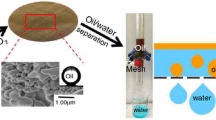
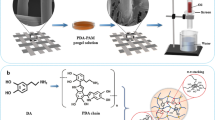
Abstract
The development of sustainable, low-cost, green and efficient oil–water separation materials is an attractive and challenging work. Oil/water separation process has been achieved by a variety of materials with special wettability, but most materials require complex instruments or involve toxic and corrosive chemicals, which might lead to some potential economic and environmental issues. Our work proposed here is a novel super-hydrophilic, underwater super-oleophobic cellulose hydrogel-coated mesh (CHCM) which produced by deep eutectic solvent, aimed for efficient gravity-driven oil/water separation. Based on this pre-wet CHCM, the separation efficiency of various oil–water mixtures was above 98.0%, and the results also showed that CHCM has good recyclability and durability, even after 20 cycles, the separation efficiency also maintained at 98.5%. Impressively, the prepared CHCM also exhibited good salt resistance; it can separate a high efficiency pump oil mixture from a saturated aqueous NaCl solution. Through XPS analysis of CHCM, the oil–water separation mechanism is due to the large number of super-hydrophilic groups on its surface. This simple, green, and efficient method overcomes an important barrier to the safe separation of oil–water mixtures and provides insights into the design of advanced materials for practical oil–water separation.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, the rapid growth of global energy demand has intensified the exploitation of crude oil (Ao et al. 2018; Zhang et al. 2018). At the same time, increasing oil leakage and oil spills have become one of the top environmental problem (Ma et al. 2016). At present, cleaning up oil spills in the water is a difficult problem in today’s world. Environmental and economic needs highlight the urgent need for functional materials that efficiently achieve oil–water separation (Ma et al. 2016; Xue et al. 2014). Traditional technologies for oil pollution remediation, such as eucalyptus oil, dissolved air flotation, polymer oil absorption, chemical dispersant degradation, etc. always have some drawbacks such as low decontamination ability, high cost, incomplete separation, secondary pollution, and complicated operation (Su et al. 2016; Wang et al. 2016). Therefore, it is extremely necessary to develop other methods to solve the above problems. At the present, special wettability materials have received extensive attention in oil–water separation; especially the metal mesh, textile/fabric, and polymer film were used as the substrate of the ultra-wetting material (Cao et al. 2013; Chen et al. 2016; Ge et al. 2016). Recent studies have shown that the use of metal mesh as a special wettable filter material was benefited to its high mechanical strength, good antifouling ability and good cycle performance (Pan et al. 2008; Xue et al. 2011). Researchers often used hydrophilic polymer, inorganic particle coatings, electrochemical anodization and chemical etching to impart special wetting properties to metal meshes (Ge et al. 2016; Ma et al. 2016). Unfortunately, these methods inevitably required the use of complex instruments and corrosive, toxic chemicals, followed resulting in high expense and environmental problem (Li et al. 2016; Yu et al. 2017; Zhou et al. 2017). In addition, plasma and femtosecond lasers were also applied to modified metal mashes (Chen et al. 2016; Yin et al. 2017) that technologies only existed in the laboratory application stage, and limited by equipment investment and cannot be mass-produced to apply (Ge et al. 2016).
In the current research, cellulose-based materials have been reported as an effective supporter in oil/water separation studies (Ao et al. 2017, 2018). As is known, cellulose is considered to be one of the most abundant natural polymer materials in nature, which is an inexhaustible and renewable resource (De France et al. 2016; Errokh et al. 2018). It has the advantages of wide source, non-toxic, degradable and renewable. Cellulose materials mostly exhibited super-lipophilic characteristics in oil/water separation, which existed a lot of shortcomings (Wang et al. 2015). For example, it was difficult to separate oil and water by gravity alone because the density of water is higher than the density of most oils, thus a barrier is formed between the material and the oil, and the oil is difficult to flow out (Yoon et al. 2014). In addition, these materials have high affinity for oil, which easily contaminated or even blocked by oil and then seriously affecting separation performance and limiting its service life (Ejaz Ahmed et al. 2014; Zhou et al. 2014). Moreover, it was difficult to separate the cellulose material after it adsorbed the oil and fat, which easily causes secondary pollution (De France et al. 2017; Wang et al. 2016). Thus, the preparation of cellulosic materials which having super-hydrophilic and underwater super-oleophobic could effectively overcome these mentioned shortcomings. Such cellulosic materials can inhibit the continuous infiltration during the filtration process by intercepting moisture and forming a water film to inhibit oil and fat (Ao et al. 2017, 2018). Until now, only rarely studies about cellulose-based materials with super-hydrophilicity and underwater super-oleophobic properties were reported in oil/water separation. Ao et al. (2018) prepared a super-hydrophilic and underwater super-oleophobic cellulose hydrogel coating mesh, which was driven by gravity alone. The hydrogel coated mesh has ability to separate different oil/water solution, and the separation efficiency reach above 98.9%. Zhou et al. (2016b) reported the preparation of a novel super-hydrophobic micro-fibrillated cellulose aerogel with a super-hydrophobic surface obtained by a simple, environmentally friendly silylation reaction in the liquid phase. Although these materials have high oil–water separation performance, but the disadvantages such as complicated preparation process, high cost, high toxicity and long reaction time obstructed the large-scale practical application of those materials in oil–water separation.
Herein, as illustrated in Scheme 1, we propose a simple, environmentally friendly method to manufacture a super-hydrophilic and underwater super-oleophobic CHCM for efficient oil/water separation. To the best of our knowledge, this is the first time that cellulose treated by deep eutectic solvent (DES) combined polymer poly (vinyl alcohol) (PVA) to prepare hydrogel for mesh. The obtained CHCM has super-hydrophilicity and underwater super-oleophobicity, which was effectively drive oil/water separation by gravity. It is worth noting that CHCM is a hydrophilic polymer containing a large amount of carboxyl groups and hydroxyl groups, which exhibit super-hydrophilic by hydrogen bonding to perform oil/water separation. As a hydrogel coating film, CHCM also exhibits excellent separation ability for different types of oil–water mixture, high separation efficiency, and good recyclability and durability. In addition, CHCM has good environmental stability under high salinity conditions.
Experimental section
Materials
Microcrystalline cellulose (MCC) was purchased from Guangdong Pharmaceutical Co., Ltd. The materials were decomposed in water, filtered, washed with ethanol and dried. Wire mesh was purchased from Shanghai Haorui Hardware Screen Co., Ltd, which was ultrasonicated in ethanol for 1.0 h before use. Other solvents, reagents and greases used in the separation experiments were provided by Shanghai Aladdin Biotechnology Co., Ltd.
Fabrication of CHCM
DESs were prepared by mixing 21.02 g of choline chloride (ChCl) and 18.98 g of ethanedioic acid (Ea) at 90 °C for about 45 min. After obtaining a clear solution, 0.2 g of microcrystalline cellulose was added and mixed for 6 h and then the 0.5 wt% cellulose solution was prepared. The pretreated wire mesh was dipped in the cellulose solution at 90 °C for 4 h to completely wet. The immersed the wet wire mesh in an aqueous solution of 50 g of polyvinyl alcohol (PVA) at room temperature for 1 h. For further gelation, the mesh was subjected to a freeze–thaw cycle (freezing at − 10 °C for 2 h and thawing at 30 °C for 2 h). The prepared sample was washed several times with distilled water to remove residual reagent (redundant oxalic acid et al.), and CHCM was prepared.
Oil/water separation
Oils such as n-hexane, pump oil, petroleum liquid, hexadecane, cyclohexane, xylene, etc. were employed in our study, and then the oil and water were mixed and tested for oil/water separation experiments. The water was dyed with methylene blue so that it can be clearly distinguished from the oil. The CHCMs were placed between two 25 mm diameter glass tubes. Firstly, the CHCMs were thoroughly wetted with water before the oil/water separation, then a mixture of oil and water (50% v/v) was poured into the upper tube drive through CHCM by gravity. The calculation method of separation efficiency was based on the following formula: η = (m1/m0) * 100, m0, m1 represented the mass of water before and after the separation process, respectively. In addition, the separation performance of the mesh was measured in different concentrations of NaCl solution.
Characterization
Field-emission scanning electron microscopy (FESEM, Zeiss SYPRA 55, Carl, Germany) was used to observe the microscopic surface. The wettability & contact angle of water and oil on the mesh surface at room temperature were tested using the OCA2000 contact angle measuring instrument from DPIC, Germany.
Results and discussion
The fabrication and morphology of CHCMs
In view of the advantages of large-scale oil/water separation ability, high mechanical strength and high filter efficiency, wire mesh was used as oil/water separation matrix in this research (Ma et al. 2017; Zhang et al. 2018; Zhou et al. 2017). More importantly, the active metal surface provided a wide range of methods for surface modification (Feng et al. 2004). The cellulose was firstly dissolved in the DES (choline chloride and oxalic acid). The detailed dissolution mechanism was that the cellulose fiber bundles unwound, and the fiber bundle was decomposed into fiber chains by DES, when cellulose is added to DES. The dissociated [Ch]+ cation and [Cl]− anion in DES diffused into the cellulose molecular chain space and destroyed hydrogen bonds. The fiber bundles are untangled into chains with the help of DES. Here DES could not only be used as a solvent for dissolving fibers, but also as a catalyst to modify the hydroxyl groups in the cellulose surface.(Li et al. 2017; Liu et al. 2017). One carboxyl group of oxalic acid dehydrated and condensed with the hydroxyl group on the cellulose surface to attach the other carboxyl group, resulting in an increase in the hydrophilicity of the cellulose surface. The large number of hydroxyl groups in PVA will firstly react with the carboxyl groups on the surface of the dissolved cellulose to bond together. As shown in Scheme 1, the wire mesh was immersed in a hydrophilic DES cellulose solution and easily coated (Thoniyot et al. 2015), the subsequent addition of PVA to the solution increased the gelation in wire mesh. In particular, freeze/thaw cross-linking method can form a stable hydrogel coating in the CHCM surface, which was a simple, easy and eco-friendly preparation process in a mild reaction condition (Chen et al. 2017).
In order to explore the mechanism of wire mesh before and after coating, the samples were characterized and analyzed by SEM. The SEM image of the initial wire mesh with different magnification was shown in Fig. 1a and d. The average wire diameter and mesh pore size was about 30 and 45 μm, respectively. The uncoated wire was relatively flat and just a little grooves and bulges on the wire mesh that might be formed during extrusion during preparation and storage (Zhang et al. 2018). After cellulose hydrogel coated, a significant change was occurred on the surface morphology of the wire mesh (Fig. 1b, e). The cellulose hydrogel was tightly bound to the surface of the wire mesh, while the pores leave only a partial blockage for the passage of water (Fig. 1b). The surface of CHCM showed microscopic roughness and was arranged of microscale holes and hydrogel flocculated particles in the magnified images (Fig. 1e). The surface roughness of CHCM and the strong hydrophilicity to water were the key to its super-hydrophilicity in air and super-oleophobicity in water (Ju et al. 2008). As shown in Fig. 1e, f, the hydrogel layer has a thickness of about 20 μm. Super-hydrophilic and micro/nanostructures were critical for functionalized mesh to adsorb and lock water to form an oil-repellent barrier, creating underwater super-oleophobicity (Li et al. 2012; Zhu et al. 2014) (see Supporting Information Fig. S1). The mapping spectra of original wire mesh (Fig. S2) showed that the major element was Fe, Mn and C. After cellulose hydrogel coated wire mesh (Fig. S3), the element of C and O was increased in the surface and the voids of the mesh, confirming the success of cellulose hydrogel coated of CHCM.
The wettability of CHCMs
The surface wetting behavior of the initial wire mesh and CHCM were measured in detail by a CA analyzer, respectively. The test results suggested that the wettability of a solid surface mainly depends on the chemical composition and micro/nanostructure of the material (Obaid et al. 2015; Zhou et al. 2016a, b). As shown in Fig. 2a and Video S1, the intrinsic water CA of the initial wire mesh was 104.98 ± 1.0° due to its smooth surface. While coated with cellulose hydrogel, CHCM displayed special wettabilities because the mesh surface of the cellulose hydrogel after DES treatment contained a large number of hydrophilic groups (including ·OH, –COOH, etc.) (Liu et al. 2017). In this case, the water droplets spread rapidly on the CHCM with a CA value of 0° (Fig. 2b and Video S2). In addition, 0° (Fig. 2c) of the contact angle was detected when CHCM was infiltrated by pump oil, which indicated its super-hydrophilicity was the result of the synergy between the inherent high surface energy and micro-roughness (Zhang et al. 2013a, b). The measured CA for 1, 2-dichloroethane droplets was ~ 138°, exhibiting excellent underwater super-oleophobicity. When the surface of the CHCM was encapsulated by water, the micro-nano roughness of the surface taped the water to form a water cushion, followed with created repulsion between non-polar (oil) molecules and the polar (water) molecules. Therefore, CHCM exhibited remarkable super-oleophobicity under water (Zhou et al. 2016a, b).
The analyses of oil repellency, oil resistance and oil/water separation ability of CHCMs
The oil repellency and resistance function of the CHCM are important performance factors for oil/water separation. As the whole treatment process demonstrated in Fig. S4d, we firstly wet the mesh in water and then in the pump oil. Subsequently, the mesh was removed into fresh water again. When original wire mesh was immersed in water, the pump oil sticks to the original screen even if it was shaken arbitrarily in the water (Fig. S4b). On the contrary, CHCM would become clean again when it was soaking in water (Fig. S4c), which indicated that CHCM has low adhesion to oil droplets. The obtained results above indicate that the cellulose hydrogel layer can effectively prevent CHCM from being contaminated or blocked by grease during the oil/water separation process, thereby providing a possibility for its good recyclability (Ao et al. 2018; Gu et al. 2014).
Video S3, Video S4, Fig. 3 and Fig. S5 showed the oil/water separation process of the original mesh and CMCH, respectively. A 50% v/v mixture of pump oil and water (methylene blue stained) was poured onto the original wire mesh or CMCH, which was pre-wetted with water and installed in the middle of the oil–water separator (Zhang and Seeger 2011). Pump oil and water was easily passed through the original wire mesh by the gravity as driving force (Fig. S5a). On the contrary, CMCH has the high efficacy to separate oil and water. Pump oil was trapped above the mesh and the water was easily passed through the super-hydrophilic CHCM (Fig. S5b). The results showed that the cellulose hydrogel coating provided a large number of super-hydrophilic functional groups and played an important role in oil/water separation. In this way, the other oil/water mixtures could also be successfully separated (Zhang et al. 2013b). Figure 3c, d is an optical microscope image of the oil–water mixture before and after separation by CHCM. A large amount of oil droplets appeared in the microscope field of the oil–water mixture before separation (Fig. 3c). However, the supernatant of the oil–water mixture separated by CHCM became transparent and colorless, which proved that the oil droplets could be effectively blocked and separated.
Photographs of the oil/water separation process using a the original wire mesh and b the CHCM. Pump oil was used as the typical oil and dyed water with methylene blue for clear observation. Optical microscope images of the oil-in-water emulsion c before and d after separation by the CHCM. Insets in c and d: photos of the emulsion c before and d after the filtration process
The CHCM was subjected to oil–water separation test using hexane, cyclohexane, petroleum liquid, pump oil, hexadecane or xylene. In the condition of underwater, the CAs and SAs test results for six oils were collected in Fig. 4a. As seen, CA was greater than 130° and CA hysteresis was less than 5°, indicating that CMCH has good underwater oil repellency. The oil intrusion pressure (Pin) indicated the maximum oil column height (hmax) that the CMCH could withstand. The intrusion pressure (Pin) was determined by the following formula (Chen et al. 2016; Xue et al. 2013):
where ρ was the density of the oil and g was the acceleration of gravity. Based on the measured hmax value, the Pin of oil was calculated as shown in Fig. 4b. The intrusion pressure of n-hexane and hexadecane was more than 3.5 kPa, and the intrusion pressure of other oils was also greater than 2.0 kPa. The CMCH has a good ability to support oil when immersed in water.
a Underwater CAs and SAs of different oils on CHCM, b intrusion pressure of the pre-wetted CHCM for different oils, c the separation efficiency and d water flux of different oil/water mixtures. Oil/water separation efficiency (η) was a separation capability which could quantitatively, e durability of the CHCM. Separation efficiency values after continuous separation of the pump oil/water mixture for 10 cycles and the hexadecane/water mixture for 10 cycles, f separation efficiency of the CHCM for separating the pump oil/water mixture in a high salt environment and immersing them in different concentrations of NaCl solution
Oil/water separation efficiency (η) was a separation capability which could quantitatively describe the CMCH (Tao et al. 2014). As seen in Fig. 4c, the separation efficiency of CHCM for the pump oil/water mixture was about 98.8%, and the separation efficiency for other oil–water mixtures was over 98.0%. The water flux was an important indicator of the separation ability by gravity of the mesh. The formula for calculating the water flux (Fwater) of CMCH was as follows (Gao et al. 2014; Huang et al. 2015; Zhou et al. 2013):
where V was the volume of water in the oil–water mixture, S was the cross-sectional area of the mesh contact oil–water mixture, and t was the time required for complete penetration of water (Xue et al. 2013). The water flux of the CMCH reached above 20,000 L m−2 h−1 for all oils tested. The water flux of hexane and hexadecane is as high as 30,000 L m−2 h−1(as shown in Fig. 4d). The water flux of CMCH was much higher than many other meshes (Table S1), and has obvious advantages in quickly separating large amounts of oil/water mixtures. It was mainly the function of unblocked pores and hydrophilic gel layer on the mesh. In many respects, CHCM’s performance in oil–water separation was superior to the various materials reported earlier (Ao et al. 2017, 2018; Chen et al. 2016; Xue et al. 2011).
The purity of the separated oil was examined by infrared spectroscopy (FTIR) as shown in Fig. S6a, all of the absorption bands were characteristically vibrated by the alkane and ester of the oil itself. No new functional groups entered through the oil/water separation process, which implied that the high purity of the separation oil. The results showed that no other impurities were present in the six separated oils (Zhou et al. 2016a, b).
The recyclability of CMCH
The durability of the CHCM was a significant issue in the practical application of oil/water separation. The recoverability of CMCH was tested by circulating oil/water separation for more than 20 times (Xue et al. 2013). The CHCM was firstly used to separate the pump oil/water mixture for 10 cycles and then hexadecane/water was separated for another 10 cycles (Chen et al. 2016; Ma et al. 2017). As shown in Fig. 4e, the separation efficiency remained above 98% for the pump oil/water mixture and the hexadecane/water mixture at 20 cycles, and the CHCM remained 20 times after the excellent underwater oil repellency reuse (Fig. S7a), which was showing good recyclability of CMCH oil/water separation. As noted above, the super-hydrophilicity resulting from hydrogel coating was critical to underwater super-oleophobic performance. After hydrogel coating, the surface of the wire mesh could be minimized to maintain a balance between the hydrophilic surface (high surface energy medium) and the hydrophobic air (low surface energy medium) (Gao et al. 2014; Xue et al. 2011). Therefore, when the sample was stored in the air, the super-hydrophilicity of the coated hydrogel surface was lower. However, when the treated CHCM was stored in water (high surface energy medium), the interface energy was low, so the wettability recovery was greatly limited (Thoniyot et al. 2015). When CMCH was used for oil–water separation, it must be wetted with water to maintain its stability well. As shown in Fig. S7b, the underwater CAs of the pump oil did not change much after immersing in water for 90 h, indicating that the underwater super-oleophobicity was stable. After 90 h of immersion, the separation efficiency of the pump oil/water mixture was maintained above 98%, indicating that the wet mesh has strong oil/water separation ability.
The anti-salty capability of CHCMs
Considering that oil spills often occur at sea, actual oil–water separation often occurs in seawater with high salt concentrations (Wang et al. 2015; Li et al. 2016; Liu et al. 2016). Conventional membrane separation materials were subject to high salt and serious pollution during oil–water separation, resulting in a sharp drop in separation flux and oil–water separation efficiency. The research and analysis of the anti-salty capability of CHCM was shown in Fig. S8. The process of CHCM separating oil and saturated NaCl mixed solution was showed in Fig. S8a, b. The AgNO3 solution was placed in a bottom beaker to detect Cl− in the separated solution. When the mixed solution was poured into CHCM, the oil was blocked on the surface of the mesh, but the saturated NaCl solution quickly penetrates into the CHCM under the action of gravity (Maguire-Boyle and Barron 2011). There was no apparent light-yellow pump oil in the white precipitate of AgCl, which was demonstrated good screening ability. For different salt concentrations of NaCl solution (1%, 5%, 10%, 15%, 25% and saturated NaCl solution) and pump oil mixture, the separation efficiency was above 98% (Fig. 4f), the results showed that the mesh has good salt resistance. The excellent salt tolerance might be due to the strong hydrogen bonding enhancing the stable cross-linked hydrogel network. These characteristics were highly desirable in actual seawater spill cleanup (Feng et al. 2004; Thoniyot et al. 2015).
The mechanism of CMCH
In order to study the super-hydrophilic characteristics of CHCM surface, XPS spectroscopy was used to determine the functional groups on the surface of the material. The high-resolution of C1s and O1s in XPS was shown at Fig. 5. The C1s spectrum of CHCM is decomposed into three peaks (Fig. 5a). The carboxyl function of carbon, such as –COOR and –COOH at 289.2 eV with hydrophilic properties occupies 14.2%. The spectrum of the O1s of the hydrogel is decomposed into four peaks (Fig. 5b). The hydroxyl content is 15.4%, and the hydrogen bond content of CHCM is as high as 41.2%. Due to the presence of these super-hydrophilic functional groups, the CHCM oil–water separation characteristics can be highlighted. The hydrogel mesh formed by the combination of DES and cellulose has super hydrophilic and oil-repellent properties. Therefore, CHCM plays the role of super-hydrophilicity and underwater oleophobicity in the process of oil–water separation. In addition, such interesting property originated from the super-hydrophilic functional groups (–OH, –COOH and –COOR) in the CHCM, which interacted with water molecules to form a thin layer of water on sample surface, and the inherent immiscibility between water and oil resulted in the repellence to oil compounds during the filtration process (Cai and Ma 2019; Huang et al. 2015; Phiri et al. 2019; Xue et al. 2011; Zhang et al. 2018).
Finally, the paper proves that the oil–water separation material obtained by combining DES with cellulose and wire mesh has certain advantages through testing and characterization. DES dissolved cellulose was a relatively environmentally friendly, easy-to-obtain process at this stage compared to other dissolved cellulose processes.
Conclusion
In summary, we report the use of DES to dissolve cellulose and coating a wire mesh to form a hydrogel for oil/water separation. The results of SEM and FTIR showed that the surface of cellulose was modified after DES dissolved cellulose, and hydrophilic carboxyl group coverage was obtained. The prepared super-hydrophilic mesh has super-oleophobic property in water, and achieve 98% oil–water separation efficiency. After several cycles of separation, CHCM could still maintain efficient oil/water separation efficiency. It was maintaining a high CA even after long-term underwater storage. It shows that CHCM has good recyclability and durability. In particular, the production scheme is simple, environmentally friendly, efficient and versatile, which could provide a new perspective on the actual solution to the pollution caused by oily industrial wastewater and oil spills.
Supporting information
Figure showing the schematic illustration of the separation mechanism, the mapping of uncoated/coated wire mesh and oil repellence and anti-oil fouling properties of the CHCM.
Process of water droplets entered an original wire mesh, showing good hydrophobicity (AVI). The cellulose hydrogel coated wire mesh droplets permeate after gravity and flow out. CHCM exhibits good hydrophilicity (AVI). Process of pump oil/water separation by uncoated wire mesh (AVI). Process of pump oil/water separation by cellulose hydrogel coated wire mesh (AVI).
References
Ao C, Yuan W, Zhao J, He X, Zhang X, Li Q, Xia T, Zhang W, Lu C (2017) Superhydrophilic graphene oxide@electrospun cellulose nanofiber hybrid membrane for high-efficiency oil/water separation. Carbohydr Polym 175:216–222
Ao C, Hu R, Zhao J, Zhang X, Li Q, Xia T, Zhang W, Lu C (2018) Reusable, salt-tolerant and superhydrophilic cellulose hydrogel-coated mesh for efficient gravity-driven oil/water separation. Chem Eng J 338:271–277
Cai D, Ma P (2019) Hydrogel-coated basalt fibre with superhydrophilic and underwater superoleophobic performance for oil–water separation. Compos Commun 14:1–6
Cao Y, Zhang X, Tao L, Li K, Xue Z, Feng L, Wei Y (2013) Mussel-inspired chemistry and michael addition reaction for efficient oil/water separation. ACS Appl Mater Interfaces 5:4438–4442
Chen F, Song J, Liu Z, Liu J, Zheng H, Huang S, Sun J, Xu W, Liu X (2016) Atmospheric pressure plasma functionalized polymer mesh: an environmentally friendly and efficient tool for oil/water separation. ACS Sustain Chem Eng 4:6828–6837
Chen J, Shi X, Ren L, Wang Y (2017) Graphene oxide/PVA inorganic/organic interpenetrating hydrogels with excellent mechanical properties and biocompatibility. Carbon 111:18–27
De France KJ, Chan KJW, Cranston ED, Hoare T (2016) Enhanced mechanical properties in cellulose nanocrystal–poly(oligoethylene glycol methacrylate) injectable nanocomposite hydrogels through control of physical and chemical cross-linking. Biomacromol 17:649–660
De France KJ, Hoare T, Cranston ED (2017) Review of hydrogels and aerogels containing nanocellulose. Chem Mater 29:4609–4631
Ejaz Ahmed F, Lalia BS, Hilal N, Hashaikeh R (2014) Underwater superoleophobic cellulose/electrospun PVDF–HFP membranes for efficient oil/water separation. Desalination 344:48–54
Errokh A, Magnin A, Putaux J, Boufi S (2018) Morphology of the nanocellulose produced by periodate oxidation and reductive treatment of cellulose fibers. Cellulose 25:3899–3911
Feng L, Zhang Z, Mai Z, Ma Y, Liu B, Jiang L, Zhu D (2004) A super-hydrophobic and super-oleophilic coating mesh film for the separation of oil and water. Angew Chem 116:2046–2048
Gao X, Xu L, Xue Z, Feng L, Peng J, Wen Y, Wang S, Zhang X (2014) Dual-scaled porous nitrocellulose membranes with underwater superoleophobicity for highly efficient oil/water separation. Adv Mater 26:1771–1775
Ge J, Zhao H, Zhu H, Huang J, Shi L, Yu S (2016) Advanced sorbents for oil-spill cleanup: recent advances and future perspectives. Adv Mater 28:10459–10490
Gu J, Xiao P, Chen J, Zhang J, Huang Y, Chen T (2014) Janus polymer/carbon nanotube hybrid membranes for oil/water separation. ACS Appl Mater Interfaces 6:16204–16209
Huang Y, Li H, Wang L, Qiao Y, Tang C, Jung C, Yoon Y, Li S, Yu M (2015) Ultrafiltration membranes with structure-optimized graphene-oxide coatings for antifouling oil/water separation. Adv Mater Interfaces 2:1400433
Ju H, McCloskey BD, Sagle AC, Wu Y, Kusuma VA, Freeman BD (2008) Crosslinked poly(ethylene oxide) fouling resistant coating materials for oil/water separation. J Membr Sci 307:260–267
Li J, Shi L, Chen Y, Zhang Y, Guo Z, Su B, Liu W (2012) Stable superhydrophobic coatings from thiol-ligand nanocrystals and their application in oil/water separation. J Mater Chem 22:9774
Li L, Zhang G, Su Z (2016a) One-step assembly of phytic acid metal complexes for superhydrophilic coatings. Angew Chem Int Ed 55:9093–9096
Li Y, Zhang H, Fan M, Zhuang J, Chen L (2016b) A robust salt-tolerant superoleophobic aerogel inspired by seaweed for efficient oil–water separation in marine environments. Phys Chem Chem Phys PCCP 18:25394–25400
Li P, Sirviö JA, Haapala A, Liimatainen H (2017) Cellulose nanofibrils from nonderivatizing urea-based deep eutectic solvent pretreatments. ACS Appl Mater Interfaces 9:2846–2855
Liu L, Chen C, Yang S, Xie H, Gong M, Xu X (2016) Fabrication of superhydrophilic-underwater superoleophobic inorganic anti-corrosive membranes for high-efficiency oil/water separation. Phys Chem Chem Phys 18:1317–1325
Liu Y, Guo B, Xia Q, Meng J, Chen W, Liu S, Wang Q, Liu Y, Li J, Yu H (2017) Efficient cleavage of strong hydrogen bonds in cotton by deep eutectic solvents and facile fabrication of cellulose nanocrystals in high yields. ACS Sustain Chem Eng 5:7623–7631
Ma Q, Cheng H, Fane AG, Wang R, Zhang H (2016) Recent development of advanced materials with special wettability for selective oil/water separation. Small 12:2186–2202
Ma Q, Cheng H, Yu Y, Huang Y, Lu Q, Han S, Chen J, Wang R, Fane AG, Zhang H (2017) Preparation of superhydrophilic and underwater superoleophobic nanofiber-based meshes from waste glass for multifunctional oil/water separation. Small 13:1700391
Maguire-Boyle SJ, Barron AR (2011) A new functionalization strategy for oil/water separation membranes. J Membr Sci 382:107–115
Obaid M, Barakat NAM, Fadali OA, Motlak M, Almajid AA, Khalil KA (2015) Effective and reusable oil/water separation membranes based on modified polysulfone electrospun nanofiber mats. Chem Eng J 259:449–456
Pan Q, Wang M, Wang H (2008) Separating small amount of water and hydrophobic solvents by novel superhydrophobic copper meshes. Appl Surf Sci 254:6002–6006
Phiri I, Eum KY, Kim JW, Choi WS, Kim SH, Ko JM, Jung H (2019) Simultaneous complementary oil–water separation and water desalination using functionalized woven glass fiber membranes. J Ind Eng Chem 73:78–86
Su B, Tian Y, Jiang L (2016) Bioinspired interfaces with superwettability: from materials to chemistry. J Am Chem Soc 138:1727–1748
Tao M, Xue L, Liu F, Jiang L (2014) An intelligent superwetting PVDF membrane showing switchable transport performance for oil/water separation. Adv Mater 26:2943–2948
Thoniyot P, Tan MJ, Karim AA, Young DJ, Loh XJ (2015) Nanoparticle–hydrogel composites: concept, design, and applications of these promising, multi-functional materials. Adv Sci 2:1400010
Wang B, Liang W, Guo Z, Liu W (2015) Biomimetic super-lyophobic and super-lyophilic materials applied for oil/water separation: a new strategy beyond nature. Chem Soc Rev 44:336–361
Wang X, Xu S, Tan Y, Du J, Wang J (2016a) Synthesis and characterization of a porous and hydrophobic cellulose-based composite for efficient and fast oil–water separation. Carbohydr Polym 140:188–194
Wang X, Yu J, Sun G, Ding B (2016b) Electrospun nanofibrous materials: a versatile medium for effective oil/water separation. Mater Today 19:403–414
Xue Z, Wang S, Lin L, Chen L, Liu M, Feng L, Jiang L (2011) A novel superhydrophilic and underwater superoleophobic hydrogel-coated mesh for oil/water separation. Adv Mater 23:4270–4273
Xue C, Ji P, Zhang P, Li Y, Jia S (2013) Fabrication of superhydrophobic and superoleophilic textiles for oil–water separation. Appl Surf Sci 284:464–471
Xue Z, Cao Y, Liu N, Feng L, Jiang L (2014) Special wettable materials for oil/water separation. J Mater Chem A 2:2445–2460
Yin K, Chu D, Dong X, Wang C, Duan J, He J (2017) Femtosecond laser induced robust periodic nanoripple structured mesh for highly efficient oil–water separation. Nanoscale 9:14229–14235
Yoon H, Na S, Choi J, Latthe SS, Swihart MT, Al-Deyab SS, Yoon SS (2014) Gravity-driven hybrid membrane for oleophobic–superhydrophilic oil–water separation and water purification by graphene. Langmuir 30:11761–11769
Yu Z, Yun FF, Gong Z, Yao Q, Dou S, Liu K, Jiang L, Wang X (2017) A novel reusable superhydrophilic NiO/Ni mesh produced by a facile fabrication method for superior oil/water separation. J Mater Chem A 5:10821–10826
Zhang J, Seeger S (2011) Polyester materials with superwetting silicone nanofilaments for oil/water separation and selective oil absorption. Adv Funct Mater 21:4699–4704
Zhang S, Lu F, Tao L, Liu N, Gao C, Feng L, Wei Y (2013a) Bio-inspired anti-oil-fouling chitosan-coated mesh for oil/water separation suitable for broad pH range and hyper-saline environments. ACS Appl Mater Interfaces 5:11971–11976
Zhang X, Li Z, Liu K, Jiang L (2013b) Bioinspired multifunctional foam with self-cleaning and oil/water separation. Adv Funct Mater 23:2881–2886
Zhang Q, Liu N, Wei Y, Feng L (2018) Facile fabrication of hydrogel coated membrane for controllable and selective oil-in-water emulsion separation. Soft Matter 14:2649–2654
Zhou X, Zhang Z, Xu X, Guo F, Zhu X, Men X, Ge B (2013) Robust and durable superhydrophobic cotton fabrics for oil/water separation. ACS Appl Mater Interfaces 5:7208–7214
Zhou K, Zhang QG, Li HM, Guo NN, Zhu AM, Liu QL (2014) Ultrathin cellulose nanosheet membranes for superfast separation of oil-in-water nanoemulsions. Nanoscale 6:10363
Zhou C, Cheng J, Hou K, Zhao A, Pi P, Wen X, Xu S (2016a) Superhydrophilic and underwater superoleophobic titania nanowires surface for oil repellency and oil/water separation. Chem Eng J 301:249–256
Zhou S, Liu P, Wang M, Zhao H, Yang J, Xu F (2016b) Sustainable, reusable, and superhydrophobic aerogels from microfibrillated cellulose for highly effective oil/water separation. ACS Sustain Chem Eng 4:6409–6416
Zhou C, Cheng J, Hou K, Zhu Z, Zheng Y (2017) Preparation of CuWO4@ Cu2O film on copper mesh by anodization for oil/water separation and aqueous pollutant degradation. Chem Eng J 307:803–811
Zhu X, Tu W, Wee K, Bai R (2014) Effective and low fouling oil/water separation by a novel hollow fiber membrane with both hydrophilic and oleophobic surface properties. J Membr Sci 466:36–44
Acknowledgments
This work was supported by the Natural Science Foundation of China (Nos. 21978248, 21676223), the special fund for Fujian Ocean High-Tech Industry Development (No. FJHJF-L-2018-1), China, and the Natural Science Foundation of Fujian, China (No. 2019J06005).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 (MP4 36295 kb)
Supplementary material 3 (MP4 54423 kb)
Supplementary material 4 (MP4 27257 kb)
Supplementary material 5 (MP4 22381 kb)
Rights and permissions
About this article
Cite this article
Wang, H., Li, J., Yu, X. et al. Facile fabrication of super-hydrophilic cellulose hydrogel-coated mesh using deep eutectic solvent for efficient gravity-driven oil/water separation. Cellulose 28, 949–960 (2021). https://doi.org/10.1007/s10570-020-03578-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03578-9