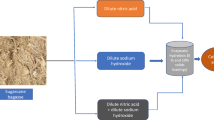
Abstract
In an enzymatically driven lignocellulosic biorefinery, pretreatment and hydrolysis modules are the two most significant cost contributors for obtaining high gravity sugar solutions. The present study aimed to reduce the use of alkali and Cellic CTec2 during the bioprocessing of sugarcane bagasse (SCB). Later its impact on the overall process economics and the environment was evaluated. During pretreatment, solid loading of 15% (w/w) and use of 2% (w/v) sodium hydroxide at 121 °C for 30 min emerged as an optimum strategy. It resulted in > 65% delignification of SCB, retaining ≥ 90% and 65% of glucan and xylan fraction, respectively, in the pretreated biomass. Two approaches were evaluated in parallel to minimize the requirement of this commercial cellulase enzyme blend. The first strategy involved its partial replacement with an in-house enzyme cocktail by blending. The second route was performing hydrolysis with reduced loadings of cellulase enzyme blend above its optimum temperature, which gave more promising results. Hydrolysis of 20% alkali pretreated SCB with cellulase enzyme blend dosed at 15 mg protein g−1 glucan led to 84.13 ± 1 and 83.5 ± 2.3% glucan and xylan conversion yields respectively in 48 h at 52.5 °C. The filtrate and wash fraction contained ≥ 165 and ≥ 65 g L−1 sugar monomers representing glucose and xylose. However, in both the fractions > 75%, sugar accounted for glucose. The techno-economic analysis revealed that the sugar production cost from SCB was 1.32 US$/kg, with the optimized bioprocess. Environmental impact study showed that the process contributed to 1.57 kg CO2 eq in terms of climate change.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lignocellulosic biomass (LCB) is the most abundant natural source of renewable carbon in the terrestrial biosphere. Two insoluble polysaccharides, namely cellulose and hemicellulose, chemically interact with complex heterogeneous aromatic polymer lignin and constitute the underlying architecture of LCB (Zoghlami and Paës 2019). Especially, structural carbohydrates are the sustainable source of sugars upon depolymerization. The released sugars can be transformed into a broad spectrum of industrially important bio-based chemicals and fuels using the biochemical platform (Chandel et al. 2020). However, the critical challenge to harness their true potential lies in overcoming the barrier of "biomass recalcitrance". Several direct and indirect factors act as negative drivers in the depolymerization of embedded sugars. These include surface area accessibility, content and lignin composition, degree of lignin condensation, chain length and crystallinity of cellulose, the particle size of LCB, pore size, and volume (Zhao et al. 2012a, b; Yoo et al. 2020).
The pretreatment is a pre-requisite step for facilitating enzymatic hydrolysis of sugar-polymers (Baruah et al. 2018; Antunes et al. 2019). Through different mechanisms, these physical, chemical, physicochemical, or biological processes and their combinations alter the structure and composition of LCB in a way that enhances the accessibility of carbohydrate-active enzymes (CAZy) for their target substrates (Baruah et al. 2018; Antunes et al. 2019; Galbe and Wallberg 2019). Irrespective of the pretreatment method chosen, this step is considered the most expensive, either being energy or capital intensive or owing to the cost of chemicals and requirements of specialized equipment for LCB processing (Harmsen et al. 2010; Valdivia et al. 2016). It can contribute to about 30–40% of the total cost of LCB processing (Kumar et al. 2019; Rahmati et al. 2020). Pretreatment has a pervasive impact on the cost of all the biological processing operations, downstream, being the first unit operation.
Similarly, after feedstock production, enzymatic saccharification is the second most cost-contributing module. The cost of enzyme alone can contribute to 30% of the total operating expenditure (OPEX), as reviewed by Valdivia et al. (2016). Ironically, the second-generation biorefinery is primarily driven by commercial biofuel cellulases, with few industries having the monopoly. Hence, a new trend is emerging, wherein researchers are combining the bioprocesses developed with economic and environmental indicators to understand potential hotspots that pose challenges to its overall sustainability (Ögmundarson et al. 2020). In this regard, techno-economic analysis (TEA) is the most popular methodology framework that discretely identifies the process lacunas, benefits, risks and uncertainties associated with the processes. Before the scale-up, its integration can help the researchers address critical issues on a priority basis, mitigate them, and improvise (Saini et al. 2020). However, it has been observed that this vital tool is primarily intended to decipher the cost of specific products. Very recently, Cheng et al. (2019) have given a compelling argument that the process economics of lignocellulosic sugar production is more crucial than particular products. In view of "sugars" serving as the "raw material", its production cost can be a key bottleneck for commercializing any second-generation bio-based product, whether biofuels or biochemicals.
Understanding the significance and criticality of this rationale, the present study focused on the cost-effective production of high-gravity sugar solutions. Improvements in pretreatment and saccharification were targeted during the biorefining of sugarcane bagasse (SCB). Later, we integrated the experimental processes with TEA and life cycle assessment (LCA) to decipher the cost of lignocellulosic sugars and assess their environmental implications.
Our previous study demonstrated an efficient alkali pretreatment that resulted in ~ 84.5% cellulose conversion and liberating ≥ 100 g L−1glucose from 18% biomass with 24 h when Cellic CTec2 was dosed at 25 mg protein g−1glucan (Baral et al. 2020a). Despite higher sugar yields, the process consumed relatively high NaOH during pretreatment, as the biomass loadings were 5% and enzyme loadings were high. Therefore the first goal was to increase solid loading during pretreatment and simultaneously preserve a significant cellulosic fraction in the pretreated solids. The second target was a minimum 20% reduction in doping of commercial cellulase enzyme blend while maintaining > 100 g L−1glucose in the saccharified broth. Two parallel approaches were taken to achieve the second goal. The first involved the partial replacement of Cellic CTec2 with in-house cellulase preparation during high-solids enzymatic saccharification (HSES) by blending them. Earlier, Adsul et al. (2020) in their comprehensive review, have proposed several alternatives like step-by-step addition, over-expression of essential enzymes for cellulase cocktail, enzyme blending and design of experiments (DOE) to reduce the overall loadings or dependence of commercial enzymes. Other than DOE, the most common and candid approach has been the blending of newer enzyme cocktails derived from various microbial sources or using them in combination with commercial cellulases (Maeda et al. 2011; Saini et al. 2016; da Silva et al. 2016; Sharma et al. 2017; Yang et al. 2018; Brar et al. 2019).The in-house cocktail obtained from mutant UV-8 of Talaromyces verruculosus IIPC 324 was used for conducting the enzyme blending trials, as described earlier (Jain et al. 2019). Though the benchmarking studies of the in-house enzyme with commercial cellulase enzyme blend using acid-pretreated SCB revealed that the latter was superior to the former, the partial replacement was still seen as an opportunity, and synergism was worth exploring (Jain et al. 2019).
The second approach was by performing extended hydrolysis with reduced dosage of cellulase enzyme blend at a temperature above its optimum. Last but not least important objective was to decipher an optimum fed-batch strategy for HSES, where the entire biomass feeding did not exceed four hours. Once all the conditions of pretreatment and HSES were optimized with reduced enzyme loads, the benefits of the improved process in this study were compared to our previous report (Baral et al. 2020a) via critical evaluation through TEA. LCA was also conducted on a preliminary basis to understand the effect of process improvements on ecology. Later, the study was concluded by briefly analyzing the economical and environmental impact of the said improvements on lactic acid as the targeted product. However, for this evaluation, the data of pretreatment and hydrolysis processes reported in this work were taken, whereas lactic acid yields and productivity were considered from the previous study (Baral et al. 2020a).
Experimental section
Raw materials and commercial enzyme
Raw SCB was provided by our industrial partner Dhampur Sugar Mills, Bijnor, India. Wheat bran for cellulase production was procured from the local market. Cellic CTec2 was purchased from Sigma-Aldrich with the Lot number being #SLCC1677. It was sold under the brand name "cellulase enzyme blend" and further would be referred with this terminology only. All the other chemicals were procured from Sigma Aldrich unless and otherwise specified. Protein estimation by Bradford assay revealed that it contained 101.11 ± 4.11 mg BSA equivalents protein g−1 enzyme (Bradford 1976). As per the IUPAC protocol, the cellulase activity of the said preparation in terms of Filter Paper Units (FPU) was found to be 127.5 ± 4.6 IU g−1 enzyme (Ghosh 1987). All the experiments in this study were performed in a minimum of duplicates unless specified.
Production and concentration of in-house enzyme
Mutant UV-8 of T. verruculosus IIPC 324 was grown on wheat bran to produce cellulases by solid-state fermentation. Later, the crude cellulase secretome was subjected to partial purification to obtain concentrated cellulase enzymes or CCE (Jain and Agrawal 2018; Jain et al. 2019). The desalted CCE contained 6.19 ± 0.34 mg BSA equivalents protein g−1 enzyme (Bradford 1976).Its FPU activity was found to be 2.98 ± 0.08 IU g−1 enzyme (Ghosh 1987).
Effect of NaOH concentration on delignification of SCB at high-solids
At any stage in a biorefinery, biomass processing at high-solids is favourable for improving the process economics. Therefore, solid loading was raised to 15% from our previously reported protocol with 5% solids (Baral et al. 2020a). Before NaOH pretreatment, the raw biomass was dried overnight at 60 °C. It was followed by size reduction in a cutting mill (Retsch SM 100, Germany) fitted with a 1 mm mesh. Delignification of 30 g dry biomass was carried out by autoclaving at 121 °C for 30 min, under static conditions with different alkali concentrations (0.5, 1, 1.5, 2 and 2.5%). After pretreatment, the solids were separated by filtering and then washed until a neutral pH was obtained. The composition of structural carbohydrates and lignin content of the pretreated biomass was determined per the National Renewable Energy Laboratory (NREL) protocol (Sluiter et al. 2010).The pretreatment in which the delignification was ≥ 65% and cellulose loss did not exceed 15% was further chosen for bulk pretreatment (600 g of dry biomass in two batches) and all the saccharification studies.
Assessing the hydrolytic potential of cellulase enzyme blend at 52.5 °C with 20% solids
Since the optimum temperature for enzymatic hydrolysis by in-house CCE was 55 °C whereas for cellulase enzyme blend, it is 50 °C, therefore, a mid-temperature of 52.5 °C was chosen for enzyme blending studies (Jain et al. 2019; Baral et al. 2020b). However, before initiating this study, it also became essential to assess the hydrolytic potential of cellulase enzyme blend at 52.5 °C. Moreover, a rate kinetic study at high-solids was crucial to decipher the minimum threshold value of cellulase enzyme blend required to release > 100 g L−1 glucose with minimum productivity of 2.4 g L−1 h−1 for selecting its right dosage during blending studies.
Enzymatic hydrolysis was performed with 20% (w/v) alkali pretreated SCB in a non-buffered medium (pH 4.5) containing PEG 6000 (0.2 g g−1 acid-insoluble lignin content) and placing the flasks in an incubator shaker set at 52.5 °C, 180 rpm. A two-step substrate feeding strategy (10% + 10%) was adopted with slight modifications (Baral et al. 2020a). Four different enzyme loadings were chosen (10, 15, 20 and 25 mg protein g−1glucan), and hydrolysis was terminated after 64 h with intermittent sampling every 16 h. After termination, the saccharified broth obtained was subjected to centrifugation at 7000 rpm for 15 min at 4 °C to separate the residual biomass from the sugar-rich filtrate fraction. Later, the residual biomass was washed with water to extract the adhered sugars and centrifuged again to obtain the wash fraction as described previously (Baral et al. 2020a). Enzyme loading was done at the start of the hydrolysis, and no intermittent addition was done.
High-solids enzymatic saccharification
Once the enzyme loading of cellulase enzyme blend and hydrolysis duration was shortlisted based on the experiment conducted in the preceding section, blending studies were initiated using CCE obtained from T. verruculosus IIPC 324. The various combinations chosen for the said study are depicted in Table S1. Neat preparations of cellulase enzyme blend (Run 1) and In-house CCE (Run 6) served as positive and negative controls, respectively. This experiment helped identify the best blending ratio of enzymes required for HSES of alkali pretreated SCB with uncompromised glucose yields and productivity. Further, it gave a fair idea of the synergism between the two enzyme cocktails and subsequent partial replacement of commercial cellulase enzyme blend with an in-house cellulase cocktail. Alternatively, hydrolysis at reduced loadings of cellulase enzyme blend was attempted at 50 and 55 °C to decipher the most favourable temperature for high-solids hydrolysis within 48 h.
Optimization of substrate feeding strategy and data validation
When the best enzyme treatment was shortlisted, the substrate feeding strategy was optimized to attain maximum hydrolysis yields. The feeding regime was restricted to a maximum of 4 h only, based on the liquefaction observed visually during experimentation. Table S2 shows the substrate feeding regime for HSES of alkali pretreated SCB. This experiment was essential to understand that which substrate feeding regime was best suited for maximizing sugar yields from alkali pretreated sugarcane bagasse. Later, using the best combination, the data reproducibility was verified and validated by performing hydrolysis at a 15 g dry substrate basis.
Analytical method
All the principal sugars were analyzed by HPLC system (Shimadzu make) equipped with Aminex HPX-87H (Bio-Rad, California, USA) column coupled with refractive index detector (RID-10A; Shimadzu Corporation Japan). The analysis was done at 55 °C under isocratic conditions with 5 mM H2SO4 as the mobile phase. The flow rate was set at 0.55 mL min−1, and the injection volume was 20 µL. Based on the retention times, all the sugars were determined qualitatively, and further, drawing calibration curves helped in their quantitative estimation.
Glucan and xylan hydrolysis (%) was calculated by the formula used in our earlier study (Nalawade et al. 2020).
where 1.11 and 1.13 is the polymerization factor for the glucan and xylan respectively.
The glucose productivity during enzymatic saccharification was calculated by dividing the glucose concentration obtained in the filtrate fraction with hydrolysis time and was expressed as g L−1 h−1.
Techno-economic and life cycle assessment for the optimized process
An assessment framework was employed to perform detailed TEA and LCA of the processes involved in the fermentable sugar production from SCB. The experimental lab-scale data was extrapolated to a biorefinery scale, with a bagasse handling capacity of 377 tonnes per day (TPD). This throughput was decided for manufacturing 100 TPD of L ( +) lactic acid, the final product of interest. The biorefinery included alkali pretreatment followed by enzymatic hydrolysis to generate fermentable sugars, which was further valorized to lactic acid. In the first place, for quantifying the benefit of process improvement, the modified process described here (termed as Process-2) was compared with the previously reported process (termed as Process-1) (Baral et al. 2020a).Table S3 compares the operating conditions for the two processes for the pretreatment and hydrolysis stages. For both TEA and LCA, a flowsheet was first developed for the mentioned throughput. The operating conditions for the pretreatment and hydrolysis steps and the process performance data such as yield and conversion were taken from the experimental results. The pretreatment liquor is being explored for biogas production, and hence this stream is not considered as a waste or an effluent in the environmental and economic assessment. Additional components of a scaled-up process were considered based on process engineering fundamental. Biorefinery was assumed to be annexed to a conventional sugar mill in India that produced sugar and generated electricity via a co-generation plant. The biorefinery's electricity demands were assumed to be supplied free of cost by the sugar mill's co-generation plant. The existing boiler and cooling tower of the sugar mill were employed for the operation of the biorefinery. Steam demands of the biorefinery plant were assumed to be met by natural gas. The TEA and LCA specific methodology is explained in the following sections.
Techno-economic assessment
The pretreatment reactor was sized for the desired throughput. The reactor design was developed, considering the process conditions. This reactor consisted of carbon steel with anti-corrosive Monel 500 lining. While estimating the cost of fabricated equipment, both the reactor's size and the raw material costs were considered. Similarly, the cost consideration for the enzyme hydrolyzer unit was based on the NREL report and included the reactors, stirrer, and other ancillary equipment (Humbird et al. 2011). Since the capacities of the two reactors during pretreatment and hydrolysis was different, the cost was adjusted using power-law expression and the corresponding scaling factor (0.6).
Further, all the equipment costs were adjusted for 2019 using the Chemical Engineering Plant Cost Index (CEPCI). The equipment cost was used to calculate the fixed capital investment (FCI), thereby calculating the working capital and total capital investment (TCI). Total operating cost (TOC) constitutes both the fixed and variable operating costs. The variable operating cost was estimated from the mass and energy balance data, including the cost of raw materials and utilities taken from the literature. The production cost was calculated by estimating the TOC and general overhead. The evaluation was performed for a plant operating 330 days a year from the energy and mass balance data developed from the experiments.
Life cycle assessment
The LCA study used a cradle to gate scope. The system boundary included all the life cycle stages of fermentable sugars production ranging from sugarcane farming, transportation to biorefining (such as size reduction, pretreatment, and hydrolysis). Open LCA®1.9.0 was used to develop the product system required for the assessment. The functional unit considered in the LCA study is 1 kg of fermentable sugars produced for biorefinery. ReCiPe Midpoint (H) methodology was employed to calculate the environmental impacts across 18 midpoint categories. Life cycle inventory data were sourced from published literature and Ecoinvent® database (version 3.3). Data about sugarcane farming and transportation in the Indian context were adapted from the LCA study published in the Indian scenario (Murali and Shastri 2019). The economic allocation was performed to divide the overall impact of sugarcane farming among sugar, molasses and bagasse. Emission data for the enzyme production was adapted from Sreekumar et al. (2020).
Effect of process improvement during pretreatment and saccharification on "Lactic acid" as final targeted product
Since lactic acid was the final targeted product, additional flowsheet simulation work was performed to model fermentation followed by downstream separation in ASPEN Plus. The separation of the lactic acid from the fermented broth was achieved by reactive distillation using ethanol and the corresponding reaction kinetics data, as mentioned in the literature (Su et al. 2013). Recovery of lactic acid via esterification and hydrolysis reactions in reactive distillation columns was simulated using ASPEN PLUS® software to achieve 99.9% pure lactic acid. Economic plant life of 10 years was considered, and the production cost was estimated. For LCA, 1 kg of lactic acid was taken as the functional unit. System boundary for this lactic acid plant included fermentation and downstream stages, including stages considered for fermentable sugar production. The details are not reported here since the focus of this work is to compare upstream process improvements. The methodological details related to the same have been discussed elsewhere (Munagala et al. 2021).
Results and discussion
Effect of NaOH concentration on delignification of SCB at high-solids
When the delignification was carried out at varying NaOH loadings, the following results were obtained as observed in Table 1.
The alkali concentration displayed a significant negative correlation (p ≤ 0.01) with solid recovery and acid-insoluble lignin (AISL) of pretreated biomass. On the other hand, it exhibited a strong positive correlation with the cellulose content of the pretreated biomass. The best delignification was obtained when the NaOH concentration was 2.5%, solubilizing nearly 90% AISL content. Simultaneously, the treatment also led to > 15% cellulose loss in the lignin-rich black liquor stream. Hence, the pretreatment of 15% SCB with 2% NaOH at 121 °C and 30 min residence time was shortlisted for all the saccharification studies. An enrichment of cellulose by 23.8% with 1% NaOH at 15% solids is comparable to the findings of Ascencio et al. where 21.8% cellulose increase was reported under identical pretreatment conditions with 10% SCB (2020).
When the pretreatment was upscaled from 30 to 300 g dry SCB, the average composition of the two batches was: cellulose: 66.83 ± 1.6%; hemicellulose- 20.63 ± 2.06%; acid-insoluble lignin: 12 ± 1.14%. Higher lignin content could be attributed to poor delignification of scale-up biomass under static conditions while autoclaving. It is likely that a time gap between biomass removal from autoclave and water washing step, lead to percolation of extracted lignin present in black liquor into the swollen cellulose-rich biomass. The complete details of the recoveries and mass balance during pretreatment optimization has been furnished in Table S4.
Earlier, we had reported a similar kind of results at 250 g scale when 5% SCB was pretreated with 2% alkali at 76 ± 2 °C in a water bath using continuous stirring (Baral et al. 2020a). Thus, by increasing the temperature from 76 to 121 °C, pretreated biomass of similar compositional analysis was obtained in relatively less duration. In this modified pretreatment, the solid loading was successfully raised from 5 to 15%, leading to a three-fold (0.4 g to 0.13 g/g biomass) reduction in NaOH consumption and curtailing the holding time from 120 min to merely 30 min. The results of bulk pretreatment are concurrence with the observations of Maryana et al. (2014). They reported a 39.9% increase in cellulose content when 250 g of SCB at 8.33% solids was treated with 4% NaOH under identical pretreatment conditions. Contrary to a 65% reduction in lignin content during the present study, they achieved a 59% reduction in lignin content.
Assessing the hydrolytic potential of cellulase enzyme blend at 52.5 °C with 20% solids
Before blending with an in-house cellulase cocktail, the rate kinetic studies were conducted with cellulase enzyme blend at 52.5 °C using 20% alkali pretreated SCB. We found that 16 h incubation was sufficient to hydrolyze > 70% cellulose and xylan, respectively, when cellulase enzyme blend was dosed at 20–25 mg protein g−1glucan (Fig. 1).
These enzyme loadings yielded 120.6 ± 6.9 and 35.5 ± 1.65 g L−1 glucose and xylose, respectively, in the filtrate fraction in merely 16 h. In the initial hours, the glucose productivities in the filtrate fraction were prominently high (5.6–7.9 g L−1 h−1) when cellulase enzyme blend was loaded between 15 and 25 mg protein g−1glucan. Our group had reported the glucose productivity of 2.65 g L−1 h−1 at 12.5% solids and further improved it to 5.27 g L−1 h−1 with 18% alkali pretreated SCB using the same enzyme (Nalawade et al. 2020; Baral et al. 2020a). This result indicated that alteration in the pretreatment method and performing hydrolysis at 52.5 °C had no adverse effect on hydrolysis; instead, it enhanced glucose and xylose yields even at 20% solids.
Even at 15 mg protein g−1glucan, cellulase enzyme blend liberated 90.2 ± 0.66 g L−1 glucose in the filtrate fraction from 20% solids within 16 h of incubation. However, more recently, Brondi et al. reported the release of ~ 45 g L−1 glucose from 15% liquid hot water pretreated SCB within 24 h when it was fortified with 12% soy protein and treated with 17.85 FPU g−1glucan Cellic CTec3 (2020). These results affirm the superiority of the present process in terms of selecting suitable pretreatment and saccharification conditions.
Extended hydrolysis of 64 h at an enzyme loading of 20–25 mg protein g−1glucan led to a release of ≥ 195 g L−1 fermentable sugars in the filtrate fraction (152.28 ± 2.04 g L−1 glucose; 44.12 ± 1.30 g L−1 xylose).
Very recently, Brar et al. (2020) reported 64.4% cellulose hydrolysis in 96 h accounting for 90.7 ± 3.2 g L−1 glucose when hydrothermally pretreated SCB was depolymerized with Cellic CTec2 dosed at 20.92 mg protein g−1glucan. The present results are far superior to recently published data, where the same enzyme at 20 mg protein g−1glucan yielded 176 ± 5.9 g L−1 glucose from 30% dilute acid pretreated wheat straw after 96 h (Kadhum et al. 2019). Kim et al. (2019) reported the release of 157.5 g L−1 fermentable sugars after 120 h when Cellic CTec2 (30 FPUg−1glucan) mediated hydrolysis was conducted with 30% pretreated oak.
During saccharification, the time course profiles indicated that when the enzyme loading exceeded 10 mg protein g−1glucan, the product saturation in terms was glucose was visible after 48 h only (Fig. 1). At the same time, the pace of xylan hydrolysis also reduced drastically. The increase in enzyme loading from 10 to 15 mg protein g−1glucan resulted in a massive improvement in saccharification yields. A similar upsurge was not observed when the loading of cellulase enzyme blend was further increased to 20 and 25 mg protein g−1glucan. The enzyme loading of 15 mg protein g−1glucan and duration of 48 h was ideal for cellulase enzyme blend being dosed alone as it led to ≥ 80% glucan and xylan hydrolysis, respectively.
On the contrary, for the blending studies, 20 mg enzyme protein g−1glucan content and 32 h were wise choices. This upper threshold limit was chosen because this loading of cellulase enzyme blend in 32 h could help us visualize synergism between the two cocktails and distinctly identify the enhancement due to the blending resulting in improved sugar titres.
High-solids enzymatic saccharification by blending enzymes
When the blending studies were conducted with cellulase enzyme blend and in-house CCE obtained from T. verruculosus IIPC 324 following results were obtained as shown in Table 2. This table indicates that fortification with an in-house enzyme derived from T. verruculosus IIPC 324 had no positive impact on the hydrolysis yields. Blending in-house enzyme with cellulase enzyme blend in the ratio of 1:1 rather reduced the glucan and xylan hydrolysis by ~ 1.42–1.46 fold.
The present results are in disagreement with the findings of Kumar et al. (2020), who reported 1.67 fold enhanced saccharification yields when they blended Cellic CTec2 with an in-house enzyme derived from Aspergillus flavus (AF-NGF1) strain in the same ratio. In the present study, the negative control released ~ 2.5 fold lesser sugars in the filtrate fraction than the positive under identical hydrolysis conditions. Substrate inhibition, product inhibition and lesser duration of hydrolysis could be the likely reasons for the underperformance of the in-house cellulase cocktail. The results of positive (cellulase enzyme blend) and negative (in-house enzyme) control are similar to the outcome of Yang et al. (2018), who obtained 2.25 fold lesser reducing sugars from the secretome of Penicillium chrysogenum P33, compared to commercial cellulase preparation from T. longibrachiatum. However, a blending ratio of 1:1 of these enzymes yielded 65.82% and 25.02% higher reducing sugars from delignified miscanthus and wheat straw, than commercial cellulase alone. Earlier, da Silva et al. (2016) concluded that their in-house enzyme preparation upon blending with Cellic CTec2 merely boosted biomass liquefaction but had no impact on final sugar yields. The latter cellulase cocktail was the best in terms of saccharification efficiency of hydrothermally pretreated SCB. Thus, enzyme blending is fruitful only when synergism exists between the enzymes, thereby promoting higher cellulose hydrolysis. The present study affirmed that blending of cellulase cocktail derived from T. verruculosus IIPC 324 with commercial cellulase enzyme blend was an unrewarding proposition.
High-solids enzymatic saccharification with reduced cellulase enzyme blend at higher temperatures
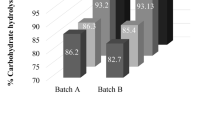
In the quest to reduce the enzyme dosage, it became necessary to perform HSES of alkali pretreated SCB at low loadings of cellulase enzyme blend. Since > 80% carbohydrate conversion yields were obtained with 15 mg protein g−1glucan at 52.5 °C in 48 h, as discussed in the earlier section, the hydrolytic potential of cellulase enzyme blend was evaluated at 50 and 55 °C also. Figure 2 depicts glucan, and xylan hydrolysis from 20% alkali pretreated SCB at three temperatures, 52.5 °C representing the midpoint.
The temperature-based hydrolysis indicated that the maximum threshold temperature for cellulase enzyme blend was 52.5 °C, beyond which a significant reduction in carbohydrate hydrolysis was observed. A rise from 50 to 52.5 °C improved glucan and xylan hydrolysis marginally by 9.6% and 13.3%, respectively. On the other hand, elevating temperature from 50 to 55 °C reduced the glucose and xylose release by > 20%. A similar observation was made when our group conducted hydrolysis with 7.5% acid pretreated SCB with the same enzyme preparation and raising the temperature from 50 to 55 °C reduced glucan hydrolysis by ~ 7% (Baral et al. 2020b). But this time, the high substrate, low enzyme loading, longer duration of hydrolysis and a different pretreatment strategy probably played a pivotal role in severely impeding the saccharification. It is likely that the cumulative effect of thermal enzyme inactivation at 55 °C and the presence of high concentrations of soluble and insoluble inhibitors released during HSES were primarily responsible for reduced hydrolysis, as discussed earlier (Hodge et al. 2008). Even the application data sheet of cellulase enzyme blend reveals that the optimum working temperature range for the said commercial enzyme is between 45 and 50 °C (Application Sheet Novozymes 2010). But in the present experiments restricting the duration of hydrolysis of 48 h, fortunately, favoured the maximum sugar release at 52.5 °C. It should be noted that Kancelista et al. (2020) have also reported optimal conditions for Cellic CTec2 being 52.7 °C and pH 5.2 using the Box Behnken model, which resulted in maximum ethanol yield during separate hydrolysis and fermentation of sweet sorghum.
Optimization of substrate feeding strategy and data validation
Fed-batch hydrolysis is always preferred over batch hydrolysis during HSES. It overcomes the constraints of mass and heat transfer, facilitating the proper mixing of the enzyme with the LCB. In the present study, complete substrate feeding was restricted to 4 h only, based on the precedence of Dunaway et al. (2010), who confirmed that rapid viscosity changes occurred with 8–10 h of saccharification, indicating the entry of hydrolysis into its exponential phase. When different substrate feeding regimes were evaluated (Table S2) to reach a final concentration of 20% solids, it was observed that in Run 3, the onset of liquefaction after the last feeding was much faster than other runs (Table 3). The plausible explanation could be low solid loading in the last two intervals.
However, when the entire saccharification trials were analyzed, the best hydrolysis was observed in Run 1. Irrespective of feeding strategy, the average glucan and xylan saccharification yields were found to be 79.74 ± 2.58 and 80.07 ± 3.92%, respectively. Cellulase enzyme blend at 15 mg protein g−1glucan loading was able to release 163.53 ± 11.93 and 66.02 ± 0.05 g L−1 fermentable sugars (glucose and xylose) from 20% solids in merely 48 h in the filtrate and wash fraction, respectively. Though cellobiose and arabinose were also detected in the filtrate fraction, these sugars couldn't be quantified.
A glucose concentration of 124.6 ± 8.2 g L−1 in the filtrate fraction of Run1 from 20% solids is superior in terms of enzyme savings and product yield obtained by Cheng et al. (2020). They attained 129.81 g L−1 glucose from 40% hydrothermally pretreated sorghum via PEG 4000 aided hydrolysis and dosing Cellic CTec2 at 36.6 mg protein g−1glucan. Gong et al. (2020) adopted a two-step substrate feeding (12% at 0 h + 7% after 3 h) strategy for hydrolysis of alkaline organosolv pretreated corn stover using Cellic CTec2 loaded at 15 mg protein g−1glucan. After 120 h of hydrolysis, the hydrolysate composition had 138.4 ± 4.0, 81.7 ± 1.2 and 33.6 ± 1.3 g L−1 glucose and xylose, respectively. Glucose productivity of 2.59 g L−1 h−1 in the present study is much higher than 0.68 g L−1 h−1 achieved by Gong et al. (2020). Even de Godoy et al. (2019), in a fed-batch process, achieved glucose productivity of 0.77–0.88 g L−1 h−1while performing hydrolysis of 24% SCB pretreated hydrothermally and using a combination of dilute acid + alkaline delignification, respectively.
From the techno-commercial viewpoint, it is necessary to obtain high-gravity sugar solutions from LCB, but maintaining high sugar productivities is equally beneficial as it increases the industry's acceptability. After optimizing fed-batch hydrolysis, it was necessary to validate the results and confirm the data reproducibility. Hence, validation experiments were performed in triplicates on a relatively higher scale (15 g dry weight). Earlier fed-batch results gave clear evidence that the carbohydrate hydrolysis fluctuated between 78 and 86% in 48 h. Therefore these trials were conducted at three-time intervals, namely 42, 48 and 54 h, to validate the optimum time for achieving > 80% hydrolysis. Table 4 highlights the total sugars recovered in the filtrate and wash fractions at different time points along with sugar and glucose productivities.
Figure 3 shows the overall saccharification yields obtained at different time points when 20% alkali pretreated SCB was hydrolyzed with cellulase enzyme blend dosed at 15 mg protein g−1glucan.
There was a marginal improvement in the concentration of total sugars in the filtrate fraction and carbohydrate conversion yields when hydrolysis duration was extended from 48 to 54 h (Table 4 and Fig. 3). The present glucose productivity of 2.42 g L−1 h−1is nearly identical to our previous batch study (Nalawade et al. 2020), with improvements in terms of substrate loading (12.5% to 20%) and reduction in enzyme dosage (25 to 15 mg protein g−1glucan). In the present study, the release of 126.85 and 51.95 g L−1 glucose and xylose, respectively, from 20% solids in 48 h, is encouraging compared to studies conducted by Liu et al. (2020). With 20% alkali pretreated SCB, they attained similar sugar concentrations after 96 h, when Cellic CTec2 was loaded at 16.4 FPU g−1glucan and fortified with 1200 IU/g substrate Cellic HTec2.
Mukasekuru et al. (2020) attempted to develop an industrially attractive process by conducting fed-batch hydrolysis of 30% SCB pretreated with alkaline-catalyzed atmospheric glycerol organosolv process. At a dosage of 5.26 FPU g−1glucan, Cellic CTec2 liberated 86 g L−1 glucose after 48 h, but the enzyme was further supplemented with 30 mg g−1 Tween 80, 20 mg g−1 BSA, and 10 mg g−1 tea saponin.
Thus, at the end of the study, we successfully extracted ~ 530 g of fermentable sugars from one kg SCB, in which 410.5 g represented glucose, and the rest was xylose. These results are superior to our previously published findings, where the 492 g monomeric sugars were obtained from one kg SCB (Baral et al. 2020a).
After validation experiments, it became essential to understand the implications of process improvements (during pretreatment and hydrolysis) on cost reduction in fermentable sugar production and lactic acid as the targeted product and linking it with LCA. Thus taking our previous study (Baral et al. 2020a) as the base case, TEA and LCA studies were conducted (Table S3), whose results are presented in the succeeding sections.
Techno-economic assessment results
Table 5 compares the original (Process 1) and the proposed improved process (Process 2) in terms of various economic parameters. The most important result was that the cost of fermentable sugar reduced by 41.3% (US$ 2.25 to 1.32 /kg) due to the proposed optimal conditions. If bagasse was considered to be free, the product cost for the optimized conditions further reduced to US$ 1.18/kg.
The noticeable decline in the production cost of fermentable sugars resulted from the savings in the raw material expenses (NaOH usage reduced by 68%; enzyme usage reduced by 40%). However, reduced enzyme loadings extended the duration of hydrolysis, which was reflected in TCI. It should be noted that TCI rose by 52%, as an additional requirement of reactors for continuous downstream processing became necessary owing to prolonged saccharification time. However, this was more than compensated by the reduction in operating costs.
Figure 4 shows the stage-wise contribution of sugars production from bagasse to the overall product cost for both the processes.
For Process-1, pretreatment and hydrolysis contributed 19% (0.44 US$/kg) and 72% (1.62 US$/kg) to the product cost, respectively. However, for Process-2, the contribution of pretreatment reduced significantly to 0.14 US$/kg, which was a 68% reduction as compared to Process-1. Additionally, hydrolysis became the major cost component, accounting for 76% of the product cost (US$ 1/kg). As mentioned previously, although the enzyme dosage requirement for Process-2 was 40% less than Process-1, the benefit was slightly offset by the higher investment for the additional equipment operating in staggering mode. However, the hydrolysis stage contribution to the product cost decreased by 0.62 US$/kg for the optimized process. Thus, the optimized process displayed substantial economic benefits on the overall production cost of sugars production.
Corroborating experimental results with TEA gave a clear insight that researchers should not merely focus on reduced enzyme loadings during cost minimization. A trade-off between the benefits of enzyme cost should be carefully and judiciously weighed against the capital investment and operating cost required to perform prolonged hydrolysis with reduced enzyme loadings. A similar conclusion was drawn by Fahmy et al. while evaluating the advantage of adding PEG 4000 for pine hydrolysis (2019).
Life cycle assessment results
Table 6 reports selected midpoint impact categories for producing 1 kg of fermentable sugar. Process-2 demonstrated lower environmental impacts than Process-1 across all impact categories, owing to increased solid loading and reduced alkali consumption during pretreatment.
The life-cycle climate change impact of Process-2 was 1.57 kg CO2eq., which was 45% lower than Process-1. Figure 5 shows the break-up of GHG emissions contributed by various stages in the bagasse-based sugar production, where the reduced contribution by pretreatment module affected the share of all other steps. The low enzyme dosage of Process-2 also resulted in a reduction of 0.55 kg CO2eq. emissions compared to Process-1.
Freshwater eutrophication potential for Process-1 and Process-2 was 86 × 10−5 and 47 × 10−5, respectively. Production of chemicals such as sodium hydroxide and sulfuric acid, coupled with farming activities that resulted in the nitrogen and phosphorous runoff to the water bodies, lead to freshwater eutrophication. In Process-1, the share of alkali towards freshwater eutrophication was 65%, but improvements in pretreatment of Process-2 reduced its contribution to 40%. However, for Process-2 with less NaOH requirement, farming activities contributed 42% of the eutrophication potential, followed by the sugar mill's co-generation activities.
LCA results for fossil depletion were 0.59 and 0.27 kg oil eq. for Process-1 and Process-2, respectively. Production routes of natural gas and alkali resulted in more significant impacts of the fossil depletion category. Reducing the alkali content resulted in the avoided use of fossil resources in alkali production via chlor-alkali process. This benefit was reflected in the fossil depletion impact value of the optimized process with the resultant impacts due to alkali lowered by 68%. Additionally, the fossil depletion potential due to natural gas usage of Process-2 decreased by 53% compared to Process-1 owing to a reduction in the steam requirement.
Water depletion potential for Process-1 and Process-2 was 7.76 and 3.29 m3, respectively. A significant contribution to water depletion for the processes under study can be attributed to the alkali, i.e., sodium hydroxide used in the pretreatment stage. Sodium hydroxide production alone resulted in the water depletion of 6.34 and 2.11 m3. In the case of Process-2, optimizing the process lowered alkali usage and thereby improving the water depletion potential. A similar trend can be observed in the case of human toxicity with the decreased impact values of kg Process-2 at 0.32 kg 1,4-DB eq., 54% lower than Process-1. The release of heavy metals to air and water during alkali production contributed majorly to human toxicity, followed by cogeneration activities. Upon comparing the environmental impacts of the two processes under study, as expected optimized process displayed strong benefits across all impact categories. Significant mitigation of impacts was observed in the midpoint impact categories of Process-2. Recovery of alkali used in the pretreatment or reducing the alkali usage can improve the overall sustainability.
Figure 6 depicts the overall scheme of pretreatment and saccharification where the bioprocessing of one kg sugarcane bagasse is envisaged and integrated with TEA and LCA. The complete details of the mass balance is furnished in the supplementary information (SI-5).
Effect of process improvement during pretreatment and saccharification on "Lactic acid" as the final targeted product
The present section briefly summarizes the impact of improvements in upstream processes that affected the TEA and LCA of "lactic acid". The cost of lactic acid obtained through Process-1 and Process-2 were 4.50 and 2.92 US$/kg, respectively. If the bagasse was free, the product cost for Process-1 and Process-2 further reduced to 4.22 and 2.64 US$/kg, respectively. The lactic acid product cost reported in the literature ranged from 1.1 US$/kg (Manandhar and Shah 2020) to 1.94 US$/kg (Pachón et al. 2018). Very recently, Li et al. used a python-based open-source platform BioSTEAM and inferred that if the price of lactic acid is between 1.7 and 2.1 US$/kg, the likelihood of biorefinery being economically viable increases (2021). In light of this supporting evidence, the benefits of optimized pretreatment and hydrolysis in this study were successfully translated into better lactic acid production economics.
Similar advantages were also observed for LCA of lactic acid. The climate change impact for the optimized process was 3.58 kg CO2 eq., which was 41% lower than that for Process-1. This value was much lower than the impacts reported for fossil-based lactic acid, ranging from 4.33 to 4.46 kg CO2 eq. per kg lactic acid (Daful et al. 2016; Ecoinvent® Database). The present results were similar to those reported in other literature for lactic acid produced from SCB (Pachón et al. 2018; Daful et al. 2016; Morão and de Bie 2019).
This study strongly corroborates with the hypothesis of Cheng et al. (2019), who emphasized that the cost of lignocellulosic sugar governs the final cost of the product. In this case, it was lactic acid.
Conclusion
The effort towards cost reduction was accomplished in the present study. The pretreatment process was improved by reducing alkali dosage by 67.5% and increasing the solid-loading from 5 to 15%. The pretreated biomass contained ≥ 65% glucan and showed equally proficient accessibility towards hydrolysis using cellulase enzyme blend. Even with 40% reduced enzyme loadings, 84.23 ± 1.0 and 82.6 ± 2.3% glucan and xylan conversion yields were obtained from 20% solids in 48 h at 52.5 °C and pH 4.5. These optimizations in the upstream resulted in substantial economic and environmental benefits compared to the previously reported process. The cost reduction of 41% in the lignocellulosic sugar production further lowered the cost of L ( +) lactic acid by 35%. It also downsized the environmental impact, especially in terms of GHG emissions.
Data availability
The raw data is available with us. On request, the same would be furnished.
References
Adsul M, Sandhu SK, Singhania RR et al (2020) Designing a cellulolytic enzyme cocktail for the efficient and economical conversion of lignocellulosic biomass to biofuels. Enzyme Microb. Technol 133:109442. https://doi.org/10.1016/j.enzmictec.2019.109442
Antunes FA, Chandel AK, Terán-Hilares R et al (2019) Overcoming challenges in lignocellulosic biomass pretreatment for second-generation (2G) sugar production: emerging role of nano, biotechnological and promising approaches. 3 Biotech 9(6):230. https://doi.org/10.1007/s13205-019-1761-1
Application Sheet of Novozymes (2010) Cellic® CTec2 and HTec2-enzymes for hydrolysis of lignocellulosic materials. https://www.yumpu.com/en/document/view/35286682/novozymes-cellicar-ctec2-and-htec2-enzymes-for-hydrolysis-of. Assessed on 15th Mar 2021
Ascencio JJ, Chandel AK, Philippini RR et al (2020) Comparative study of cellulosic sugars production from sugarcane bagasse after dilute nitric acid, dilute sodium hydroxide and sequential nitric acid-sodium hydroxide pretreatment. Biomass Convers Biorefin 10(4):813–822. https://doi.org/10.1007/s13399-019-00547-6
Baral P, Pundir A, Kumar V et al (2020a) Expeditious production of concentrated glucose-rich hydrolysate from sugarcane bagasse and its fermentation to lactic acid with high productivity. Food Bioprod Process 124:72–81. https://doi.org/10.1016/j.fbp.2020.08.005
Baral P, Jain L, Kurmi AK et al (2020b) Augmented hydrolysis of acid pretreated sugarcane bagasse by PEG 6000 addition: a case study of Cellic CTec2 with recycling and reuse. BioprocBiosysEngg 43(3):473–482. https://doi.org/10.1007/s00449-019-02241-3
Baruah J, Nath BK, Sharma R et al (2018) Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front Energy Res 6:141. https://doi.org/10.3389/fenrg.2018.00141
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. AnalBiochem 72(1–2):248–254. https://doi.org/10.1006/abio.1976.9999
Brar KK, Agrawal D, Chadha BS et al (2019) Evaluating novel fungal secretomes for efficient saccharification and fermentation of composite sugars derived from hydrolysate and molasses into ethanol. Bioresour Technol 273:114–121. https://doi.org/10.1016/j.biortech.2018.11.004
Brar KK, Santo MC, Pellegrini VO (2020) Enhanced hydrolysis of hydrothermally and autohydrolytically treated sugarcane bagasse and understanding the structural changes leading to improved saccharification. Biomass Bioenergy 139:105639. https://doi.org/10.1016/j.biombioe.2020.105639
Brondi MG, Elias AM, Furlan FF et al (2020) Performance targets defined by retro-techno-economic analysis for the use of soybean protein as saccharification additive in an integrated biorefinery. Sci Rep 10(1):1–13. https://doi.org/10.1038/s41598-020-64316-6
Chandel AK, Garlapati VK, Jeevan Kumar SP et al (2020) The role of renewable chemicals and biofuels in building a bioeconomy. Biofuel Bioprod Bioref. 14:830–844. https://doi.org/10.1002/bbb.2104
Cheng MH, Huang H, Dien BS et al (2019) The costs of sugar production from different feedstocks and processing technologies. Biofuel BioprodBioref 13(3):723–739. https://doi.org/10.1002/bbb.1976
Cheng MH, Kadhum HJ, Murthy GS et al (2020) High solids loading biorefinery for the production of cellulosic sugars from bioenergysorghum. BioresourTechnol 318:124051. https://doi.org/10.1016/j.biortech.2020.124051
da Silva AS, de Souza MF, Ballesteros I et al (2016) High-solids content enzymatic hydrolysis of hydrothermally pretreated sugarcane bagasse using a laboratory-made enzyme blend and commercial preparations. ProcBiochem 51(10):1561–1567. https://doi.org/10.1016/j.procbio.2016.07.018
Daful AG, Haigh K, Vaskan P et al (2016) Environmental impact assessment of lignocellulosic lactic acid production: integrated with existing sugar mills. Food Bioprod Process 99:58–70. https://doi.org/10.1016/j.fbp.2016.04.005
de Godoy CM, Machado DL, da Costa AC (2019) Batch and fed-batch enzymatic hydrolysis of pretreated sugarcane bagasse–assays and modeling. Fuel 253:392–399. https://doi.org/10.1016/j.fuel.2019.05.038
Dunaway KW, Dasari RK, Bennett NG (2010) Characterization of changes in viscosity and insoluble solids content during enzymatic saccharification of pretreated corn stover slurries. Bioresour Technol 101(10):3575–3582. https://doi.org/10.1016/j.biortech.2009.12.071
Fahmy M, Sohel MI, Vaidya AA et al (2019) Does sugar yield drive lignocellulosic sugar cost? Case study for enzymatic hydrolysis of softwood with added polyethylene glycol. ProcBiochem 80:103–111. https://doi.org/10.1016/j.procbio.2019.02.004
Galbe M, Wallberg O (2019) Pretreatment for biorefineries: a review of common methods for efficient utilization of lignocellulosic materials. Biotechnol Biofuels 12(1):1–26. https://doi.org/10.1186/s13068-019-1634-1
Ghosh TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268
Gong Z, Wang X, Yuan W et al (2020) Fed-batch enzymatic hydrolysis of alkaline organosolv-pretreated corn stover facilitating high concentrations and yields of fermentable sugars for microbial lipid production. Biotechnol Biofuels 13(1):13. https://doi.org/10.1186/s13068-019-1639-9
Harmsen PF, Huijgen W, Bermudez L et al (2010) Literature review of physical and chemical pretreatment processes for lignocellulosic biomass. Research Report ECN-E-10-013
Hodge DB, Karim MN, Schell DJ, McMillan JD (2008) Soluble and insoluble solids contributions to high-solids enzymatic hydrolysis of lignocellulose. Bioresourc Technol 99(18):8940–8948. https://doi.org/10.1016/j.biortech.2008.05.015
Humbird D, Davis R, Tao L et al (2011) Process design and economics for biochemical conversion of lignocellulosic biomass to ethanol: dilute-acid pretreatment and enzymatic hydrolysis of corn stover. Natl Renew Energy Lab. https://doi.org/10.2172/1107470
Jain L, Agrawal D (2018) Rational approach for mutant selection of Talaromycesverruculosus IIPC 324 secreting biofuel cellulases—assessing saccharification potential. Ind CropProd 114:93–97. https://doi.org/10.1016/j.indcrop.2018.01.078
Jain L, Kurmi AK, Agrawal D (2019) Benchmarking hydrolytic potential of cellulase cocktail obtained from mutant strain of Talaromycesverruculosus IIPC 324 with commercial biofuel enzymes. 3Biotech 9(1):23. https://doi.org/10.1007/s13205-018-1547-x
Kadhum HJ, Mahapatra DM, Murthy GS (2019) A comparative account of glucose yields and bioethanol production from separate and simultaneous saccharification and fermentation processes at high solids loading with variable PEG concentration. BioresourTechnol 283:67–75. https://doi.org/10.1016/j.biortech.2019.03.060
Kancelista A, Chmielewska J, Korzeniowski P et al (2020) Bioconversion of sweet sorghum residues by Trichodermacitrinoviride C1 enzymes cocktail for effective bioethanol production. Catalysts 10(11):1292. https://doi.org/10.3390/catal10111292
Kim DH, Park HM, Jung YH et al (2019) Pretreatment and enzymatic saccharification of oak at high solids loadings to obtain high titers and high yields of sugars. BioresourTechnol 284:391–397. https://doi.org/10.1016/j.biortech.2019.03.134
Kumar MN, Ravikumar R, Thenmozhi S et al (2019) Choice of pretreatment technology for sustainable production of bioethanol from lignocellulosic biomass: bottlenecks and recommendations. Waste Biomass Valor 10(6):1693–1709. https://doi.org/10.1007/s12649-017-0177-6
Kumar M, Pandey AK, Kumari S et al (2020) Secretome produced by a newly isolated Aspergillus flavus strain in engineered medium shows synergy for biomass saccharification with a commercial cellulase. Biomass Convers Bioref. https://doi.org/10.1007/s13399-020-00935-3
Li Y, Bhagwat SS, Cortés-Peña Y et al (2021) Sustainable lactic acid production from lignocellulosic biomass. ACS Sustain ChemEng 9:1341–1351. https://doi.org/10.1021/acssuschemeng.0c08055
Liu Y, Cao Y, Yu Q et al (2020) Enhanced sugars production with high conversion efficiency from alkali-pretreated sugarcane bagasse by enzymatic mixtures. BioResources 15(2):3839–3849
Maeda RN, Serpa VI, Rocha VA et al (2011) Enzymatic hydrolysis of pretreated sugar cane bagasse using Penicilliumfuniculosum and Trichodermaharzianumcellulases. ProcBiochem 46(5):1196–1201. https://doi.org/10.1016/j.procbio.2011.01.022
Manandhar A, Shah A (2020) Techno-economic analysis of bio-based lactic acid. Processes 8(2):199. https://doi.org/10.3390/pr8020199
Maryana R, Ma’rifatun D, Wheni AI et al (2014) Alkaline pretreatment on sugarcane bagasse for bioethanol production. EnergProcedia 47:250–254. https://doi.org/10.1016/j.egypro.2014.01.221
Morão A, de Bie F (2019) Life cycle impact assessment of polylactic acid (PLA) produced from sugarcane in Thailand. J Polymer Environ 27(11):2523–2539. https://doi.org/10.1007/s10924-019-01525-9
Mukasekuru MR, Kaneza P, Sun H et al (2020) Fed-batch high-solids enzymatic saccharification of lignocellulosic substrates with a combination of additives and accessory enzymes. Ind Crop Prod 146:112156. https://doi.org/10.1016/j.indcrop.2020.112156
Munagala M, Shastri Y, Konde K et al (2021) Valorization of sugarcane bagasse to lactic acid: life cycle assessment and economic evaluation in Indian scenario. Waste Manag 126:52–64. https://doi.org/10.1016/j.wasman.2021.02.052
Murali G, Shastri YS (2019) Biofuels Life cycle assessment based comparison of different lignocellulosic ethanol production routes. Biofuels. https://doi.org/10.1080/17597269.2019.1670465
Nalawade K, Baral P, Patil S et al (2020) Evaluation of alternative strategies for generating fermentable sugars from high-solids alkali pretreated sugarcane bagasse and successive valorization to L (+) lactic acid. Renew Energy 157:708–717. https://doi.org/10.1016/j.renene.2020.05.089
Ögmundarson Ó, Sukumara S, Herrgård MJ et al (2020) Combining environmental and economic performance for bioprocess optimization. Trends Biotechnol 38(11):1203–1214. https://doi.org/10.1016/j.tibtech.2020.04.011
Pachón ER, Vaskan P, Raman JK et al (2018) Transition of a South African sugar mill towards a biorefinery. A feasibility assessment. Applenerg 229:1–17. https://doi.org/10.1016/j.apenergy.2018.07.104
Rahmati S, Doherty W, Dubal D et al (2020) Pretreatment and fermentation of lignocellulosic biomass: reaction mechanisms and process engineering. ReactChemEng 5(11):2017–2047. https://doi.org/10.1039/D0RE00241K
Saini JK, Singhania RR, Satlewal A et al (2016) Improvement of wheat straw hydrolysis by cellulolytic blends of two Penicillium spp. Renew Energy 98:43–50. https://doi.org/10.1016/j.renene.2016.01.025
Saini R, Osorio-Gonzalez CS, Hegde K et al (2020) Lignocellulosic biomass-based biorefinery: an insight into commercialization and economic standout. Curr Sustain Renew Energy Rep 23:1–5. https://doi.org/10.1007/s40518-020-00157-1
Sharma S, Kuila A, Sharma V (2017) Enzymatic hydrolysis of thermochemically pretreated biomass using a mixture of cellulolytic enzymes produced from different fungal sources. Clean TechnolEnviron Policy 19(5):1577–1584. https://doi.org/10.1007/s10098-017-1346-9
Sluiter A, Hames B, Ruiz R et al (2010) Determination of structural carbohydrates and lignin in biomass. Laboratory Analytical Procedure (TP-510-42618)
Sreekumar A, Shastri Y, Wadekar P et al (2020) Life cycle assessment of ethanol production in a rice-straw-based biorefinery in India. Clean Technol Environ Policy 22:409–422. https://doi.org/10.1007/s10098-019-01791-0
Su CY, Yu CC, Chien IL et al (2013) Plant-wide economic comparison of lactic acid recovery processes by reactive distillation with different alcohols. IndEngChem Res 52:11070–11083. https://doi.org/10.1021/ie303192x
Valdivia M, Galan JL, Laffarga J et al (2016) Biofuels 2020: biorefineries based on lignocellulosic materials. MicrobBiotechnol 9(5):585–594. https://doi.org/10.1111/1751-7915.12387
Yang Y, Yang J, Liu J et al (2018) The composition of accessory enzymes of Penicilliumchrysogenum P33 revealed by secretome and synergistic effects with commercial cellulase on lignocellulose hydrolysis. BioresourTechnol 257:54–61. https://doi.org/10.1016/j.biortech.2018.02.028
Yoo CG, Meng X, Pu Y et al (2020) The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: a comprehensive review. BioresourTechnol 301:122784. https://doi.org/10.1016/j.biortech.2020.122784
Zhao X, Zhang L, Liu D (2012a) Biomass recalcitrance. Part I: the chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels BioprodBioref 6(4):465–482. https://doi.org/10.1002/bbb.1331
Zhao X, Zhang L, Liu D (2012b) Biomass recalcitrance. Part II: fundamentals of different pretreatments to increase the enzymatic digestibility of lignocellulose. Biofuels BioprodBioref 6(5):561–579. https://doi.org/10.1002/bbb.1350
Zoghlami A, Paës G (2019) Lignocellulosic biomass: understanding recalcitrance and predicting hydrolysis. Front Chem 7:874. https://doi.org/10.3389/fchem.2019.00874
Acknowledgments
The authors are thankful to Dr Anjan Ray, Director CSIR-Indian Institute of Petroleum, for his valuable inputs and guidance. We are grateful to our industrial partner Dhampur Sugar Mills, Bijnor, India, for providing sugarcane bagasse.
Funding
The authors express their gratitude to the Department of Biotechnology (DBT), India, Biotechnology and Biological Sciences Research Council (BBSRC) and Innovate UK for funding the vWa Project (GAP 3513) under Indo-UK Industrial Waste 2017 grant.
Author information
Authors and Affiliations
Contributions
PB: Investigation, Methodology, Formal analysis, Writing-Original Draft; MM: Writing-Original Draft, Software, Formal analysis; YS: Supervision, Software, Resources, Data curation; VK: Writing- Review & Editing; DA- Conceptualization, Supervision, Writing-Original Draft, Project Management and Fund acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that that have no conflicts of interest.
Human and animal rights statement
No animal or human trials were undertaken or conducted by any of the authors during the said study.
Consent to participate
All the authors are aware of the submission and have given their consent to participate.
Consent to publish
All the authors have read the manuscript before submission. All authors read and approved the final manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Baral, P., Munagala, M., Shastri, Y. et al. Cost reduction approaches for fermentable sugar production from sugarcane bagasse and its impact on techno-economics and the environment. Cellulose 28, 6305–6322 (2021). https://doi.org/10.1007/s10570-021-03940-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-021-03940-5