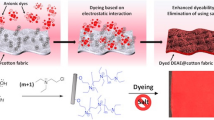
Abstract
Traditional dyeing process of cotton has caused a large amount of wastewater with high salt content, which seriously damages the ecological environment. In this work, cotton fabric was modified with 3-chloro-2-hydroxypropyl trimethyl ammonium chloride in order to decrease the electrostatic repulsion with the anionic dyes. A systematic optimization of the process has been performed. The acid dyes were applied instead of the common reactive dyes to dye the modified fabrics. The advantage of the high dye-uptake of acid dyes is studied. The levelness of the fabric dyed by reactive dyes and acid dyes were compared. The adsorption process and the bindings between the modified fabric and anionic acid dyes were further explored. The dyeing process were optimized. Finally, the dye-uptake of the modified fabric dyed by optimized conditions achieves to about 92%, which is comparable to traditional cotton dyeing process.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cotton fibers with high air permeability, comfort, antistatic property and biodegradability have always been considered the most important raw materials (Li et al. 2021). In general, the dyes used for cellulose are reactive dyes (Ahmed et al. 2020). However, the coulomb repulsion between the negatively charged dye molecules and the negatively charged cellulose macro-molecules seriously limits the dye-uptake of reactive dyes (Peng et al. 2021). To solve these problems, a large amount of inorganic salts are added into the dye bath to shield the negative charges on the cellulose surfaces (Pei et al. 2021). Currently, one of the biggest challenges in textile printing and dyeing industry is the treatment of dyeing wastewater, which causes great damage to resources if it has not been disposed of properly. And it will cause great damage to land resources if it has been not disposed properly. The high concentration of electrolytes in the dyeing bath also results in hydrolysis of the anionic dye molecules, which reduces the utilization rate of dyes (Wang et al. 2020a). The removal of the hydrolyzed dyes adsorbed on the fiber surfaces requires massive water resources (Mu et al. 2019). In addition, dyeing baths with high content of inorganic salts will greatly pollute the domestic water (Charola et al. 2018). Therefore, how to reduce the amount of inorganic salts during the dyeing reactions has become an important problem (Siddiqua et al. 2017). During the past years, great efforts have been made to solve the above problems. The sewage device at the end of the pipeline is installed currently, which is inefficient and expensive. The kernel of low-salt or non-salt dyeing technology is to decrease the electrostatic repulsion between the cellulose fiber and dye molecules (Wang et al. 2019). Currently, the main studies focus on four directions. The first way is to optimize the dyeing process (Khatri et al. 2015). The second method is to develop salt-free dyeing auxiliary (Zhang et al. 2007). The third approach is to design dye molecular structures with high-affinity (Nallathambi and Rengaswami 2017). The fourth strategy is to modify the fibers with cationic organics. The negative charges on the fiber surfaces will be converted to positive charges by introducing cationic modification, which could facilitate the adsorption between the fiber and dye molecules (Teng et al. 2011). For instance, Patiño et al. (2011) applied (3-acrylamide propyl) trimethylammonium chloride (AAHTAPC) to modify the cotton fabric through dip-rolling process under alkaline. The treated fibers showed a good dyeing performances without adding any salts. Niu et al. (2020) modified the cotton with a bifunctional cationic polymer, which significantly improved the dyeability of the modified fabric compared with the conventional one. However, the overquick dyeing speed in the dyeing and fixation stage leads to poor levelness of the dyed fabric, which limits the industrial application of cationic cottons (Ristic and Ristic 2012).
Small-molecule modifiers can modify the fibers uniformly because of their good solubility, which could solve the uneven dyeing of the cationic fibers (Park et al. 2010). Among various chemical reagents, 3-chloro-2-hydroxypropyl trimethyl ammonium chloride (CHPTAC) is considered a promising cationic modifier (Yu et al. 2019). Wang et al. (2020b) prepared a hybrid ink composed of CHPTAC and reactive dye to print plain-woven cotton. The printed fabric showed high K/S value (21.68) and dye fixation (91.56%). Ghazal et al. (El-Masry 2020) treated the cotton with CHPTAC silver (Ag) nanoparticles, which showed a satisfactory antibacterial performance. CHPTAC, as a simple polymer with low molecular weight, could penetrate into the fiber easily, achieving a more uniform reaction with the fiber (Pal et al. 2005). In addition to the application in printing technology and antibiotic finishing. Rengaswami et al. (2017) used CHPTAC to modify the cotton for reactive dyeing. The usage of CHPTAC and cotton in the article was very large, which proves the feasibility of CHPTAC in industrial process. Besides, they explored the influence of different concentration CHPTAC on reactive dyeing (Rengaswami 2016). The CHPTAC to alkali concentration was optimized as 2:1 mol. Liang et al. (2020) reported a cellulose-based adsorbent by CHPTAC, which preliminarily explored the combination between cotton and modifier. Tabba et al. (2001) compared the dyeing effects of reactive, acidic and direct dyes on modified cotton under the same process condition. Cotton modified with CHPTAC can be dyed without electrolytes to obtain great colour yields with a variety of direct and reactive dyes. Arivithamani and Dev (2018) reported a Comprehensive comparison of reactive dyed cationized cotton hosiery fabrics with traditional dyed cotton. Correia et al. (2021) explored cotton treated with plasma and cationization for dyeing. And two cationic agents were used. The plasma treatment followed by cationization can facilitate the even dyeing.
Acid dyes with with low molecular weight and low affinity might be a viable option to dye the modified fabrics due to the reduced initial dyeing rate. Besides, no fixation step of acid dyes will simplify the dyeing process and reduce energy consumption, which has been rarely addressed by previous studies (Dong et al. 2020). And the structure–activity relationship and mechanism of action between the acid dyes and the modified fabrics have not been studied. Currently, there are few studies devoted to the systematic optimization of CHPTAC modification process for acid dyeing. The adsorption mechanism, thermodynamics and kinetics between dye molecules and cationic cotton have also been rarely reported. In this work, both the modification reaction and the acid dyeing process were optimized, which has a significant improvement on dyeing effects in comparison with unsuitable conditions. The adsorption process and the bindings between the modified fabric and anionic acid dyes were further explored. CHPTAC was etherified with anion oxygen of cellulose macro-molecules under alkaline conditions. The influences of NaOH dosage, modification temperature, reaction time on modification effects and its physical properties were explored. The usage of acid dyes with low molecular mass could be expected to decrease the dyeing rate on the above modified cotton fabrics to achieve high levelness. Dyeing mechanisms of modified cotton fabrics and pure cotton were studied by using molecular simulations and kinetic thermodynamic curves. The modified cotton treated by the further optimized dyeing process had a comparable dyeing performance than that of traditional one. The optimized process conditions will provide a valuable reference for other modification applications. The large-scale application of this salt-free dyeing technology will undoubtedly be of great significance to improve the quality of dyeing wastewater.
Experimental details
Materials and chemical reagent
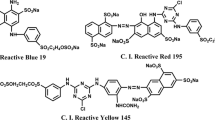
Cotton (136 g m2); 3-Chloro-2-hydroxy-propyl trimethyl ammonium chloride (CHPTAC), Sodium hydroxide (NaOH), sodium carbonate (NaCO3), and Acetic acid (CH3COOH) were bought from Sinopharm Chemicals (Shanghai, China). Acid red GR, Weak acid yellow 2G, acid black, and reactive brilliant red dyes were bought from Ningbo Stanou Co., Ltd. The structures of dyes and CHPTAC are shown in Table S1.
Cationic modification of the fabric
Impregnation method was used to achieve the combination of cotton fabric and CHPTAC. The weight ratio of modifier to fabric was 1:20. For convenience, the modified cotton was labeled as MdC. And the raw cotton was labeled as RC. For comparison, the padding process was carried out.
Modified solution: CHPTAC 30 g/L, NaOH 10 g/L and bath ratio 1: 20.
Impregnation modification process: dipping (1.5 °C/min of the heating rate, 90 °C of the holding temperature and 15 min of the reaction time) → Cooling to room temperature (about 25 °C) and picking up (10 °C of the cooling rate) → drying (80 °C for 5 min).
Padding process: two time of padding (100% of liquid rate) → drying (140 °C for 5 min).
Dyeing process
Three acid dyes (4% owf %) were used to dye cotton fabrics with the bath ratio of 20:1. Dyeing process curve could be seen in Fig. 1. For comparision, salt-free dyeing process with reactive dyes for MdC, traditional salt dyeing procedure of reactive dyes and conventional acid dyes for untreated cotton were carried out as can be seen in the Fig. S2. The influences of NaOH dosage on the dyeing effect of untreated cotton and modified cotton were studied.
Structure characterizations
SEM was used to observe the surface morphology of the fabric before and after the modification of the cotton fabric. The Varian 640 Fourier Transform Infrared Spectrometer (FT-IR) was used to test the infrared absorption spectrum of the fabrics. The zeta potential of the aqueous solution of Cotton-PDMAEMA was measured by using a Nano-10A nanoparticle size and potential analyzer (Malvern, UK) in the range of pH = 3–11. The STA449F3 synchronous thermal analyzer was used for thermogravimetric analysis before and after cotton fabric modification. The instrument heating rate was 10 °C/min, the test temperature was 25 °C to 600 °C, and the color depth K/S of the fabric surface was measured under a D65 light source by a color measuring color meter SF600 PSUS (Datacolor, USA) at a 10° field of view.
Dyeing properties measurements
Dye exhaustion, color measurements, sorption processes, fastnesses tests were carried out to measure the dyeing performances. The details of the formulas could be found in supporting information (SI). The rubbing fastness of dyed fabrics was evaluated according to GB/T 3920–2008. The samples were carried out to rub 10 times for dry and wet condition with the moisture of 95% water by fastness tester (Y571B). The washing fastness of dyed fabrics was tested according to GB/T 3920–2008. The samples were washed under 2 g/L soap solution condition by water bath shaker (DLS-1000 A, Daelim Starlet Co., Ltd). The colour change was rated according to the appropriate gray scale value. Colour fading to light was tested according to GB/T 8427–2019 and the degree of fading was assessed by the Society of Dyers and Colourist (SDC) blue wool scales.
Results and discussions
Optimization of modification process
Figure 2a–c shows the influences of different reaction conditions on the modification effect. Zeta potential of modified cotton was measured before dyeing. K/S, and Sr values were tested after dyeing. The variables kept constant in Fig. 2a–c could be found in the Table 1. Zeta potential of the MdCs is enhanced firstly and then received a steady with the increased CHPTAC concentration, which could be ascribed to the limited active sites binding to the modifier on the MdC surfaces (Yang et al. 2021). The K/S values of the dyed MdC exhibites similar trends than those of Zeta potential as the cationic groups could improve the coulomb force between dye molecules and cellulose fibers (Correia et al. 2020). Therefore, more dye molecules could be adsorbed by the fiber with the concentration of CHPTAC increasing. However, excessive CHPTAC concentrations (more than 30 g/L) not resulted in continued improvement of the tinctorial yield due to the saturated adsorption state of the fibers (Wang and Kan 2020). And the Sr (standard deviation of the K/S values) are increased rapidly at high CHPTAC concentrations. The number of reactive groups on the fiber surfaces was greatly affected by the concentration of hydroxide ions in the solution as CHPTAC need to be reacted with cellulose under alkaline environment. Thus, less NaOH dosage made the catalytic activation reaction insufficient, and too high NaOH concentration would lead to the hydrolysis of the modifier (Niu et al. 2020). As for the temperature of the modification process, excessive temperature will lead to a fast decomposition rate of the etherified products and low temperature made the holes in the fibers swell not sufficiently (Mohtashim et al. 2020). Inadequate reaction time was not able to achieve the adsorption equilibrium state between dye molecules and cellulose fiber, however, further prolonging the reaction time has no significance to improve the modification effect after reaching the adsorption equilibrium. Zhai et al. (2021a). Padding method and dipping process were compared to dye the modified cotton. Figure 2f displays the dyeing performance of MdC with three acid dyes by padding method and dipping process. The K/S values of cottons treated by dipping method (MdC-D) are larger than those of padding method (MdC-P), demonstrating the combination between MdC-D and dye molecules is more sufficient than that of MdC-P. Although 80°C of the drying temperature is less effective than 140°C, the damage for the breaking strength of the fabric is decreased visibly (Fig. S7). Therefore, dipping is more suitable for MdC dyeing. Taking all of the above into consideration, the suitable modification conditions can be defined as 30 g/L CHPTAC, 10 g/L NaOH, 90 °C of modification temperature, 15 min of reaction time and 80 °C of drying temperature. The cotton modified by optimized condition parameters is labeled as MdC-Op.
Structural characterization of MdC-Op
TG curves of MdC-Op and raw cotton fabrics (RC) are shown in Fig. 2d. Two distinct peaks of RC located at 290 and 420 °C are corresponding to the thermal decomposition of cellulose macromolecules (Dong et al. 2020). Then, MdC-Op has an additional peak located at about 200 °C, which is attributed to the thermal decomposition of CHPTAC polymers (Li et al. 2017). Compared to RC, the initial decomposition temperature of MdC-Op is decreased as can be see from the DTG curves of MdC-Op and RC in Fig. 2e, which might be due to that the thermal decomposition capacity of CHPTAC is lower than that of cotton (Yu et al. 2019). The half life temperature and weightlessness rate of MdC-Op is increased slightly demonstrating the improved thermal stability of MdC-Op (Yao et al. 2021).
Figure 3a shows the SEM image of MdC-Op. Compared to RC (Fig. S8) with a smooth surface, a thin film (CHPTAC) is loaded onto the surfaces of MdC-Op homogeneously. No cracks are found on the MdC-Op surfaces, demonstrating that the CHPTAC has no damage to the cotton morphologies. The same X-ray characteristic diffraction peaks of MdC-Op and RC prove that the CHPTAC modifier has no obvious effect on the crystal structures (Fig. 3c). The Zeta potential of untreated cotton is negative at all pH value attributed to the negative ionization of the cellulose macro-molecule (Fig. 3b). Zeta potential of MdC-Op is positive in the pH value range of 3–9.8 as the cationic group of the modifier shielded the negative charge on the fiber surface, which could improve the dyeing performances of the fabric. The FT-IR spectrums of MdC-Op and RC are shown in Fig. 3d. The stretching vibration peaks located at 3300 cm−1 and 2900 cm−1 in RC are corresponding to -OH and -C-H of polysaccharide molecular skeleton (Kamtsikakis et al. 2021). MdC-Op shows a more obvious peak at 1060 cm−1, which is recognized as C–O–C generated by the etherification between CHPTAC and RC (Liu et al. 2021). And the peaks at 1290 cm−1 and 1477 cm−1 are corresponding to -CH3 and N-CH3 of CHPTAC (Fang et al. 2021). Figure 3e–h are the EDS images of MdC-Op. The existing of nitrogen (N) element further confirms that the CHPTAC was grafted on the RC surfaces successfully. The bonding states of RC and MdC-Op were tested by XPS (Fig. 4). C and O elements of RC and MdC-Op are derived from cellulose macromolecular chains. The extra element of N elements in MdC-Op is come from CHPTAC, which is consistent with EDS and FT-IR results. Figure 4b and d show high-resolution XPS spectra of C element in RC and MdC-Op. The peaks located at 284.7 eV, 286.7 and 287.8 eV are relative to C–C/C–H, C–O and O–C–O, respectively (Gao et al. 2021). Compared to RC, the extra peak of MdC-Op located at 289.2 eV is ascribed to C-N in CHPTAC (Jiang et al. 2021), which further confirms the combination of CHPTAC and RC. Then, the peaks at 531.5 eV in Fig. 4c and e are attributed to O-C of RC and MdC-Op (Zhai et al. 2020). Figure 4f is the high-resolution XPS spectra of N element in MdC-Op. The N 1 s spectra could be divided into two peaks located at 398.8 eV and 399.9 eV. The peak at 398.8 eV was attributed to the N–C bonds in CHPTAC (Zhu et al. 2021). Nitrogen-atoms in MdC-Op might have grabbed hydrogen from the chain and formed hydrogen bonds with other hydroxyl group. Therefore, the other energy state nitrogen at 399.9 eV might be put down to the N–H hydrogen bond (Qin et al. 2011).
Dyeing mechanisms of MdC-Op
Figure 5a is the schematic diagram of the modification process of MdC-Op. CHPTAC formed an intermediate under alkaline condition and then combined with cellulose by covalent bond as shown in Fig. S1. The modified cellulose has a positive charge in the dye bath, which greatly improves the binding force between the fabric and dye molecules. Based on the UV–Visible spectrum (Fig. 5b), the linear relation between concentrations and absorbance of the acid red GR were fitted as shown in Fig. 5c. The thermodynamics and kinetics curves of the dyeing process could be measured according to the standard solution working curve. The initial dyeing rate of acidic dyes on MdC-Op is significantly increased in comparison with that of RC (Fig. 5d). The dye-uptake of MdC-Op could be reached to about 80% within 20 min of the dyeing time, which is much higher than that of RC dyed by conventional process. The electrostatic repulsion between the fabric and dye molecules is changed to electrostatic attraction as the negative charges of cotton fabric are shielded by cationic CHPTAC, which enhances the initial dyeing rate. When the dyeing time is 30 min, the saturated adsorption state could be achieved and the dye-uptake of MdC-Op could be reached to the highest of about 90%. The dyeing rate of RC is slower and the dyeing time is longer due to the strong repulsion between anionic dyes and RC in conventional dyeing process. Besides, the salts are still needed in traditional dyeing processes, which leads to a high cost of sewage treatment. The dyeing thermodynamics curves of MdC-Op are shown in Fig. 5e and f. Compared to Freundlich-type adsorption model (R2 = 0.96662), the Langmuir-type model (R2 = 0.99888) is more suitable to the dyeing process of MdC-Op. This result demonstrates that the combination between MdC-Op and dye molecules is achieved by location adsorption process. Therefore, the positional adsorption is the main combination way between the dye molecules and the fabric. Figure 5g is the optical photograph of the dye bath of MdC-Op before and after dyeing process. The raffinate is nearly transparent, which further proves the high utilization of dyestuff.
(a) Schematic diagram of modified process; (b) UV–Visible spectrum of dyed untreated cotton and dyed modified cotton; (c) Standard working curve of acid red GR; (d) Dyeing rate curve of Acid Red; (e, f) Langmuir and Freundlich adsorption isotherm of acid red dyeing; (g) Residual liquid of dyeing bath of untreated cotton and modified cotton
Optimization of dyeing process
The relationship between the dyeing effect and dye concentration is shown in Fig. 6a. The K/S value of the dyed fabric is increased with the increased concentration of dyes. The optimal K/S value of the dyed fabric can reach to about 21.99 when the dye concentration is 2 g/L. The small Sr values at these concentrations indicates that the modified fiber could be dyed homogeneously in a wide range of dye concentrations (Wang et al. 2021). The suitable dyeing temperature and dyeing time could be obtained based on single factor experiments (Fig. 6b–e). The molecular motion would be accelerated and the dye-uptake of the fabric would be enhanced with the increase of temperature as the dyeing reaction is an endothermic process (Eltaweil et al. 2020). However, the effect of temperature on the dye-uptake became smaller at high temperature region (Fig. 6b). The Fig. 6c and 6d show is the relation between dyeing time and E%, as well as the K/S values of modified cotton. The adsorption and desorption of dye molecules tended to equilibrium state with the further increase of dyeing time as the number of cationic spots on cellulose surfaces brought by quaternary ammonium salt is definite (Hou et al. 2009). After Comprehensive consideration of the energy consumption and dyeing performances, 80 °C of temperature, 30 min of dyeing time are defined as the optimum conditions for MdC-Op dyeing. The macro-molecular structures of cellulose would be damaged at highly acidic (Urbina et al. 2021). And too high pH value would lead to the rapid hydrolysis of dyes (Fig. 6e). Thus, 5.5–6.5 of pH value is defined for MdC-Op dyeing. Figure 6f–i show the visual dyeing effect of RC and MdC-Op. It is seen that the dye utilization rate and tinctorial yield of MdC-Op are much better than for RC.
(a) K/S and Sr value of dyed modified cotton at different dye concentrations; (b) E%, K/S and Sr value of dyed modified cotton at different dyeing temperature; (c, d) E%, K/S and Sr value of dyed modified cotton at different dyeing time; (e) K/S value of dyed modified cotton at different pH value; (f) Optical photograph during the dyeing process; (g) Comparison of dyed modified cotton and dyed cotton; (h–i) Optical microscope image of dyed modified cotton and dyed cotton
Molecular adsorption model of dye molecule-RC and dye molecule-MdC-Op
For the convenience of the calculation, the dimer of glucose (DG) was used as substitute for RC. The molecular DG and MdC-Op are shown in Fig. S9a-S9b. Oxygen anions generated by hydroxyl groups of cellulose macro-molecules (DG as the substitute) are occupied by the cationic modifier in the solution. The molecular orbitals of the cellulose macromolecules combined with modifier are looser ascribed to the hybridization between cellulose and CHPTAC (Zhai et al. 2021b) (Fig. S9c and S9d). The density state of MdC-Op contained both cellulose molecules and CHPTAC, demonstrating that the stable covalent bond could be formed between RC and CHPTAC (Fig. S9e and S10) (Fahim and Magd 2021). Figure S9f and S9h show the hybrid orbitals of MdC-Op/dyes and RC/dyes. The molecular orbitals of RC/dyes are almost composed of two separate components, which is because the bindings between dye molecules and RC are only rely on Van der "Waals" forces and hydrogen bonds (Kaushik et al. 2015). The molecular orbitals of MdC-Op/dyes are more cohesive due to the existence of the covalent bonds between dye molecules, CHPTAC and cellulose macromolecules (Morgan et al. 2016). The combination of cellulose and modifier is achieved by strong covalent bonds combined with Van der "Waals" forces and hydrogen bonds, and the calculated adsorption energy between MdC-Op and dyes is 0.43 eV, which is much lower than 1.74 eV of RC-dyes (Fig. S11). Hence, these results further confirm the good dyeing properties of MdC-Op.
Dye adaptability of the optimized process
The MdC-Op was dyed with acid yellow 2G and acid black to explore the dye adaptability. The adsorption dynamics of both two dyes are the same as the acid red GR (Fig. S12). The dyeing performances of RC and MdC-Op are shown in Fig. 7a and b. The color depth of the modified fabric is obviously improved, which indicates a mass of dye molecules had been absorbed into the fabric. Color tests of the fabric before and after modification are shown in Table S2. The chroma of the dyed MdC-Op is obviously enhanced. Red a* and yellow b* of the dyed MdC-Op is increased to 57.8 and 78.4, which is much higher than those of the dyed RC (27.2 of a* and 26.2 of b*). The brightness (L*) of the modified fabric is more dimmer than untreated fabric, illustrating the high dyeing depth of MdC-Op.
The comparisons between traditional cotton dyeing process and cationic cotton salt-free dyeing process
MdC-Op has a much higher dye utilization than RC, which could significantly reduce the difficulty of the sewage treatment.The influences of solution environment (NaOH) on the dyeing effect of MdC-Op with salt-free dyeing and RC with conventional dyeing were shown in Fig. 7c, d. The dyeing rate of MdC-Op could be optimized to about 90%, which is higher than that of reactive dyes on RC (85–87% of optimized dyeing rate and 82–85% of optimized fixation rate). The dyeing curves could be seen in Fig. S12 and S13. The values of the variables kept constant in Fig. 7c were 4% owf of reactive dyes, 60 °C of dyeing temperature, 30 min of dyeing time. The values of the variables kept constant in Fig. 7d was 4% owf of acid dyes, 80 °C of temperature and 30 min of dyeing time. The needed accessory ingredient for the optimized dyeing rate of MdC-Op is less than RC, which could reduce the process cost. The dyeing performances of acid dye and reactive dye on MdC-Op are shown in Fig. 7e. The comparable dyeing rate and less Sr of acid dye further confirm the superiority of acid dyes for dyeing. The dyeing fastnesses of the dyed fabrics are shown in Table 2. Acid dyes had very poor dyeing properties on untreated cotton fabrics. However, the dyeing fastnesses of the MdC-Op dyed by acid dyes were greatly improved, which is comparable to MdC-Op dyed by reactive dyes (with fixation). Therefore, acid dyes were confirmed further for salt-free dyeing MdC-Op.
Conclusions
In this study, CHPTAC as a micro-molecular quaternary amine is grafted onto the cotton. The modified cotton is dyed by acid dyes without salt, which is comparable to the dyeing performances of reactive dyes for untreated fabric. Both the modification reaction and the dyeing process are optimized. Adsorption models between dye molecules and fibers based on the theoretical simulation were explored. The preferable modification conditions are 30 g/L of modifier concentration, 10 g/L of alkali dose, 15 min of reaction time and 90 °C of modification temperature. The suitable dyeing conditions for acid dyes dyeing are 5.5 ~ 6.5 of pH value, 80 °C of temperature, 30 min of dyeing time. The adsorption mode of anionic acid dye and cationic cotton is Langmuir type due to the strong electrostatic attraction between modified fabric and dye molecules. The dyeing rate of the modified fabric can reach up to about 92% and the K/S value can reach to 21.99, which proves the great application prospects of MdC-Op in the field of salt-free dyeing for textile.
References
Ahmed HB, Mikhail MM, El-Sherbiny S, Nagy KS, Emam HE (2020) pH responsive intelligent nano-engineer of nanostructures applicable for discoloration of reactive dyes. J Coll Interface Sci 561:147–161. https://doi.org/10.1016/j.jcis.2019.11.060
Arivithamani N, Giri Dev VR (2018) Characterization and comparison of salt-free reactive dyed cationized cotton hosiery fabrics with that of conventional dyed cotton fabrics. J Clean Prod 183:579–589. https://doi.org/10.1016/j.jclepro.2018.02.175
Charola S, Yadav R, Das P, Maiti S (2018) Fixed-bed adsorption of Reactive Orange 84 dye onto activated carbon prepared from empty cotton flower agro-waste. Sustain Environ Res 28(6):298–308. https://doi.org/10.1016/j.serj.2018.09.003
Correia J, Mathur K, Bourham M, Oliveira FR, Siqueira Curto Valle RDC, Valle JAB, Seyam A-FM (2021) Surface functionalization of greige cotton knitted fabric through plasma and cationization for dyeing with reactive and acid dyes. Cellulose 28(15):9971–9990. https://doi.org/10.1007/s10570-021-04143-8
Correia J, Rainert KT, Oliveira FR, de Cássia Siqueira Curto Valle R, Valle JAB, (2020) Cationization of cotton fiber: an integrated view of cationic agents, processes variables, properties, market and future prospects. Cellulose 27(15):8527–8550. https://doi.org/10.1007/s10570-020-03361-w
Dong W, Zhou M, Li Y, Zhai S, Jin K, Fan Z, Zhao H, Zou W, Cai Z (2020) Low-salt dyeing of cotton fabric grafted with pH-responsive cationic polymer of polyelectrolyte 2-(N, N-dimethylamino)ethyl methacrylate. Coll Surf A. https://doi.org/10.1016/j.colsurfa.2020.124573
El-Masry AAM, Mosaad RM (2020) Multifunction finishing of cellulose based fabrics via 3-Chloro-2-hydroxypropyl trimethyl ammonium chloride (Quat-188) and silver nanoparticles (AgNPs) to improve its dyeability and antibacterial. Bio Res Appl Chem 11(4):11666–11678. https://doi.org/10.33263/briac114.1166611678
Eltaweil AS, Elgarhy GS, El-Subruiti GM, Omer AM (2020) Carboxymethyl cellulose/carboxylated graphene oxide composite microbeads for efficient adsorption of cationic methylene blue dye. Int J Biol Macromol 154:307–318. https://doi.org/10.1016/j.ijbiomac.2020.03.122
Fahim AM, Magd EEA-E (2021) Enhancement of Molecular imprinted polymer as organic fillers on bagasse cellulose fibers with biological evaluation and computational calculations. J Mol Struct. https://doi.org/10.1016/j.molstruc.2021.130660
Fang L, Ding L, Ren W, Hu H, Huang Y, Shao P, Yang L, Shi H, Ren Z, Han K, Luo X (2021) High exposure effect of the adsorption site significantly enhanced the adsorption capacity and removal rate: a case of adsorption of hexavalent chromium by quaternary ammonium polymers (QAPs). J Hazard Mater 416:125829. https://doi.org/10.1016/j.jhazmat.2021.125829
Gao S, Wang X, Song C, Zhou S, Yang F, Kong Y (2021) Engineering carbon-defects on ultrathin g-C3N4 allows one-pot output and dramatically boosts photoredox catalytic activity. Appl Catal B Environ. https://doi.org/10.1016/j.apcatb.2021.120272
Hauser PJ, Tabba AH (2001) Improving the environmental and economic aspects of cotton dyeing using a cationised cotton†. Color Technol 117(5):282–288. https://doi.org/10.1111/j.1478-4408.2001.tb00076.x
Hou A, Shi Y, Yu Y (2009) Preparation of the cellulose/silica hybrid containing cationic group by sol–gel crosslinking process and its dyeing properties. Carbohydr Polym 77(2):201–205. https://doi.org/10.1016/j.carbpol.2008.12.022
Jiang J, Duan D, Ma J, Jiang Y, Long R, Gao C, Xiong Y (2021) Van der waals heterostructures by single cobalt sites-anchored graphene and g-C3N4 nanosheets for photocatalytic syngas production with tunable CO/H2 ratio. Appl Catal B Environ. https://doi.org/10.1016/j.apcatb.2021.120261
Kamtsikakis A, Delepierre G, Weder C (2021) Cellulose nanocrystals as a tunable nanomaterial for pervaporation membranes with asymmetric transport properties. J Membr Sci 635:119473. https://doi.org/10.1016/j.memsci.2021.119473
Kaushik M, Basu K, Benoit C, Cirtiu CM, Vali H, Moores A (2015) Cellulose nanocrystals as chiral inducers: enantioselective catalysis and transmission electron microscopy 3D characterization. J Am Chem Soc 137(19):6124–6127. https://doi.org/10.1021/jacs.5b02034
Khatri A, Peerzada MH, Mohsin M, White M (2015) A review on developments in dyeing cotton fabrics with reactive dyes for reducing effluent pollution. J Clean Prod 87:50–57. https://doi.org/10.1016/j.jclepro.2014.09.017
Li M-C, Wu Q, Moon RJ, Hubbe MA, Bortner MJ (2021) Rheological aspects of cellulose nanomaterials: governing factors and emerging applications. Adv Mater 33(21):2006052. https://doi.org/10.1002/adma.202006052
Li M, Zhang L, Qiu M, Zhang Y, Fu S (2017) Dyeing property of fluorescent pigment latex on cationic knitted cotton fabrics. Text Res J 89(3):422–433. https://doi.org/10.1177/0040517517748494
Liang X, Liang B, Wei J, Zhong S, Zhang R, Yin Y, Zhang Y, Hu H, Huang Z (2020) A cellulose-based adsorbent with pendant groups of quaternary ammonium and amino for enhanced capture of aqueous Cr(VI). Int J Biol Macromol 148:802–810. https://doi.org/10.1016/j.ijbiomac.2020.01.184
Liu Q, Meng Z, Korpi A, Kontturi E, Kostiainen MA (2021) Cationic cellulose nanocrystals for fast, efficient and selective heparin recovery. Chem Eng J 420:129811. https://doi.org/10.1016/j.cej.2021.129811
Mohtashim Q, Rigout M, Carr C (2020) Investigation into the sequential application of cationic/anionic fixing agents to improve the oxidative washing resistance of CI Leuco Sulphur Black 1-dyed cotton fabric. Color Technol 136(3):263–269. https://doi.org/10.1111/cote.12460
Morgan JLW, McNamara JT, Fischer M, Rich J, Chen H-M, Withers SG, Zimmer J (2016) Observing cellulose biosynthesis and membrane translocation in crystallo. Nature 531(7594):329–334. https://doi.org/10.1038/nature16966
Mu B, Liu L, Li W, Yang Y (2019) High sorption of reactive dyes onto cotton controlled by chemical potential gradient for reduction of dyeing effluents. J Environ Manage 239:271–278. https://doi.org/10.1016/j.jenvman.2019.03.062
Nallathambi A, Venkateshwarapuram Rengaswami GD (2016) Salt-free reactive dyeing of cotton hosiery fabrics by exhaust application of cationic agent. Carbohydr Polym 152:1–11. https://doi.org/10.1016/j.carbpol.2016.06.087
Nallathambi A, Venkateshwarapuram Rengaswami GD (2017) Industrial scale salt-free reactive dyeing of cationized cotton fabric with different reactive dye chemistry. Carbohydr Polym 174:137–145. https://doi.org/10.1016/j.carbpol.2017.06.045
Niu T, Wang X, Wu C, Sun D, Zhang X, Chen Z, Fang L (2020) Chemical modification of cotton fabrics by a bifunctional cationic polymer for salt-free reactive dyeing. ACS Omega 5(25):15409–15416. https://doi.org/10.1021/acsomega.0c01530
Pal S, Mal D, Singh RP (2005) Cationic starch: an effective flocculating agent. Carbohydr Polym 59(4):417–423. https://doi.org/10.1016/j.carbpol.2004.06.047
Park S, Baker JO, Himmel ME, Parilla PA, Johnson DK (2010) Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnol Biofuels 3(1):10. https://doi.org/10.1186/1754-6834-3-10
Patiño A, Canal C, Rodríguez C, Caballero G, Navarro A, Canal JM (2011) Surface and bulk cotton fibre modifications: plasma and cationization. Influence on dyeing with reactive dye. Cellulose 18(4):1073–1083
Pei L, Gu X, Wang J (2021) Sustainable dyeing of cotton fabric with reactive dye in silicone oil emulsion for improving dye uptake and reducing wastewater. Cellulose 28(4):2537–2550. https://doi.org/10.1007/s10570-020-03673-x
Peng H, Xie R, Fang K, Cao C, Qi Y, Ren Y, Chen W (2021) Effect of diethylene glycol on the inkjet printability of reactive dye solution for cotton fabrics. Langmuir 37(4):1493–1500. https://doi.org/10.1021/acs.langmuir.0c03016
Qin Y, Chen L, Wang X, Zhao X, Wang F (2011) Enhanced mechanical performance of poly(propylene carbonate) via hydrogen bonding interaction with o-lauroyl chitosan. Carbohydr Polym 84(1):329–334. https://doi.org/10.1016/j.carbpol.2010.11.045
Ristic N, Ristic I (2012) Cationic modification of cotton fabrics and reactive dyeing characteristics. J Eng Fibers Fabrics 7(4):155892501200700400. https://doi.org/10.1177/155892501200700408
Siddiqua UH, Ali S, Iqbal M, Hussain T (2017) Relationship between structure and dyeing properties of reactive dyes for cotton dyeing. J Mol Liq 241:839–844. https://doi.org/10.1016/j.molliq.2017.04.057
Teng X, Zhang S, Ma W (2011) Application of a hydrolyzable cationic agent, poly(acryloxyethyl trimethylammonium chloride), in salt-free reactive dyeing for good dyeing properties. J Appl Polym Sci 122(4):2741–2748. https://doi.org/10.1002/app.34023
Urbina L, Corcuera MÁ, Gabilondo N, Eceiza A, Retegi A (2021) A review of bacterial cellulose: sustainable production from agricultural waste and applications in various fields. Cellulose. https://doi.org/10.1007/s10570-021-04020-4
Wang A, Xia L, Zhou S, Guo H, Wang Y, Xu W (2020a) Solvent assisted reactive dyeing of cotton with high exhaustion in the absence of salt. J Clean Prod 267:122062. https://doi.org/10.1016/j.jclepro.2020.122062
Wang L, Yan K, Hu C, Ji B (2020b) Preparation and investigation of a stable hybrid inkjet printing ink of reactive dye and CHPTAC. Dyes Pigments. https://doi.org/10.1016/j.dyepig.2020.108584
Wang R, Fang K, Ren Y, Song Y, Zhang K, Bukhari MN (2019) Jetting performance of two lactam compounds in reactive dye solution. J Mol Liq 294:111668. https://doi.org/10.1016/j.molliq.2019.111668
Wang W-Y, Kan C-W (2020) An eco-friendly dyeing method: bromophenol blue (BPB) applied for dyeing cotton fabrics coated with cationic finishing agents. Cellulose 27(15):9045–9059. https://doi.org/10.1007/s10570-020-03375-4
Wang W, Hu J, Zhang R, Yan C, Cui L, Zhu J (2021) A pH-responsive carboxymethyl cellulose/chitosan hydrogel for adsorption and desorption of anionic and cationic dyes. Cellulose 28(2):897–909. https://doi.org/10.1007/s10570-020-03561-4
Yang Y, Li M, Fu S (2021) Colored cotton fabric with hydrophobicity prepared by monodispersed cationic colored polymer nanospheres. Coll Polym Sci. https://doi.org/10.1007/s00396-021-04855-0
Yao Y, Zhou D, Shen Y, Wu H, Wang H (2021) Morphology-controllable amphiphilic cellulose microgels made from self-assembly of hydrophobic long-chain bromide-alkylated-cellulose/gelatin copolymer. Carbohydr Polym 269:118265. https://doi.org/10.1016/j.carbpol.2021.118265
Yu C, Lu Y, Chang Y, Liang S, Tao K (2019) Facile salt-free process for cotton fabric dyeing: pad-irradiate-pad-steam process using 3-chloro-2-hydroxypropyl trimethyl ammonium chloride. Environ Prog Sustain Energy 38(6):e13252. https://doi.org/10.1002/ep.13252
Zhai S, Fan Z, Jin K, Zhou M, Zhao H, Zhao Y, Ge F, Li X, Cai Z (2020) Synthesis of zinc sulfide/copper sulfide/porous carbonized cotton nanocomposites for flexible supercapacitor and recyclable photocatalysis with high performance. J Coll Interface Sci 575:306–316. https://doi.org/10.1016/j.jcis.2020.04.073
Zhai S, Li M, Jin Z, Sun J, zhao h, Cai Z, Zhao Y, (2021a) Fabrication of flexible wearable electrodes based on carbon nanotubes/nickel/nickelous hydroxide ternary composites by a facile single side printing technology. Dalton Trans. https://doi.org/10.1039/D1DT01679B
Zhai S, Luo Y, Fan Z, Zhou M, Hou K, Zhao H, Hu M, Xiao Y, Jin K, Zhao Y, Li X, Cai Z (2021b) Porous carbonized cotton loaded with Zn–Cu–M(M=O, S) nanocomposites for electrochemical energy storage and oxygen evolution reaction. Mat Today Energy 21:100806. https://doi.org/10.1016/j.mtener.2021.100806
Zhang F, Chen Y, Lin H, Lu Y (2007) Synthesis of an amino-terminated hyperbranched polymer and its application in reactive dyeing on cotton as a salt-free dyeing auxiliary. Color Technol 123(6):351–357. https://doi.org/10.1111/j.1478-4408.2007.00108.x
Zhu Z, Huang Z, Huang W, Wen H, Zhang J, Wang P, Peng Y, Liu C (2021) Polymer brush-grafted cotton fiber for the efficient removal of aromatic halogenated disinfection by-products in drinking water. J Coll Interface Sci 597:66–74. https://doi.org/10.1016/j.jcis.2021.03.084
Acknowledgments
This work was funded by the Fundamental Research Funds for the Central Universities and Graduate Student Innovation Fund of Donghua University (CUSF-DH-D-2020065) and China Scholarship Council (No. 202006630075).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhai, S., Li, Y., Dong, W. et al. Cationic cotton modified by 3-chloro-2-hydroxypropyl trimethyl ammonium chloride for salt-free dyeing with high levelling performance. Cellulose 29, 633–646 (2022). https://doi.org/10.1007/s10570-021-04295-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-021-04295-7