Abstract
The surface carboxyl and sulfate groups on cellulose nanowhiskers were quantified via the adsorption of toluidine blue O (TBO), a cationic dye. Here, simple and rapid protocols, such as mixing the nanowhisker suspensions with a dye solution, separating the supernatants via centrifugation, and determining the excess dye concentration via visible light absorbance techniques, were used to obtain reproducible results comparable with those obtained via titration. In addition to facilitating the discrete quantification of the sulfate and carboxyl groups, the TBO adsorption method enabled carboxyl quantification in the presence of mercapto groups, which was difficult to achieve via titration. The adsorption of TBO onto the carboxyl groups was completed within 30 min, enabling the rapid treatment of many samples within a short period.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Various nanocellulose materials, including cellulose nanowhiskers (CNWs) (Araki 2013; de Souza Lima and Borsali 2004; Eichhorn 2011), cellulose nanofibers (CNFs) (Isogai et al. 2011), and cellulose nanocrystals (CNCs) (Habibi et al. 2010), contain different types of surface functional groups, especially ionic groups. These ionic surface functional groups include sulfates (sulfate half-esters) which are introduced onto the surface of CNWs (Araki 2013; Araki et al. 1998; de Souza Lima and Borsali 2004; Eichhorn 2011) or CNCs (Habibi et al. 2010) during sulfuric acid-mediated hydrolysis. The carboxyl or phosphate groups can also be introduced via the surface oxidation of CNWs/CNFs (Araki 2013; de Souza Lima and Borsali 2004; Eichhorn 2011; Isogai et al. 2011) and the post-phosphorylation of CNWs (Araki et al. 2000a), respectively. These ionic moieties work as charged groups through their dissociation in water, which avoid the aggregation and sedimentation of the nanocellulose particles/fibers because of electrostatic repulsions, resulting in a good stability of the aqueous nanowhisker dispersions (Araki 2013; de Souza Lima and Borsali 2004; Eichhorn 2011; Habibi et al. 2010; Isogai et al. 2011). Therefore, the introduction of these charged surface groups is crucial for achieving good dispersion without aggregation. Furthermore, various types and contents of such charged surface groups affect the properties of the nanocellulose aqueous suspensions, including the suspension viscosity (Araki et al. 1998, 1999, 2000a), liquid–crystal formation (Araki et al. 2000b; Araki and Kuga 2001), or heat resistance (Fukuzumi et al. 2010). The controlled introduction of these surface functional groups will help in grafting other functional molecules and polymers as possible reactive sites (Araki et al. 2001, 2002). Given all the above factors, techniques for quantitatively determining the charged surface group contents are indispensable for targeted control of the properties of nanocellulose, thereby furthering research efforts on various functional materials containing nanocellulose.
Many techniques have been developed and utilized for the quantification of the charged surface groups of CNWs, CNFs, and CNCs. One of the most typical methods is acid–base titration in which the pH (Batmaz et al. 2014) and conductivity (Araki et al. 1998, 2000a; Bondeson et al. 2006; ISO 21400, 2018; Johnston et al. 2018) of the samples are monitored. Other methods, including headspace gas chromatography (Chai et al. 2003; Wu et al. 2018), gel permeation chromatography after fluorescent derivatization (Bohrn et al. 2006), 13C NMR spectroscopy (Kumar and Yang 1999), and zeta potential measurements (Zhu et al. 2013), were also examined. Among the various methods available, conductometric titration is the simplest and most widely used method because it facilitates the quantification of strong and weak acid moieties (e.g., sulfate and carboxyl groups, respectively). However, the titration method exhibits some disadvantages, including a lengthy measurement time with laborious procedures, the use of expensive apparatus for automation, and a lengthy and complicated pretreatment procedure for the samples (e.g., ion exchange resin treatment may take several hours) (ISO 21400, 2018). Therefore, an alternative method is required for rapid and facile quantification using common analytical instruments.
The use of surface adsorption techniques using cationic dyes, such as methylene blue (MB) or toluidine blue O (TBO), has gained popularity as another method for quantitative analysis of the carboxyl content of cellulosic materials. This method is based on the stoichiometric formation of ionic pairs between the dye molecules and the respective carboxyl groups. Quantification based on MB adsorption was developed early in the 1940s (Davidson 1948; Wilson and Mandel 1961) and was incorporated into the TAPPI guidelines for pulp and paper (TAPPI T237 om-93). Although the method was later excluded from the TAPPI standards because of its complexity and time-consuming nature, the recent TAPPI description agrees that the MB adsorption method is still useful for analyzing small sample quantities or samples with a very low carboxyl content. Further, the dye adsorption method was applied for quantification of the carboxyl contents on various solid supports (Rödiger et al. 2011; Sano et al. 1993; Tiraferra and Elimelech 2012). Majority of the reported protocols include multistep processes, including the addition of excess dye molecules relative to the carboxyl content to be determined, thorough washing of the unadsorbed dye, and quantitative liberation and measurements of the adsorbed dye. However, the method initially used to analyze cellulosic materials (Wilson and Mandel 1961) seems to be simpler and more straightforward; namely, the carboxyl content could be accurately quantified by directly determining the unadsorbed dye amounts in the supernatant after a reaction of the solid cellulosic materials in an excess dye solution. Although this method offers many advantages, it seems to be difficult to apply it to nanocellulose materials because of the difficulty associated with the separation of the supernatant dye solution from the dye-saturated nanocellulose solid particles. The filtration of the nanosized samples seems to be unreasonable, and collection via centrifugation seems to be implausible because majority of the nanocelluloses are well-dispersed colloids or nanofibers. Therefore, carboxyl groups quantification by the dye adsorption method have never been applied to nanocellulose materials. Very recently (just after the first submission of the present article), quite a similar approach of uses of MB dye adsorption for surface charge quantification of nanocellulose samples was published (Yan et al. 2021).
Herein, the dye adsorption method was examined to quantify the anionic surface groups, especially carboxyl groups, of CNWs to establish optimized protocols. The developed methods exhibited excellent reproducibility, and the results were sufficiently comparable to those obtained via conventional titration methods. One major advantage is the trace amounts of the samples (0.5–2.5 mg per measurement) required for accurate results, as well as brief and facile procedures. In addition to the discrete quantification of the sulfate and carboxyl contents in CNW samples, which could also be achieved using the conventional titration methods, the newly developed method was extremely selective toward the carboxyl groups, even in a complex sample matrix containing other weakly acidic groups such as mercapto groups. Carboxyl quantification in such a complex sample matrix was improbable using the conventional titration method.
Materials and methods
Complete experimental details about the measurement procedures, sample preparation, and analytical protocols are presented in the Supplementary Information. An overview of the protocol is presented in Scheme 1. Briefly, an aqueous suspension of the CNWs was mixed with a TBO standard solution of a known concentration in water (for quantifying the carboxyls or total anionic surface groups) or in aqueous HCl under pH = 1 (for quantifying strong acid groups). After a sufficiently long shaking period, the supernatant was separated from the dye-adsorbed CNWs via centrifugation. Then, the dye concentration in the pipetted supernatant was determined after being diluted using the appropriate dilution factor. The concentration of the dye in the supernatant was calculated, and the results were subtracted from the initial dye content to obtain the content of the anionic surface groups in the CNWs. The relevant equation is presented in the Supplementary Information.
As noted in the Supplementary material, it is crucial to avoid the use of any kinds of glassware during the experiment because the positively charged TBO molecules are readily adsorbed onto the glass surface, causing the results to become inaccurate.
Results and discussion
Figure 1 shows the relation between the carboxyl content obtained via the titration and TBO adsorption methods for various samples, including CNWs, TEMPO-oxidized CNF, CCNWs with different degrees of oxidation, CNW/CCNW100 mixtures containing various composition ratios. It should be noted that the titration measurements were not directly conducted for the series of CNW/CCNW100 mixed samples; instead, their “titration” values were calculated using the titration values of CNW and CCNW100 based on their mixing ratios. Figure 1 clearly indicates that the values obtained using the dye adsorption methods were consistent with those by titration. The developed method exhibited excellent reproducibility, as indicated by the narrow deviations in Fig. 1, which indicates sufficiently narrow deviations to be hidden by the symbols. Although previous studies performed the dye adsorption processes for the surface carboxyls under buffered or conditioned pH experimental conditions (Davidson 1948; Fardim et al. 2005; Rödiger et al. 2011; Sanoet al. 1993; Tiraferri and Elimelech 2012; Wilson and Mandel 1961), the results of this study indicated that quite satisfactory results could be obtained (for the stoichiometric adsorption of TBO on the surface carboxyls) without an addition of electrolytes. The use of electrolytes should be avoided because the presence of some types of electrolytes suppressed the dye adsorption process, affecting the experimental results (Soedjak 1994). Figure 1 indicates the results of similar dye adsorption measurements performed under “pH = 1” conditions (red symbols. see Supplementary Information for details); here, the calculated apparent carboxyl content results were almost negligible at pH = 1 under the employed measurement conditions.
Comparisons of the carboxyl contents determined via titration and TBO adsorption. The black and red symbols correspond to the measurements conducted “in water” and those in “pH = 1,” respectively. Filled circle and cross, CNWs; open circles, CCNWs with different levels of surface carboxylation; open diamonds, CNWs/CCNW100 mixtures at different mixing ratios; and filled triangles, TEMPO-oxidized CNF
At pH = 1, the TBO adsorption onto the surface carboxyls, which are present in their acidic form (–COOH), is suppressed. In contrast, the surface sulfate groups are expected to still interact with TBO under these conditions because they are dissociated. Therefore, the sulfate and carboxyl contents can be estimated by focusing on the differences in the dye adsorption values obtained under “neutral water” and “pH = 1” conditions (in the actual calculation, vary trace TBO consumption at pH = 1 were also taken into consideration for more accurate estimation; see Supplementary Information). Thus, the dye adsorption measurements were performed both in water and at pH = 1 for several samples obtained via sulfuric acid-mediated hydrolysis under various hydrolyzing conditions containing different amounts of surface sulfate/carboxyl groups (Table 1).
The content of the surface sulfate/carboxyl groups in the nanocellulose materials was estimated based on the protocol described in the Supplementary Information (also see Table 1). The values obtained via TBO adsorption were consistent with those obtained using the conventional titration technique for majority of the tested samples. Although there seems to be some differences between the sulfate group amounts obtained by titration and those by TBO adsorption, plotting of them indicated quite a satisfactorily linear relationship between them, as shown in Fig. 2. Therefore, the sulfate amounts obtained by TBO adsorption can be readily converted into relevant “titrated” values using the relationship shown in Fig. 2 (i.e. y = 0.831x) and can be compared with values in previous publications. On the other hands, some of the carboxyl contents obtained by TBO adsorption in Table 1 indicated quite a trace negative values. It seems to be because that CNWs obtained by sulfuric acid hydrolysis have almost no carboxyls; in other words, their carboxyl contents might be below detection limits, which may lie around 0.05 mmol/g. Such low carboxyl contents would be also difficult to be detected accurately by titration.
Comparisons of the sulfate/carboxyl contents of the CNWs in Table 1, determined via titration and TBO adsorption. Filled circles, sulfate group contents; open circles, carboxyl group contents. The dotted line indicates a linear relationship of y = 0.831x
All the results presented in Table 1 and Fig. 2 exhibited general agreement between the two quantitative methods within a comparable order of magnitude, indicating that the TBO adsorption method could be applied for the quantification of the surface sulfate/carboxyl groups of nanocellulose materials with high accuracy and reproducibility.
The TBO adsorption method may offer another advantage over the traditional titration method because it facilitates the quantification of carboxyl content in the presence of other weakly acidic groups such as mercapto groups, which is considerably difficult to achieve using the titration method. Previously, surface mercapto groups were introduced onto the PEG-grafted CCNWs by grafting 2-aminoethanethiol into the surface carboxyl groups (Urata and Araki 2020). It was difficult to determine the carboxyl and mercapto contents separately by titration, because both functional groups were weakly acidic. Therefore, it was almost impossible to differentiate the results obtained using the grafted 2-aminoethanethiol and those obtained using the residual carboxyl groups. Because these two functional groups had different pKa values, i.e., 3–4 and 11–13 for the carboxyl and mercapto groups, respectively, it was theorized that maintaining the pH of the system within a defined range (e.g., approximately 7) would promote the preferential dissociation of one group (in this case, the carboxyls) over the other group. At pH 7, the mercapto groups remain protonated, whereas the carboxyl groups are dissociated. Thus, TBO would only adsorb onto the carboxyls under pH 7, whereas the mercapto groups would remain unaffected.
This strategy was verified by mixing the CCNW50 sample with various amounts of silica containing surface—SH groups and subjecting the mixtures to both the quantitative methods. The results are summarized in Table 2. The values obtained via the titration method are listed as “weakly acidic group contents” and not as “carboxyl contents.” The conductometric titration curves of these samples indicated that there were only two inflection points that corresponded to the neutralization (end point) of the strongly and weakly acidic groups. The former corresponded to the known concentration of HCl added prior to titration, whereas the latter indicated the sum of the carboxyl and mercapto groups because of no differentiation within the weakly acidic region. Based on Table 2, the amount of weakly acidic groups quantified via titration was not equal to the sum of the carboxyl and mercapto groups. Against our initial presumption, the weak acidic group amounts indicated relatively minor fluctuations, not an increase with an increase of mercapto groups. Although a reasonable explanation for this observation has not yet been obtained, Table 2 indicates the unnegligible effect exerted by the mercapto groups on carboxyl quantification. The carboxyl content values obtained via TBO adsorption were consistent with the results obtained using the CCNW50 sample regardless of the mixing ratios because there was no interference due to the presence of the mercapto groups. These observations indicate the superiority of the TBO adsorption method because it facilitated accurate carboxyl quantification even in the presence of mercapto groups, which was an impossible task when using the titration method.
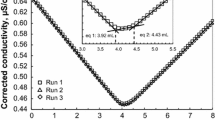
Finally, the changes in the adsorbed amount of TBO in the given mixing period were examined. Here, aliquots were sequentially pipetted from one stock reaction vessel at a given time, followed by the analysis of each aliquot to determine the adsorption amounts and the apparent carboxyl content with respect to the reaction/mixing time. If the TBO adsorption required considerable reaction/mixing times, the initial adsorption process became slower with a gradual increase as time progressed, affecting the apparent carboxyl content. Figure 3 showed that the apparent carboxyl content values were similar to the actual carboxyl content values 30 min after mixing and that the value remained constant for a further 24 h. The carboxyl content values after the 24-h TBO adsorption process are expressed using the dotted lines and numbers in Fig. 3. The results showed that the interactions between the surface carboxyl groups and TBO were complete after 30 min. Although all of the TBO adsorption results in the above-mentioned studies were obtained after overnight treatment, Fig. 3 clearly indicates that a 2-h mixing time is sufficient for the stoichiometric adsorption of TBO.
The actual values of all measurements, shown by Figs. 1 and 2 and Tables 1 and 2, were summarized in Table S1 in Supplementary Information, together with standard deviation values and analysis of variance (ANOVA) results. Most of the compared values indicated significant differences (e.g. P < 0.05 or P < 0.01) due to quite an excellent reproducibility (in other words, narrow standard deviations) of both titration and TBO adsorption methods.
Since CNWs are inherently well-dispersed colloidal particles, it is also quite important to prove a complete absence of TBO-adsorbed CNWs in the supernatant after adsorption and centrifugation steps. Their incomplete sedimentation by centrifugation will lead to an increase in TBO amount in the supernatant, resulting in underestimation of adsorbed TBO. The author conducted FT-IR and wide angle X-ray measurements of freeze-dried supernatant solutions obtained from different CNW examinations (W-70C10M, W-45C60M, and C-45C30M); both the results indicated quite a perfect absence of CNWs in the supernatant liquid, as shown in Figure S2.
Carboxyl quantification utilizing the TBO adsorption method developed in this study exhibited various advantages that the titration protocol could not offer. The TBO method yielded reproducible, quantitative results using trace samples of 0.5–2.5 mg of solid per measurement, and the values obtained were comparable with those obtained via titration. Unlike the lengthy, laborious efforts necessary for the titration method, the TBO adsorption required just a simple mixing of the samples with the dye solution and a subsequent 2-h shaking process that could be easily automated. The simple, rapid post-procedure protocols of dilution and measurement using a common UV–Vis spectrophotometer (unlike the unpopular automatic titrator) enabled the high-throughput analysis of large numbers of samples. Several tens of measurements can be conducted within a day, which was totally impossible to achieve using the titration method. Further, the TBO adsorption method facilitated the separate quantification of strongly and weakly acidic groups, and the targeted carboxyl quantification could be performed in the presence of different weak acidic moieties.
The nature of TBO adsorption indicated that all the stages, ranging from the mixing of the samples with the dye solutions to the application of the measurement protocols, provided the results automatically and arithmetically, thereby allowing almost no introduction of a human error into the entire process. On the other hand, the titration method is susceptible to biases because the interpretations of the results, especially line fitting of each area, were influenced by the operator. The author anticipate that the TBO adsorption method will find widespread applicability for the rapid and precise quantification of the surface functional groups in nanocellulose materials.
Supplementary information
Full experimental details, including the starting materials and reagents as well as protocols for conducting the TBO adsorption measurements “in water” and under “pH = 1,” are reported in the Supplementary material. An example of the calibration curves indicating the relations between TBO concentrations and the absorbance at 628 nm both “in water” and in “pH = 1” are shown in the Supplementary Information. The results of FT-IR and wide angle X-ray diffraction analyses of the freeze-dried supernatants are also shown, together with those of recrystallized TBO. All of the sulfate/carboxyl contents obtained by both titration and TBO adsorption were summarized in Table S2 together with probability parameters obtained by ANOVA analyses.
The following files are available free of charge.
An Excel worksheet for automatic calculation of surface carboxyl/sulfate groups (Worksheet for dye adsorption.xlsx).
These materials are available free of charge via the Internet.
Change history
01 August 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10570-022-04758-5
References
Araki J (2013) Electrostatic or steric?–Preparations and characterizations of well-dispersed systems containing rod-like nanowhiskers of crystalline polysaccharides. Soft Matter 9:4125–4141
Araki J, Kuga S (2001) Effect of trace electrolyte on liquid crystal type of cellulose microcrystals. Langmuir 17:4493–4496
Araki J, Kuga S, Magoshi J (2002) Influence of reagent addition on carbodiimide-mediated amidation for poly(ethylene glycol) grafting. J Appl Polym Sci 85:1349–1352
Araki J, Wada M, Kuga S (2001) Steric stabilization of a cellulose microcrystal suspension by poly(ethylene glycol) grafting. Langmuir 17:21–27
Araki J, Wada M, Kuga S, Okano T (1998) Flow properties of microcrystalline cellulose suspension prepared by acid treatment of native cellulose. Coll Surf A 142:75–82
Araki J, Wada M, Kuga S, Okano T (1999) Influence of surface charge on viscosity behavior of cellulose microcrystal suspension. J Wood Sci 45:261–258
Araki J, Wada M, Kuga S, Okano T (2000a) Influence of surface charge on viscosity anomaly of microcrystalline cellulose suspensions. In: Nishinari K (ed) Hydrocolloids: physical chemistry and industrial application of gels, polysaccharides, and proteins. Elsevier, Amsterdam, pp 283–288
Araki J, Wada M, Kuga S, Okano T (2000b) Birefringent glassy phase of a cellulose microcrystal suspension. Langmuir 16:2413–2415
Batmaz R, Mohammed N, Zaman M, Minhas G, Berry RM, Tam KC (2014) Cellulose nanocrystals as promising adsorbents for the removal of cationic dyes. Cellulose 21:1655–1665
Bohrn R, Potthast A, Schiehser S, Rosenau T, Sixta H, Kosma P (2006) The FDAM method: determination of carboxyl profiles in cellulosic materials by combining group-selective fluorescence labeling with GPC. Biomacromol 7:1743–1750
Bondeson D, Mathew A, Oksman K (2006) Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 13:171–180
Chai XS, Hou QX, Zhu JY, Chen S, Wang SF, Lucia L (2003) Carboxyl groups in wood fibers. 1. Determination of carboxyl groups by headspace gas chromatography. Ind Eng Chem Res 42:5440–5444
Davidson GF (1948) 6 – The acidic properties of cotton cellulose and derived oxycelluloses. Part II. The absorption of methylene blue. J Textile Institute Trans 39:T65–T86
de Souza Lima MM, Borsali R (2004) Rodlike cellulose microcrystals: Structure, properties and applications. Macromol Rapid Commun 25:771–787
Eichhorn SJ (2011) Cellulose nanowhiskers: promising materials for advanced applications. Soft Matter 7:303–315
Fardim P, Moreno T, Holmbom B (2005) Anionic groups on cellulosic fiber surfaces investigated by XPS, FTIR-ATR, and different sorption methods. J Colloid Interface Sci 290:383–391
Fukuzumi H, Saito T, Okita Y, Isogai A (2010) Thermal stabilization of TEMPO-oxidized cellulose. Polym Degrad Stab 95:1502–1508
Habibi Y, Lucia LA, Rojas OJ (2010) Cellulose nanocrystals: chemistry, self-assembly, and applications. Chem Rev 110:3479–3500
ISO 21400 (2018). Pulp – Determination of cellulose nanocrystal sulfur and sulfate half-ester content. https://www.iso.org/standard/70861.html
Isogai A, Saito T, Fukuzumi H (2011) TEMPO-oxidized cellulose nanofibers. Nanoscale 3:71–85
Johnston LJ, Jakubek ZJ, Beck S, Araki J, Cranston ED, Danumah C, Fox D, Li H, Wang J, Mester Z, Moores A, Murphy K, Rabb SA, Rudie A, Stephan C (2018) Determination of sulfur and sulfate half-ester content in cellulose nanocrystals: an interlaboratory comparison. Metrologia 55:872
Kumar V, Yang T (1999) Analysis of carboxyl content in oxidized celluloses by solid-state 13C CP/MAS NMR spectroscopy. Int J Pharm 184:219–226
Rödiger S, Ruhland M, Schmidt C, Schröder C, Grossmann K, Böhm A, Nitschke J, Berger I, Schimke I, Schierack P (2011) Fluorescence dye adsorption assay to quantify carboxyl groups on the surface of poly(methyl methacrylate) microbeads. Anal Chem 83:3379–3385
Sano S, Kato K, Ikada Y (1993) Introduction of functional groups onto the surface of polyethylene for protein immobilization. Biomaterials 14:817–822
Soedjak HS (1994) Colorimetric determination of carrageenans and other anionic hydrocolloids with methylene blue. Anal Chem 66:4514–4518
Tiraferri A, Elimelech M (2012) Direct quantification of negatively charged functional groups on membrane surfaces. J Membrane Sci 389:499–508
Urata T, Araki J (2020) Adsorption of silver nanoparticles onto cellulose nanowhiskers incorporating thiol groups. Manuscript in preparation (presented at Tsukuba Global Science Week 2020 (TGSW2020), September 2020, Tsukuba, Japan)
Wilson WK, Mandel J (1961) Determination of carboxyl in cellulose. Comparison of various methods TAPPI-ACS-ASTM-ICCA subcommittee on determination of carboxyl. Tappi 44:131–137
Wu N, Wan X, Liu B, Chai XS (2018) Headspace A simple and efficient headspace gas chromatographic method for the determination of carboxyl groups in nanofibrillated cellulose. Cellulose 25:953–959
Yan N, Chai XS, Runge T (2021) A simple multi-wavelength spectroscopic method for the determination of carboxyl group content in nanocellulose. Cellulose 28:2805–2811
Zhu S, Panne U, Rurack K (2013) A rapid method for the assessment of the surface group density of carboxylic acid-functionalized polystyrene microparticles. Analyst 138:2924–2930
Acknowledgments
The author is grateful to Hyogo Okuda, Minami Nakajima, Takafumi Shimizu, Torino Kosaka, Teppei Ueda, Naoya Ohsawa, Yoichi Sasaki, Itsuki Takebe, Yasumasa Honmura, Erika Kotaki, and Takahiro Suzuki (Shinshu University) for conducting the titration experiments of the various nanowhisker samples. Yusuke Okada and Sachiko Yoshioka (Shinshu University) are acknowledged for their kind assistance in X-ray diffraction and FT-IR measurements, respectively. The author acknowledges DKS Co. Ltd. for kind donation of the TEMPO-oxidized cellulose nanofiber sample.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that they are relevant to the content of this article.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Araki, J. Dye adsorption revisited: application of the cationic dye adsorption method for the quantitative determination of the acidic surface groups of nanocellulose materials. Cellulose 28, 7707–7715 (2021). https://doi.org/10.1007/s10570-021-04035-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-021-04035-x