 6, the control sample burns out rapidly after igniting and burns completely with little residue and the LOI value is only 19.0%. The afterflame time and afterglow time are 6 s and 30 s, respectively. When it comes to flame-retardant fabrics, the flame spread slows down and the fire even self-extinguishes. In detail, the flame is obviously suppressed for FR1 and slowly reaches the top of sample with no afterglow, exhibiting the LOI value of 24.6% and remaining a complete and soft char frame. Furthermore, FR2 and FR3 are difficult to catch the fire and easily self-extinguish immediately when the igniter is removed. Both afterflame and afterglow phenomenon are not observed in the whole test. The damage length dramatically decreases with 83 mm and 73 mm as shown in Fig. S1. And the corresponding LOI value is 25.7% and 28.3% for FR2 and FR3, respectively. Apparently, the cotton fabric is transformed to flame-retardant material from flammable material thanks to the PCS layer on its surface. It is suggested that the coating containing P and N is highly effective in suppressing flame spread and improving flame retardancy.
To fully assess the fire-retardnacy and fire safety of flame-retardant cotton fabrics in fire scenario, the cone calorimeter test (CCT) was employed and the results are summarized in Fig. 7 and Table 2. The peak value of heat release rate (PHRR) for FR2 reduce sharply by 88.2% in contrast with that of the control sample. The value of total heat release (THR) decreases by 59.4% from 3.4 MJ/m2 of control to 1.3 MJ/m2 of FR2. Particularly, FR3 cannot be ignited at the heat flux of 25 kW/m2, exhibiting highly flame retardancy and fire safety. Furthermore, the reduction of fire growth rate index (FIGRA is defined as PHRR divided by the time to PHRR) from 1.8 to 0.1 kW/ (m2 s) suggests that the flame propagation is inhibited. That means there is enough time to evacuate people or to extinguish the fire in an event. In addition, the declined weight loss rate of FR2 is detected in comparison with the control cotton as listed in Fig. 7c. The residue of FR2 is significantly increased to 24.6 wt% from 6.0 wt% of the control. The control almost burns out after CCT, while FR2 maintains the original shape and cellulose structure as presented in Fig. 7d, implying the possible condensed-phase flame-retardant mechanism. The aforementioned results of VFT, LOI and CCT all demonstrate that treated cotton fabrics exhibit high efficiency flame-retardant property.
Flame-retardant mechanism
In order to further clarify the flame-retardant mechanism, SEM was applied to observe the morphology and the corresponding EDX was used to analyze the element composition and distribution of residue after burning as shown in Fig. 8. The residue of control is fragile and disorderly after burning, displaying damaged morphology of cotton fabrics as shown in Fig. 8a. However, the residues of coated cotton fabrics are much more continuous and almost have no broken fibers as shown in Fig. 8b–d, which maintain the structure of cotton fabrics and integrity of cellulose, especially for FR2 and FR3. As shown in the EDX images in Fig. 8e, phosphorus element is well-distributed and enriched in char residue, signifying the condensed-phase flame-retardant mechanism of PCS. Notably, there are some small particles and small bubbles appearing on the fibers, which indicates the presence of intumescent char layer. It is known that chitosan is a kind of typical char-forming agent and PCS could work as acid source and carbon source at same time (Li et al. 2021; Yang et al. 2021). The cellulose units in cotton fabrics can serve as carbon source. Therefore, polyphosphoric acid can be generated during the combustion due to the decomposition of PCS and promote the dehydration and carbonization of cotton fabrics to form stable P-containing compact shield. The generated shield can isolate the transform of heat and oxygen, suggesting the condensed-phase flame-retardant activity of PCS functional coatings (Chen et al. 2020b, a; Guo et al. 2020).
To further investigate the flame-retardant mechanism of functionalized cotton fabrics, TG-FTIR was employed to analyze the pyrolysis volatiles. For control as shown in Fig. 9, there are almost no characterized peaks of flammable volatiles below 300 °C. The highest intensity of the most bands around at Tmax ranges from 300 to 400 °C. For control as seen in Fig. 9a, gaseous water and organic products containing O–H groups (3500–3800 cm−1), hydrocarbons (2700–3200 cm−1), carbon monoxide (CO, 2100–2200 cm−1), carbon dioxide (CO2, 2350 cm−1), carbonyl compounds (1750 cm−1), aromatic compounds (the skeletal vibration, 1510 cm−1) and ether compounds (the stretching vibration, 1120 cm−1) are all detected (Gu et al. 2021; Zhou et al. 2019; Zhu et al. 2018). In comparison, the absorption intensity of the flammable volatiles all weaken and the peaks of hydrocarbons around at 2700–3200 cm−1 disappear. These results indicate that functionalized cotton fabrics reduce the generation of flammable volatiles during the thermal degradation of cellulose.
To further explore the change of some typical flammable volatiles during thermal degradation of cellulose, FTIR spectra of the released gases at maximum decomposition temperature and the absorption intensity versus time of selected groups are given in Fig. 9c-f). On the base of Lambert–Beer law, the concentration of gas and the absorption intensity of a specific wavenumber have a linear relationship (Wang et al. 2020a, b; Zhang et al. 2021). Compared with the control cotton, the absorption intensities of all the flammable volatiles are greatly suppressed, indicating remarkably reduced releasing amount of the flammable gases. These gases may be changed to carbonaceous residue before release, which leads to the lower transformation and feedback of heat and mass to the fire zone, resulting in the greatly increased residue after burning.
Overall, combined the above discussion, the flame-retardant mechanism of the eco-friendly biomass PCS coating for cotton fabrics is proposed as illustrated in Fig. 10. PCS can generate phosphoric acid and polyphosphoric acid during degradation to accelerate the dehydration and carbonization due to the excellent charring of CS, forming a stable and dense char barrier. The generated char barrier can prevent the transfer of heat, oxygen and flammable volatiles to feedback from the fire zone, also protect the underlying cotton. Meanwhile, H2O, CO2, NH3 and some nonflammable volatiles are produced to dilute the flammable volatiles and oxygen, and also cool down the temperature of the system. As a consequence, the obtained cotton fabrics exhibit high efficiency flame retardancy.
Washing durability
Washing durability of the flame-retardant cotton fabrics was explored with the assistance of LOI and VFT tests after washing with ionic detergent. As shown in Fig. 11 and Table S2, the weight remains on samples decreased to 7.5 wt% from 11.3% wt% for FR1 and to 11.0 wt% from 14.1 wt% for FR3 after 10 washing cycles (10 LCs). However, the cotton fabrics treated with PCS still behave satisfied flame retardancy with LOI value of 21% for FR1 and 23.5% for FR3, which are superior to the control sample. After VFT, the shapes and morphologies of the char residues still maintain after washing, though all the damaged lengths reach 300 mm as shown in Fig. S2. Such decreased weight gain can be explained that a portion of PCS is constructed on the interface of the fibers mechanically rather than grafted on fibers, which are washed off by the detergent. However, a new covalent bond of P-O-C can also be formed between PCS and the hydroxyl groups of the cellulose during the heating and curing process in the presence of dicyandiamide (Chen et al., 2020b, a). Therefore, the retained flame retardant on cotton fabrics will not peel off and can maintain stable flame retardancy for cotton fabrics. In fact, most of the unreacted PCS is lost in the first few washing and it retains stability thereafter.
The mechanical properties of cotton fabrics are illustrated in Table 3. The tensile strength of cotton fabric treated with 10 wt% PCS is slightly decreased in both weft and warp directions in comparison with that of the control fabrics. The phenomenon is probably caused by the breaking of acidic PCS solution to a few C–O–C bonds of cotton cellulose at a higher temperature of 180 °C (Feng et al. 2017). Nonetheless, the retained strength is still strong enough for using as clothing fabrics and decoration textiles. Additionally, the elongation at break in both weft and warp directions are almost unchanged, indicating that the treated fabrics exhibit good anti-deformability and the PCS is well-distributed on the cotton fibers (Li et al. 2019a, b).
Conclusion
In this work, an eco-friendly biomass-based coating involved phosphorylated chitosan is fabricated and constructed on the surface of cotton fabrics to improve the durable flame retardancy through a dip-pad-dry-cure process. The coating is thoroughly characterized by FTIR, XPS, XRD and SEM–EDX. The coating is used as charring agent and acid source to catalyze and carbonize cellulose. Therefore, the treated cotton fabrics exhibit superior flame retardancy with a LOI value of 28.3% for FR3 and extinguish immediately after removing the ignitor in vertical flame test. More specially, FR3 is not ignited under a 25 kW/m2 heat flux. The values of PHRR and THR of FR2 have sharp reductions of 88.2% and 59.4%, respectively, in comparison with that of control cotton. A typical condensed-phase flame-retardant mechanism by forming a phosphorus-rich intumescent and carbonaceous char layer and diluting effect of the non-flammable volatiles in gas-phase is certified by the char analysis and TG-FTIR. Furthermore, the flame retardancy of treated cotton fabric is still superior to the control after 10 washing cycles due to the partial chemical grafting of PCS on cellulose, indicating improved durable flame retardancy. In addition, an acceptable tensile strength and almost maintained elongation at break are obtained. Therefore, this eco-friendly biomass-based flame-retardant coating combined with the typical fabricating technique provides a promising strategy for fabricating durable flame-retardant fabrics.
Supporting information
The detailed TGA data, digital photos recorded from VFT, the digital photos of cotton fabrics before and after washing, the LOI value and weight gain of different cotton fabrics before and after washing.
References
Abdelrahman MS, Khattab TA (2019) Development of one-step water-repellent and flame-retardant finishes for cotton. ChemistrySelect 4:3811–3816. https://doi.org/10.1002/slct.201900048
Affes S, MaalejH LSM, Abdelhedi R, Nasri R, Nasri M (2022) Effect of glucose substitution by low-molecular weight chitosan-derivatives on functional, structural and antioxidant properties of maillard reaction-crosslinked chitosan-based films. Food Chem 366:13. https://doi.org/10.1016/j.foodchem.2021.130530
Chen Y, Wang D, Liu S, Lu Y, Zhang GX, Zhang FX (2020a) A novel P-N-based flame retardant with multi-reactive groups for treatment of cotton fabric. Cellulose 27:9075–9089. https://doi.org/10.1007/s10570-020-03387-0
Costes L, Laoutid F, Brohez S, Dubois P (2017) Bio-based flame retardants: when nature meets fire protection. Mat Sci Eng R 117:1–25. https://doi.org/10.1016/j.mser.2017.04.001
Fang F, Ran S, Fang Z, Song P, Wang H (2019) Improved flame resistance and thermo-mechanical properties of epoxy resin nanocomposites from functionalized graphene oxide via self-assembly in water. Compos B Eng 165:406–416. https://doi.org/10.1016/j.compositesb.2019.01.086
Gao BB, Yang JH, Zhang SD, Li XY (2021) Green fabrication of thermally-stable oxidized cellulose nanocrystals by evolved fenton reaction and in-situ nanoreinforced thermoplastic starch. Cellulose 28:8405–8418. https://doi.org/10.1007/s10570-021-04039-7
Feng YJ, Zhou Y, Li DK, He S, Zhang FX, Zhang GX (2017) A plant-based reactive ammonium phytate for use as a flame-retardant for cotton fabric. Carbohydr Polym 175:636–644. https://doi.org/10.1016/j.carbpol.2017.06.129
Gao D, Li R, Lv B, Ma J, Tian F, Zhang J (2015) Flammability, thermal and physical-mechanical properties of cationic polymer/montmorillonite composite on cotton fabric. Compos B Eng 77:329–337. https://doi.org/10.1016/j.compositesb.2015.03.061
Gou T, Wu X, Zhao Q, Chang S, Wang P (2021) Novel phosphorus/nitrogen-rich oligomer with numerous reactive groups for durable flame-retardant cotton fabric. Cellulose 28:7405–7419. https://doi.org/10.1007/s10570-021-03980-x
Gu JJ, Yan XF, Li JW, Qian YW, Zhu CK, Qi DM (2021) Durable flame-retardant behavior of cotton textile with a water-based ammonium vinyl phosphonate. Polym Degrad Stab 191:109658. https://doi.org/10.1016/j.polymdegradstab.2021.109658
Guo WW, Wang X, Huang J, Zhou Y, Cai W, Wang J, Hu Y (2020) Construction of durable flame-retardant and robust superhydrophobic coatings on cotton fabrics for water-oil separation application. Chem Eng J 398:125661. https://doi.org/10.1016/j.cej.2020.125661
Jian RK, Ai YF, Xia L, Zhao LJ, Zhao HB (2019) Single component phosphamide-based intumescent flame retardant with potential reactivity towards low flammability and smoke epoxy resins. J Hazard Mater 371:529–539. https://doi.org/10.1016/j.jhazmat.2019.03.045
Lazar S, Carosio F, Davesne AL, Jimenez M, Bourbigot S, Grunlan JC (2018) Extreme heat shielding of clay/chitosan nanobrick wall on flexible foam. ACS Appl Mater Inter 10:31686–31696. https://doi.org/10.1021/acsami.8b10227
Leistner M, Abu-Odeh AA, Rohmer SC, Grunlan JC (2015) Water-based chitosan/melamine polyphosphate multilayer nanocoating that extinguishes fire on polyester-cotton fabric. Carbohydr Polym 130:227–232. https://doi.org/10.1016/j.carbpol.2015.05.005
Lewis DM, Hawkes JA, Hawkes L, Mama J (2020) A new approach to flame-retardant cellulosic fabrics in an environmentally safe manner. Color Technol 136:512–525. https://doi.org/10.1111/cote.12504
Li P, Liu C, Wang B, Tao Y, Xu YJ, Liu Y, Zhu P (2021) Eco-friendly coating based on an intumescent flame-retardant system for viscose fabrics with multi-function properties: Flame retardancy, smoke suppression, and antibacterial properties. Prog Org Coat 159:106400. https://doi.org/10.1016/j.porgcoat.2021.106400
Li P, Wang B, Liu YY, Xu YJ, Jiang ZM, Dong CH, Zhu P (2020) Fully bio-based coating from chitosan and phytate for fire-safety and antibacterial cotton fabrics. Carbohydr Polym 237:116173. https://doi.org/10.1016/j.carbpol.2020.116173
Li S, Zhong L, Huang S, Wang D, Zhang FX, Zhang GX (2019a) A novel flame retardant with reactive ammonium phosphate groups and polymerizing ability for preparing durable flame retardant and stiff cotton fabric. Polym Degrad Stab 164:145–156. https://doi.org/10.1016/j.polymdegradstab.2019.04.009
Liu XY, Xie RY, Chen T, He L, Wang T, Liao W, Liu ZG, Chen MJ (2021a) Improvement of polyurethane film strength by H-bonding crosslinking with hydroxylated melamine. J Appl Polym Sci 138:51411. https://doi.org/10.1002/app.51411
Li Z, Liu Z, Dufosse F, Yan L, Wang DY (2018) Interfacial engineering of layered double hydroxide toward epoxy resin with improved fire safety and mechanical property. Compos B Eng 152:336–346. https://doi.org/10.1016/j.compositesb.2018.08.094
Li Z, Liu Z, Zhang J, Fu C, Wagenknecht U, Wang DY (2019b) Bio-based layered double hydroxide nanocarrier toward fire-retardant epoxy resin with efficiently improved smoke suppression. Chem Eng J 378:122046. https://doi.org/10.1016/j.cej.2019.122046
Liang T, Jiang Z, Wang C, Liu J (2017) A facile one-step synthesis of flame-retardant coatings on cotton fabric via ultrasound irradiation. J Appl Polym Sci 134:45114. https://doi.org/10.1002/app.45114
He L, Chen T, Zhang Y, Hu LR, Wang T, Han R, He JL, Luo W, Liu ZG, Deng JN, Chen MJ (2022) Imide-DOPO derivative endows epoxy resin with excellent flame retardancy and fluorescence without losing glass transition temperature. Compos B Eng 230:109553. https://doi.org/10.1016/j.compositesb.2021.109553
Lin D, Zeng X, Li H, Lai X, Wu T (2019) One-pot fabrication of superhydrophobic and flame-retardant coatings on cotton fabrics via sol-gel reaction. J Colloid Interface Sci 533:198–206. https://doi.org/10.1016/j.jcis.2018.08.060
Ling C, Guo L (2020) Preparation of a flame-retardant coating based on solvent-free synthesis with high efficiency and durability on cotton fabric. Carbohydr Polym 230:115648. https://doi.org/10.1016/j.carbpol.2019.115648
Liu L, Pan Y, Wang Z, Hou Y, Gui Z, Hu Y (2017a) Layer-by-layer assembly of hypophosphorous acid-modified chitosan based coating for flame-retardant polyester-cotton blends. Ind Eng Chem Res 56:9429–9436. https://doi.org/10.1021/acs.iecr.7b02303
Liu ZY, Xu MJ, Wang Q, Li B (2017b) Preparation and properties of flame retardant cotton fabric by surface chemical grafted modification. Chem J Chin Univ-Chin 38:1477–1483. https://doi.org/10.7503/cjcu20170032
Lu Y, Jia Y, Zhou Y, Zou J, Zhang GX, Zhang FX (2018) Straightforward one-step solvent-free synthesis of the flame retardant for cotton with excellent efficiency and durability. Carbohydr Polym 201:438–445. https://doi.org/10.1016/j.carbpol.2018.08.078
Chen MJ, Lazar S, Kolibaba TJ, Shen R, Quan Y, Wang QS, Chiang HC, Palen B, Grunlan JC (2020b) Environmentally benign and self-extinguishing multilayer nanocoating for protection of flammable foam. ACS Appl Mater Inter 12:49130–49137. https://doi.org/10.1021/acsami.0c15329
Liu Z, Yu Z, Qiaolin T, Kaixin Z, Weihao D, Lewen Z, Rong W, Chen J, Deng JJ, Liao W, Wang QW, Chen MJ, Liu ZG (2021b) Highly efficient flame-retardant and transparent epoxy resin. Polym Adv Technol 32:2940–2952. https://doi.org/10.1002/pat.5306
Shi T, Zhang SD, Shi XX (2021a) Oxidized regenerated celluloses to fabricate high fire safety for epoxy resin with super expansion char layer. Cellulose 28:2995–3015. https://doi.org/10.1007/s10570-021-03723-y
Liu BW, Zhao HB, Wang YZ (2021c) Advanced flame-retardant methods for polymeric materials. Adv Mater. https://doi.org/10.1002/adma.202107905
Rehan M, El-Naggar ME, Mashaly HM, Wilken R (2018) Nanocomposites based on chitosan/silver/clay for durable multi-functional properties of cotton fabrics. Carbohydr Polym 182:29–41. https://doi.org/10.1016/j.carbpol.2017.11.007
Rehman ZU, Huh SH, Ullah Z, Pan YT, Churchill DG, Koo BH (2021) LBL generated fire retardant nanocomposites on cotton fabric using cationized starch-clay-nanoparticles matrix. Carbohydr Polym 274:118626. https://doi.org/10.1016/j.carbpol.2021.118626
Shi XH, Li XL, Li YM, Li Z, Wang DY (2022a) Flame-retardant strategy and mechanism of fiber reinforced polymeric composite: a review. Compos B Eng 233:109663. https://doi.org/10.1016/j.compositesb.2022.109663
Rosace G, Castellano A, Trovato V, Iacono G, Malucelli G (2018) Thermal and flame retardant behaviour of cotton fabrics treated with a novel nitrogen-containing carboxyl-functionalized organophosphorus system. Carbohydr Polym 196:348–358. https://doi.org/10.1016/j.carbpol.2018.05.012
Shi HL, Zhao WJ, Zhao XW, Li ZW, Li XH, Zhang ZJ (2021b) Fabrication of bismuth oxychloride nanosheets decorated with chitosan and phytic acid for improvement of flexible poly(vinyl chloride) flame retardancy. Fib Polym 36:1375–1384. https://doi.org/10.1007/s12221-021-0678-6
Shi XH, Chen L, Liu BW, Long JW, Xu YJ, Wang YZ (2018a) Carbon fibers decorated by polyelectrolyte complexes toward their epoxy resin composites with high fire safety. Chinese J Polym Sci 36:1375–1384. https://doi.org/10.1007/s10118-018-2164-1
Shi XH, Xu YJ, Long JW, Zhao Q, Ding XM, Chen L, Wang YZ (2018b) Layer-by-layer assembled flame-retardant architecture toward high-performance carbon fiber composite. Chem Eng J 353:550–558. https://doi.org/10.1016/j.cej.2018.07.146
Shi XH, Chen L, Zhao Q, Long JW, Li YM, Wang YZ (2020) Epoxy resin composites reinforced and fire-retarded by surficially-treated carbon fibers via a tunable and facile process. Compos Sci Technol 187:107945. https://doi.org/10.1016/j.compscitech.2019.107945
Wang B, Li P, Xu YJ, Jiang ZM, Dong CH, Liu Y, Zhu P (2020a) Bio-based, nontoxic and flame-retardant cotton/alginate blended fibres as filling materials: thermal degradation properties, flammability and flame-retardant mechanism. Compos B Eng 194:108038. https://doi.org/10.1016/j.compositesb.2020.108038
Wang W, Guo J, Liu X, Li H, Sun J, Gu X, Li W (2020b) Constructing eco-friendly flame retardant coating on cotton fabrics by layer-by-layer self-assembly. Cellulose 27:5377–5389. https://doi.org/10.1007/s10570-020-03140-7
Wang X, Wu T, Hong J, Dai J, Lu Z, Yang C, Dai L (2021) Organophosphorus modified hollow bimetallic organic frameworks: Effective adsorption and catalytic charring of pyrolytic volatiles. Chem Eng J 421:129697. https://doi.org/10.1016/j.cej.2021.129697
Xu DH, Gao ZY, Xu B, Ren H, Zhao XS, Zhang YN, Zhu P (2020) A facile and effective flame-retardant coating for cotton fabric with alpha-aminodiphosphonate siloxane. Polym Degrad Stab 180:109312. https://doi.org/10.1016/j.polymdegradstab.2020.109312
Xue CH, Wu Y, Guo XJ, Liu BY, Wang HD, Jia ST (2020) Superhydrophobic, flame-retardant and conductive cotton fabrics via layer-by-layer assembly of carbon nanotubes for flexible sensing electronics. Cellulose 27:3455–3468. https://doi.org/10.1007/s10570-020-03013-z
Yang YH, Bolling L, Priolo MA, Grunlan JC (2013) Super gas barrier and selectivity of graphene oxide-polymer multilayer thin films. Adv Mater 25:503–508. https://doi.org/10.1002/adma.201202951
Yang ZY, Li HK, Niu G, Wang J, Zhu DC (2021) Poly(vinylalcohol)/chitosan-based high-strength, fire-retardant and smoke-suppressant composite aerogels incorporating aluminum species via freeze drying. Compos B Eng 219:108919. https://doi.org/10.1016/j.compositesb.2021.108919
Yuan B, Zhang JM, Mi QY, Yu J, Song R, Zhang J (2017) Transparent cellulose-silica composite aerogels with excellent flame retardancy via an in situ sol-gel process. ACS Sustain Chem Eng 5:11117–11123. https://doi.org/10.1021/acssuschemeng.7b03211
Zhang AN, Zhao HB, Cheng JB, Li ME, Li SL, Cao M, Wang YZ (2021) Construction of durable eco-friendly biomass-based flame-retardant coating for cotton fabrics. Chem Eng J 410:128361. https://doi.org/10.1016/j.cej.2020.128361
Shi XH, Liu QY, Li XL, Du AK, Niu JW, LiYM LiZ, Wang M, Wang DY (2022b) Construction phosphorus/nitrogen-containing flame-retardant and hydrophobic coating toward cotton fabric via layer-by-layer assembly. Polym Degrad Stabil 197:109839. https://doi.org/10.1016/j.polymdegradstab.2022.109839
Zhang Y, Tian W, Liu L, Cheng W, Wang W, Liew KM, Hu Y (2019) Eco-friendly flame retardant and electromagnetic interference shielding cotton fabrics with multi-layered coatings. Chem Eng J 372:1077–1090. https://doi.org/10.1016/j.cej.2019.05.012
Zhao PH, Xiong KK, Wang WT, Liu YQ (2017) Preparation of a halogen-free P/N/Si flame retardant monomer with reactive siloxy groups and its application in cotton fabrics. Chin J Chem Eng 25:1322–1328. https://doi.org/10.1016/j.cjche.2016.09.015
Zhou X, Mu X, Cai W, Wang J, Chu F, Xu Z, Hu Y (2019) Design of hierarchical NiCo-LDH@PZS hollow dodecahedron architecture and application in high-performance epoxy resin with excellent fire safety. ACS Appl Mater Inter 11:41736–41749. https://doi.org/10.1021/acsami.9b16482
Zhu P, Gu Z, Hong S, Lian H (2018) Preparation and characterization of microencapsulated LDHs with melamine-formaldehyde resin and its flame retardant application in epoxy resin. Polym Adv Technol 29:2147–2160. https://doi.org/10.1002/pat.4323
Acknowledgments
This research is financially sponsored by Natural Science Foundation of Chongqing (cstc2021jcyj-msxmX0370, cstc2021jcyj-msxmX0697), International Science and Technology Cooperation and Exchange Program of Sichuan Science and Technology Department (211552), National Natural Science Foundation of China (21975208), the Sichuan Science and Technology Program (2020JDJQ0062) and Project of Science Foundation in Chongqing Jiaotong University (20JDKJC-B049).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, XL., Shi, XH., Chen, MJ. et al. Biomass-based coating from chitosan for cotton fabric with excellent flame retardancy and improved durability. Cellulose 29, 5289–5303 (2022). https://doi.org/10.1007/s10570-022-04566-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-022-04566-x