Abstract
The ubiquitous nature of lignocellulosic biomass on planet earth and its economic viability attracted a great deal of attention from researchers and becomes foremost feedstock for biofuel production particularly bioethanol. However, due to complexity in structure, its pretreatment is essentially required prior to actual use. In the present study, a promising approach has been proposed through the development of acid-functionalized magnetic nanocatalysts. Two different acid-functionalized magnetic nanocatalysts i.e. alkylsulfonic acid functionalized magnetic nanoparticles (Fe3O4-MNPs-Si-AS) and butylcarboxylic acid functionalized magnetic nanoparticles (Fe3O4-MNPs-Si-BCOOH) were developed and their efficacy was studied in the pretreatment of sugarcane straw at varying concentrations (100, 200, 300, 400, 500 mg/g of straw). The enhanced concentration dependent production of sugar (xylose) was reported in case of both the nanocatalysts. The maximum 17.06 g/L for Fe3O4-MNPs-Si-AS and 15.40 g/L for Fe3O4-MNPs-Si-BCOOH sugar was reported at 500 mg which is comparatively higher than normal acid (H2SO4) (14.63 g/L) and non-treated (0.24 g/L) sugarcane straw. Further, both the nanocatalysts were recovered by applying an external magnetic field and reused for the next two subsequent cycles of pretreatment. It was observed that with every reuse of nanocatalysts the concentration of sugar production was reduced. Moreover, generation of very less amount of toxic inhibitors was reported in the hemicellulosic hydrolyzate obtained in the present study. Considering these facts, it is believed that such nanocatalysts can be used as an effective, eco-friendly and economically viable alternative to the conventional pretreatment agents like mineral acids.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanotechnology is an emerging branch of science and has enormous potential to provide solutions for most complex issues in various fields. It is confirmed from the various studies that nanotechnology in general and nanomaterials, in particular, have huge applications in biorefineries for the production of biofuels and value-added chemicals (Shuttleworth et al. 2014). Most of the conventional biorefining technologies currently in use are very old and hence, there is a great challenge of developing novel alternative methods that can be implemented in order to revolutionize systems while enabling greater efficiency and advantages to the detriment of those previously agreed upon. One of such common biorefining technology is the pretreatment of lignocellulosic biomasses (Galbe and Wallberg 2019; Bhati et al. 2020).
Lignocellulosic biomasses are the most preferably used feedstocks in the second generation biorefineries for the production of various valuable bio-based products due to its universal availability at low cost (Kucharska et al. 2018). However, the complex structure of these biomasses requires their pre-processing to release the carbohydrate (hemicellulose and cellulose) and non-carbohydrate (lignin) polymers present in it. Hemicellulose and cellulose are the prime sources of fermentable sugars and hence, they can be released through the pretreatment of biomass. Many physical, chemical and biological pretreatment methods have been proposed and routinely used depending on the type of lignocellulosic biomass (Ingle et al. 2019). Among the chemical pretreatment approaches, concentrated and dilute acid hydrolysis using acids such as H2SO4 and HCl are most preferably used for a variety of feedstocks due to its simple nature and easy operation. However, both concentrated and dilute acid pretreatments have some disadvantages e.g. concentrated acid pretreatment is comparatively effective in biomass hydrolysis but it is expensive, and required specialized reactor or equipments as it is highly corrosive. Moreover, thus used acid needs to be removed from the reaction mixture after pretreatment through extensive washing which again increases the process cost (Keskin et al. 2019).
On the contrary, dilute acid is preferred over concentrated for the pretreatment of biomass, but it also has some of the above-mentioned limitations. In addition, the generation of toxic inhibitors due to acid pretreatment is another major concern. The presence of toxic inhibitors such as acetic acid, furfural and hydroxymethylfurfural (HMF) in the hydrolyzate affects the effectiveness of the further processes like enzymatic hydrolysis and also degrade the sugars released during hydrolysis. Therefore, removal of such toxic inhibitors is essentially required but needs extensive washing and detoxification using different agents before the fermentation of sugars (Jong and Gosselink 2014).
All the above-discussed concerns associated with acid pretreatment methods create an urgent need to search and develop novel catalysts for effective pretreatment of lignocellulosic biomasses. In this context, some of the efforts were made and a few nanotechnology-based approaches have been proposed (Arora et al. 2020). However, there were only a few reports available on the direct application of nanomaterials in the pretreatment of lignocellulosic biomass and hence, considering the potential of nanotechnology further extensive studies are essentially required. As far as pretreatment of lignocellulosic biomasses is concerned Pena and colleagues were the first researchers who proposed the application of perfluoroalkylsufonic (PFS) and alkylsulfonic acid-functionalized magnetic nanoparticles (MNPs) for the pretreatment of wheat straw. The authors recorded promising efficacy of thus used acid-functionalized MNPs, moreover, they have also recovered and reused the nanocatalyst for multiple cycles of pretreatment (Pena et al. 2012). Further, Lai et al. (2011) demonstrated the synthesis of sulfonated silica MNPs composites and used these nanocomposites as a catalyst in the hydrolysis of lignocellulosic biomass. Moreover, the results obtained suggested the promising application of nanocatalysts in the hydrolysis of biomass. Apart from these, carbon-based solid catalysts functionalized with sulfonic acid have been used for the pretreatment of corncob. The results obtained reported the release of the high yield of xylose (78.1%) (Qi et al. 2018). Similarly, Su et al. (2015) demonstrated the synthesis of sulfonated magnetic carbonaceous acid nanoparticles and evaluated their efficacy for the hydrolysis of various lignocellulosic biomasses such as jatropha, bagasse, and Plukenetia hulls with significant levels of conversion. Moreover, Wang et al. (2015) reported the promising catalytic activity of sulfonic acid functionalized silica-coated crystalline Fe/Fe3O4 core/ shell MNPs in the hydrolysis of lignocellulosic biomass and for biofuel production.
In addition to the above-mentioned reports, there are few studies in which the authors studied the efficacy of acid-functionalized MNPs in the hydrolysis of commercial biomolecules which are directly or indirectly present in the lignocellulosic biomasses such as cellobiose, sucrose, starch, etc. Dhepe and Sahu (2010) reported the synthesis of sulfonic acid-modified mesoporous silica and studied their catalytic efficacy in the hydrolysis of sucrose and starch. The results obtained suggested promising catalytic efficacy of thus used nanocatalyst with a high degree of hydrolysis. In another study, magnetic silica-protected cobalt-spinel ferrite nanoparticles were synthesized followed by the functionalization with different acids such as perfluoroalkylsulfonic acid, alkylsulfonic acid and butylcarboxylic acid. Further, thus developed nanocatalysts were tested for their catalytic activity in the cleavage of β-(1–4) glycosidic bonds of cellobiose. The maximum efficacy (78% conversion of cellobiose) was recorded in the case of alkylsulfonic functionalized nanoparticles. Moreover, the authors also proposed the possibilities for the reuse of these nanocatalysts (Pena et al. 2011). Recently, Ingle et al. (2020a) have also synthesized three different acid-functionalized MNPs i.e. alkylsulfonic, butylcarboxylic and sulphonic acid-functionalized MNPs and demonstrated their catalytic efficacy in the hydrolysis of cellobiose. The results obtained indicated the promising catalytic nature of thus synthesized nanocatalyst at ambient reaction conditions.
All the above-discussed studies proposed the feasibility of nanocatalysts in the effective pretreatment of different lignocellulosic biomass and it was also suggested that the magnetic nature of nanocatalysts helps in easy recovery and reuse of same catalysts in multiple cycles of pretreatment thereby minimizing cost involved in the process. Considering these facts, we have synthesized two different nanocatalysts through the functionalization of MNPs with two different acids i.e. alkylsulfonic acid (Fe3O4-MNPs-Si-AS) and butylcarboxylic acid (Fe3O4-MNPs-Si-BCOOH) MNPs and studied their efficacy in the pretreatment of sugarcane bagasse. The results obtained suggested significant efficacy of thus used nanocatalysts with recovery and reuse for two more cycles of pretreatment (Ingle et al. 2020b). Therefore, in the present study, we planned to extend the use of the thus developed nanocatalysts (Fe3O4-MNPs-Si-AS and Fe3O4-MNPs-Si-BCOOH) for the pretreatment of another lignocellulosic biomass i.e. sugarcane straw (dried leaves and small stalk of sugarcane).
Materials and methods
Materials
Chemicals and reagents
All the chemicals and reagents used in different experiments of the present study e.g. FeSO4.7H2O (ferrous sulfate), NH4OH (ammonium hydroxide), NaOH (sodium hydroxide), isopropanol and methanol were purchased from LabSynth Laboratory Products Ltd. (Sao Paulo, Brazil). C2H5OH (ethanol-96%) and H2O2 (hydrogen peroxide), were purchased from Chromolab Laboratory Products Ltd. (Sao Paulo, Brazil). H2SO4 (sulfuric acid-98%), HCl (hydrochloric acid), MPTMS (3-mercaptopropyltrimethoxysilane—95%) and KBr (potassium bromide—IR grade) were purchased from Scharlab Laboratory Products Ltd (Sao Paulo, Brazil). Similarly, TEOS (Tetraethylorthosilicate—99.99%), and CPTES [4-(triethoxysilyl)-butyronitrile—98%] were purchased from Sigma-Aldrich Brazil Ltd. (Sao Paulo, Brazil) and SepPak C18 filter columns required for HPLC were purchased from Water Technologies Ltd. (Brazil).
Collection of sugarcane straw and determination of its chemical composition
The sugarcane straw used in the present study was collected from Ipiranga Agroindustrial (Descalvado, SP). After collection, the sugarcane straw was dried under the sun for 4–5 days and then it was milled in a blender followed by sieving with 10 mesh sieve to obtain particles with a uniform size of about 4–5 mm and stored at room temperature in plastic bags to avoid the moisture. Moreover, the chemical composition of natural sugarcane straw i.e. cellulose, hemicellulose and lignin contents was determined according to the methods proposed by Gouveia et al. (2009).
Methods
Synthesis and surface coating of magnetic nanoparticles (Fe3O4-MNPs)
The same Fe3O4-MNPs which were already synthesized and used in our previous study (i.e. Ingle et al. 2020b) were used in the present study. However, these MNPs were synthesized according to the method proposed by Gaikwad et al. (2019) with some modifications. Here, 2% solution of FeSO4.7H2O was stirred at 80 °C on the magnetic stirrer with continuous addition (dropwise) of 2 M NaOH till the pH reached 11. Later, the resulting reaction mixture was heated in a microwave oven at 320 watts for 2–5 min followed by centrifugation at 4000 rpm for 10–15 min to obtain pellet of Fe3O4-MNPs. Further, the pellet of thus synthesized Fe3O4-MNPs was washed several times with distilled water and a final wash with ethanol. Finally, the MNPs were dried in the hot-air oven at 60 °C.
The silica coating of Fe3O4-MNPs, was performed using a method proposed by Rajkumari et al. (2017) with some modifications. For this, a mixture of 100 mL ethanol, 15 mL distilled water, and 1 mL TEOS was prepared and 2 g of dried Fe3O4-MNPs were added in it. Further, this dispersion was sonicated for about 3 h followed by addition of 15 mL of 2.5 M NaOH. Later, this mixture was stirred on a magnetic stirrer for 2 h at room temperature. Finally, the surface silica-coated MNPs (Fe3O4-MNPs-Si) were separated by applying an external magnetic field followed by washing with distilled water for 3–4 times and ethanol. Further, these samples were dried in a hot-air oven at 100 °C.
Preparation of nanocatalysts
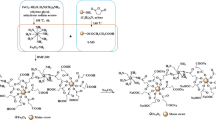
The nanocatalysts used in the present study are the different acid-functionalized Fe3O4-MNPs. These nanocatalysts were prepared by functionalization of silica-coated Fe3O4-MNPs with two different acids i.e. alkylsulfonic acid (Fe3O4-MNPs-Si-AS) and butylcarboxylic acid (Fe3O4-MNPs-Si-BCOOH) as per the method proposed by Pena et al. (2012) with some modifications as follows.
Fe3O4-MNPs-Si-AS nanocatalyst
This nanocatalyst was prepared by the addition of 1 g of Fe3O4-MNPs-Si (silica-coated nanoparticles) in a mixture of 50 mL of ethanol, 50 mL of water and 4 mL of MPTMS. The resulting mixture was then sonicated for 2 h and stirred at 80 °C for 24 h for the attachment of thiol groups. After attachment of thiol groups, the Fe3O4-MNPs-Si (i.e. Fe3O4-MNPs-Si-SH) were magnetically separated and washed 4–5 times with distilled water. Thus, recovered Fe3O4-MNPs-Si-SH nanoparticles were further added in a mixture containing 30 mL of each 50% H2O2, distilled water, and methanol. Later the mixture was kept at room temperature for two days to oxidize the thiol groups to sulfonic acid groups. The product of the oxidation step was recovered by applying a magnetic field and washed 5–6 times with a sufficient amount of distilled water and re-acidified with 50 mL of 2 M H2SO4 by incubating in a shaker at room temperature for 24 h at 200 rpm. Finally, thus prepared Fe3O4-MNPs-Si-AS nanocatalyst was again washed 3–4 times with distilled water and dried in a hot-air oven at 100 °C for 24 h.
Fe3O4-MNPs-Si-BCOOH nanocatalyst
Here, 1 g Fe3O4-MNPs-Si (silica-coated MNPs) were added to a mixture of 200 mL of 0.5 N HCl and 4 mL of CPTES and sonicated for 2 h followed by mechanical stirring at 80 °C for 24 h in a shaking incubator. Thus obtained intermediate product was then magnetically separated from the mixture and washed 3–4 times with distilled water. Further, this product was acidified by incubating it in a 2 M H2SO4 solution for 24 h to oxidize the cyano groups to carboxylic acid groups. Finally, the carboxylic acid-functionalized nanocatalyst (Fe3O4-MNPs-Si-BCOOH) was recovered using an external magnetic field from the solution and again washed with a sufficient amount of distilled water for 4–5 times and dried in the hot-air oven at 100 °C.
Characterization of Fe3O4-MNPs, Fe3O4-MNPs-Si and nanocatalysts
All the MNPs prepared in the present study i.e. Fe3O4-MNPs, Fe3O4-MNPs-Si, Fe3O4-MNPs-Si-AS and Fe3O4-MNPs-Si-BCOOH were characterized using different techniques mentioned below-.
Fourier transform infrared (FTIR) spectroscopy
FTIR analysis of all the above mentioned MNPs was performed to get an idea about different functional groups present on the surface of thus synthesized and functionalized MNPs and nanocatalysts. For this analysis, IR grade KBr and all above MNPs were dried at 100 °C overnight in a hot air oven and then the samples were prepared by mixing 1 mg of each MNPs and nanocatalysts with 100 mg of KBr. The measurements were made at wavenumbers 400–4000/cm, with the detector reading at 4/cm resolution and 32 scans per sample using a Perkin Elmer® SpectrumTM GX (Shelton, USA).
X-ray diffraction (XRD) analysis
XRD analysis was performed with an X’Pert Pro PANalytical diffractometer using various parameters and conditions mentioned below: K-Alpha 1 wavelength (λ = 1.54056 Å), K-Alpha 2 wavelength (λ = 1.54439 Å), generator voltage of 40 kV, a tube current of 35 mA and the count time of 0.5 s per 0.02° in the range of 5°–90° with a copper anode.
Transmission electron microscopy (TEM)
TEM analysis was performed to determine the shape and size of thus synthesized MNPs. For this, a drop of solution for all the above type of MNPs and nanocatalysts was placed on the carbon-coated copper grids and these samples were kept it in infrared light for drying before loading it onto a specimen holder. TEM micrographs were recorded using Zeiss Libra 120, accelerating voltage of 120 kV instrument.
Energy dispersive X-ray Spectroscopy (EDX)
EDX analysis was used to determine the elemental composition of thus synthesized Fe3O4-MNPs and acid-functionalized nanocatalysts using Hitachi S520 SEM (Hitachi, Tokyo, Japan). For this analysis, all the dried samples of MNPs and nanocatalysts were initially mounted on aluminum stubs, sputter-coated (JEOL JFC-1600) with a silver layer, and used for scanning and EDX analysis using X-ray detector of same SEM machine.
Pretreatment of sugarcane straw
The catalytic efficacy of both the acid-functionalized magnetic nanocatalysts (i.e. Fe3O4-MNPs-Si-AS and Fe3O4-MNPs-Si-BCOOH) was assessed for the pretreatment of sugarcane straw at different concentrations. For the pretreatment, 1 g of sugarcane straw was mixed with 10 mL of distilled water and various concentrations (100 mg, 200 mg, 300 mg, 400 mg, and 500 mg) of both the acid-functionalized magnetic nanocatalysts. Then the mixture was heated in a simple electrical autoclave (SOC. FABBE LTDA, Sao Paulo, Brazil, capacity 70 L) at 120 °C and 15 psi pressure for 15 min. In addition, normal acid-pretreatment [with 100 mg of H2SO4 per gram of biomass (100% dry)], and control (without any pretreatment) was also performed for the comparative evaluation of normal acid-mediated pretreatment and acid-functionalized magnetic nanocatalysts mediated pretreatments. All the experiments were performed in triplicate.
Collection of xylose and separation of nanocatalysts
After pretreatment of sugarcane straw, the hydrolyzate (liquid fraction) having xylose was separated from the solid fraction (biomass + nanocatalysts) using a 200-mesh sieve. Further, the solid fraction was mixed with a sufficient amount of distilled water and nanocatalysts were magnetically separated from sugarcane straw. Thus recovered nanocatalysts were washed 3–4 times with distilled water and dried in the hot-air oven at 60 °C and reused in the next consecutive cycles of pretreatment (second and third cycle). Moreover, the hydrolyzate containing xylose obtained from normal acid-pretreatment and non-treated sugarcane straw was also collected for sugar concentration analysis.
Reuse of nanocatalysts for further pretreatment
The acid-functionalized magnetic nanocatalysts recovered after the first cycle of pretreatment were reused in the second and third cycles of pretreatment of sugarcane straw; the same procedures mentioned above were followed for the pretreatment of sugarcane straw, collection of sugar and recovery of the used nanocatalysts.
Determination of xylose and toxic inhibitor concentrations using High-Performance Liquid Chromatography (HPLC)
The amount of xylose and acetic acid released after pretreatment of sugarcane straw in the hydrolyzate (in case of normal acid and nanocatalysts mediated pretreatments) was assessed by HPLC with a refraction index detector (Waters 410, Milford, MA, USA). The samples were diluted in a ratio of 1:1 and filtered through a SepPak C18 filter (Water Technologies Limited, Brazil). Further, samples were injected into the chromatograph, column BIO-RAD Aminex HPX-87H (7.8 × 300 mm) (Bio-Rad, Hercules, CA, USA), a temperature of 45 °C, eluent: 0.5 N H2SO4, flow 0.6 mL/min in a sample volume of 20 µL was used.
Moreover, furfural and 5-hydroxymethylfurfural (5-HMF), which are other common toxic inhibitors apart from acetic acid usually generated during acid-pretreatment and causes serious issues during enzymatic hydrolysis. Therefore, toxic inhibitors generated in the present study were analyzed using HPLC according to the method proposed by Hernández-Pérez et al. (2016). For the analysis, the samples were filtered using Minisart membranes and analyzed using ultraviolet light detector (SPD-10A UV-VIS, Waters Corp., Milford, MA, USA) with the following conditions: RP 18 (200 × 4.6 mm) column (Hewlett-Packard, Palo Alto, CA, USA) at 25 °C; injection volume (20 µL); mobile phase (acetonitrile/water 1:8) and 10% acetic acid as eluent with a flow rate of 0.8 mL/min.
Results and discussion
Chemical characterization of natural sugarcane straw
The presence of a high amount of sugars (C5- xylose; C6- glucose) and lignin in sugarcane straw attracted a great of attention from scientists around the globe. Such a rich chemical composition of sugarcane straw makes it as one of the important feedstocks for biorefining industries for the production of various valuable products. In the present study, it was observed that it contains about 32.8 ± 0.8% cellulose, 28.2 ± 0.4% hemicellulose, 29.8 ± 0.2% lignin and 2.5 ± 0.1% ash contents. These obtained results showed a resemblance with the findings reported in many of the previous studies. Hernández-Pérez et al. (2016) used sugarcane straw as promising lignocellulosic biomass for the production of xylitol, however, their observations about the chemical composition of sugarcane straw showed the presence of 31.7% cellulose, 27.0% hemicellulose, 31.1% lignin and 1.5% ash contents. Similarly, when findings of the present study were compared with available reports it was found to be in agreement with all those reports showing some minor differences in the amount of each chemical component. However, it was proposed that the variations in chemical compositions of lignocellulosic biomass are obvious and it was dependent on the various factors like the material-collecting site, climatic conditions, stage of plant development, and the variety of sugarcane (Gomez et al. 2010; Santos et al. 2012). Table 1 represents the comparative analysis of the chemical composition of sugarcane straw used in the present study and other reports available.
Synthesis and characterization of nanocatalysts
As informed above both the acid-functionalized Fe3O4-MNPs i.e. Fe3O4-MNPs-Si-AS and Fe3O4-MNPs-Si-BCOOH used in the present study were the same which synthesized and used in our previous study (Ingle et al. 2020b). These acid-functionalized magnetic nanocatalysts were prepared through the functionalization alkylsulfonic acid (Fe3O4-MNPs-Si-AS) and butylcarboxylic acid (Fe3O4-MNPs-Si-BCOOH) on silica-coated Fe3O4-MNPs. Figure 1 showed the highly magnetic Fe3O4-MNPs. Moreover, these nanocatalysts were characterized using different techniques such as FTIR, XRD, TEM, and EDX. Data obtained from the characterization of each of these techniques revealed that strongly magnetic Fe3O4-MNPs were synthesized and appropriately coated with silica which was confirmed from the FTIR and EDX analysis. Moreover, the synthesized nanocatalysts showed crystalline structure which was confirmed from XRD analysis. Similarly, TEM analysis performed showed that thus synthesized nanocatalysts were found to be spherical having a size range of 20–80 nm. The complete information on characterization and detailed discussion for these findings have already been reported in our recently published paper i.e. Ingle et al. (2020b).
Screening of nanocatalysts for their efficacy in pretreatment of sugarcane straw
Above mentioned acid-functionalized magnetic nanocatalysts (Fe3O4-MNPs-Si-AS and Fe3O4-MNPs-Si-BCOOH) were evaluated for their efficacy in the pretreatment of sugarcane straw at 120 °C for 15 min at 15 psi and different concentrations (i.e. 100 mg, 200 mg, 300 mg, 400 mg and 500 mg of each nanocatalyst per gram of biomass i.e. sugarcane straw). Moreover, similar experiments were also carried out for normal acid pretreatment and non-treated samples (control). The observations recorded in the present study clearly indicated that both the nanocatalysts showed promising efficacy towards pretreatment of sugarcane straw and it was concentration-dependent. The increase in the concentration of nanocatalysts also reported to increase the pretreatment efficacy. In the first cycle, maximum pretreatment efficacy was observed at 500 mg/ gram of biomass which was confirmed from the amount of xylose liberated after pretreatment i.e. 17.06 g/L for Fe3O4-MNPs-Si-AS and 15.40 g/L for Fe3O4-MNPs-Si-BCOOH (Fig. 2). This concentration was comparatively higher than normal acid (H2SO4) (14.63 g/L) and non-treated (0.24 g/L) sugarcane straw samples.
As discussed earlier the magnetic nature is the most promising and novel property of thus used nanocatalysts and hence such nanocatalysts have been referred to as effective and economically viable alternatives to normal acid-hydrolysis approach. In this context, after the first cycle of pretreatment, both the nanocatalysts were recovered from the reactions mixture by applying an external magnetic field and reused in the next two successive cycles for pretreatment. The same pattern discussed above was reported for second (first reuse) and third (second reuse) cycle, i.e. both the nanocatalysts at 500 mg showed maximum efficacy and it gets reduced with decrease in the concentration of nanocatalysts. However, there was a gradual decline in the pretreatment efficiencies of both the nanocatalysts was observed in each successive cycle of pretreatment (Fig. 3). It was proposed that such decrease in the pretreatment efficacy may be due to the loss of acid groups present on the surface of nanocatalysts (Pena et al. 2011, 2012). Pena et al. (2012) and Ingle et al. (2020b) have reported a gradual decrease in the concentration of sugars after pretreatment of different lignocellulosic biomass in consecutive cycles of pretreatment. However, there is a possibility to modify the process of acid-functionalization of MNPs to obtain more strongly acid-bonded nanocatalysts with high efficacy.
To date, only few studies are available on the application of any kind of nanomaterials in the pretreatment of lignocellulosic biomass. Therefore, there is limited literature available on this topic, however, the observations recorded in the present study were found in agreement with these studies. Pena et al. (2012) developed two different acid-functionalized MNPs, i.e. Perfluoroalkylsulfonic (PFS) and alkylsulfonic (AS) acid-functionalized MNPs and studied their potential for the pretreatment of wheat straw at two different conditions: 80 °C for 24 h and 160 °C for 2 h. The results obtained showed that at 80 °C for 24 h, PFS functionalized MNPs showed comparatively higher solubilization of hemicelluloses (24.0% ± 1.1%) than AS functionalized MNPs (9.1% ± 1.7%). Whereas, at 160 °C, both PFS, and AS functionalized MNPs showed promisingly higher amounts of hemicelluloses i.e. 46.3% ± 0.4% and 45% ± 1.2%, respectively.
Similarly, the same research group in their other work, studied the potential of propyl-sulfonic (PS) acid-functionalized MNPs for pretreatment of corn stover at different catalyst loads, i.e. 0.1, 0.2, and 0.3 g of catalyst/ gram of biomass at three different temperatures, 160, 180, and 200 °C for 1 h under very high pressure using specialized equipment, Parr reactor. Their observations revealed that the load of catalysts did not affect the process of hydrolysis at 160 °C, and the average glucose yield obtained from hydrolysis of corn stover at this temperature was found to be 59.0%. However, samples with a catalyst load of 0.2 g and incubated at 180 °C showed a maximum glucose yield of 90%, whereas complete hydrolysis of corn stover was reported at 200 °C (Pena et al. 2014). But, it is very important to note here that both the above-mentioned studies of Pena and groups, especially later study required very high temperature, pressure and specific equipment like Parr reactor for the pretreatment which is contradictory to the normal guidelines proposed for the development of any ideal technology or novel approach, because novel approach should involve operation at ambient reaction conditions (e.g. temperature, pressure, etc.), simple and easy operational procedure and most important it should be cost effective. Although the efficiency of these studies was good, it does not follow any of the above-proposed guidelines. Therefore, we believed that the approach proposed for the pretreatment in the present study is simple, more convenient and cost-effective. In addition, the data recorded in the present study showed a resemblance to our recent study (Ingle et al. 2020b).
Overall, all the available reports proposed that due to the strong magnetic properties, acid-functionalized MNPs can be repeatedly used for multiple cycles of pretreatment which ultimately reduce the cost involved in the process. Hence, it helps to develop a simple, effective, eco-friendly and cost-effective alternative to the normal acid-hydrolysis approach which has many disadvantages like the generation of toxic inhibitors, corrosive action, tedious washing procedure, etc. Figure 4 represents the schematic illustration of nanocatalyst mediated pretreatment of sugarcane straw, recovery and reuse of nanocatalyst. As discussed earlier, only a few studies have been performed so far in the area, hence, considering the potentials of nanocatalysts there is a need to perform extensive studies on this topic so as to develop the most effective nanocatalysts.
Determination of toxic inhibitors
It is a well-known fact and widely proved that the generation of toxic inhibitors such as acetic acid, furfural and 5-HMF during acid pretreatment is one of the major concerns. The presence of these inhibitors in the hydrolysate adversely affect the enzymatic hydrolysis of cellulose fraction and hence their removal is essentially required before further processing (Kumar and Sharma 2017). Moreover, a complete removal of such inhibitors can only be perform through the process of detoxification using different detoxifying agents which ultimately makes the down-stream processing very tedious and also increases the cost of the process.
On the contrary, in the present study, the comparatively lower amount of above-mentioned inhibitors was generated when magnetic nanocatalysts were used for the pretreatment of sugarcane straw. The concentration for acetic acid, 5HMF and furfural in case of normal acid pretreatment were 3.4 g/L, 0.8 g/L and 0.5 g/L respectively. The generation of these inhibitors in case of nanocatalysts mediated treatment was found to be concentration dependent. The samples treated with 500 mg of nanocatalysts showed higher concentration of inhibitors, however, these concentrations are much lower when compared with the concentration of inhibitors generated during normal acid pretreatment. In case of Fe3O4-MNPs-Si-AS mediated pretreatment at 500 mg, the concentration recorded for acetic acid, 5HMF and furfural were 2.30 g/L, 0.10 g/L and 0.00 g/L respectively. Whereas, in case of Fe3O4-MNPs-Si-BCCOH-mediated pretreatment at 500 mg it was found to be 2.70 g/L, 0.20 g/L and 0.00 g/L respectively. The comparison between normal and nanocatalysts mediated pretreatment clearly indicated that nanocatalysts-mediated pretreatment generates very low concentration of toxic inhibitors which is very promising output of present study. Table 2 showed the concentrations of different inhibitors generated after pretreatment of sugarcane straw using normal acid and nanocatalysts.
These observations are in complete agreement with the results reported by Hernández-Pérez et al. (2016). In their study, the authors found that the concentrations of acetic acid (2.23 g/L) was higher compared to that of 5-HMF (0.57 g/L) and furfural (0.33 g/L). Moreover, these authors also proposed that the concentrations of these toxic inhibitors are usually higher in the sugarcane straw hemicellulosic hydrolyzate than those found in the hydrolyzates of other biomasses like sugarcane bagasse, sorghum straw, rice straw, etc., however, there may be some exceptions. As stated earlier, very less amount of inhibitors was recorded in the present study which is the important merit of this approach. These findings are also in correspondence with the observations recorded by Pena et al. (2012) for acid-functionalized MNPs mediated pretreatment of wheat straw, where authors reported lower concentration of inhibitors when compared to normal acid-pretreatment.
Overall, thus obtained results clearly stated the feasibility of present nanocatalysts mediated approach in the pretreatment of lignocellulosic biomasses. The generation of very less amounts of toxic inhibitors makes the down-stream processing simple and convenient. Moreover, there is no requirement of detoxification of hydrolysate, extensive washing to remove the excess of acids, no need of specialized non-corrosive reactors or equipments, and hence, the present approach was found to be simple, environment friendly and cost-effective.
Conclusions
Various lignocellulosic biomasses are the most important and suitable feedstocks for biorefining industries in the production of different high-value products including bioethanol. Moreover, the complex structure of these biomasses necessitates the pre-processing of these feedstocks through their pretreatment. Hence, pretreatment is considered as the most important primary step in the process of bioethanol production. The concentrated and dilute acid pretreatments are routinely used methods due to their simple and easy operation. However, these methods have several limitations such as tedious down-stream processing, extensive washing, generation of toxic inhibitors and many more. In this context, the approach proposed in the present study for the pretreatment of sugarcane straw using different acid-functionalized magnetic nanocatalysts was found to be feasible, simple, effective and more convenient. It was observed that the present approach can be operated at a comparatively lower temperature using simple equipment like electric autoclave, which is contradictory to some of the approaches reported in literature which used specialized equipment operated at very high temperatures (160–200 °C), pressure and long period. As far as the production of fermentable sugar (xylose) is concerned, it was found to be higher as compared to normal acid pretreatment of sugarcane straw. Moreover, recovery and reuse of thus developed nanocatalysts for multiple cycles of pretreatments is the most attractive feature of this approach. It makes the process economically viable, which is very important in the biorefinery point of view.
Similarly, the generation of less amount of toxic inhibitors is among the promising advantages of this approach because this feature will help to make the down-stream processing very easy and cost-effective. Although the nanocatalysts mediated approach developed in the present study primarily showed many advantages over conventional acid-pretreatment methods, there is the necessity to perform extensive studies in the area to draw a concrete conclusion because to date, there are only 3–4 studies that are available. Moreover, there is mixed opinion about the toxicological concern of nanomaterials used in any application, therefore, more studies are also required on this aspect. Similarly, more efforts are required to develop even more efficient nanocatalysts, so that such nanocatalysts can be used as promising alternatives and completely replace the existing conventional acid pretreatment method.
References
Arora A, Nandal P, Singh J, Verma ML (2020) Nanobiotechnological advancements in lignocellulosic biomass pretreatment. Mater Sci Energy Technol 3:308–318. https://doi.org/10.1016/j.mset.2019.12.003
Bhati SK, Jagtap SS, Bedekar AA, Bhatia RK, Patel AK, Pant D, Banu JR, Rao CV, Kim GY, Yang YH (2020) Recent developments in pretreatment technologies on lignocellulosic biomass: effect of key parameters, technological improvements, and challenges. Bioresour Technol 300:122724. https://doi.org/10.1016/j.biortech.2019.122724
Costa SM, Mazzola PG, Silva JCAR, Pahl R, Pessoa A Jr, Costa SA (2013) Use of sugarcane straw as a source of cellulose for textile fiber production. Ind Crop Prod 42:189–194. https://doi.org/10.1016/j.indcrop.2012.05.028
Dhepe P, Sahu R (2010) A solid-acid-based process for the conversion of hemicellulose. Green Chem 12:2153–2156. https://doi.org/10.1039/C004128A
Gaikwad S, Ingle AP, da Silva SS, Rai M (2019) Immobilized nanoparticles mediated enzymatic hydrolysis of cellulose for clean sugar production: a novel approach. Curr Nanosci 15(3):296–303. doi:https://doi.org/10.2174/1573413714666180611081759
Galbe M, Wallberg O (2019) Pretreatment for biorefineries: a review of common methods for efficient utilization of lignocellulosic materials. Biotechnol Biofuels 12:294. https://doi.org/10.1186/s13068-019-1634-1
Gomez EO, Souza RTG, Rocha GJM, Almeida E, Cortez LAB (2010) Sugarcane straw as a raw material for second-generation processes. In: Cortez LAB (ed) Bioethanol sugarcane. Blucher Publisher, Brazil, pp 636–659 (original article is in Portuguese)
Gouveia ER, Nascimento RT, Souto-Maior AM, Rocha GJM (2009) Validation of methodology for the chemical characterization of sugar cane bagasse. Quim Nova 32:1500–1503 (original article is in Portuguese)
Hernández-Pérez AF, de Arruda PV, Felipe MGA (2016) Sugarcane straw as a feedstock for xylitol production by Candida guilliermondii FTI 20037. Braz J Microbiol 47:489–496. https://doi.org/10.1016/j.bjm.2016.01.019
Ingle AP, Chandel AK, Antunes FAF, Rai M, da Silva SS (2019) New trends in application of nanotechnology for the pretreatment of lignocellulosic biomass. Biofuels Bioprod Biorefining 13:776–788. https://doi.org/10.1002/bbb.1965
Ingle AP, Philippini RR, Rai M, da Silva SS (2020a) Catalytic hydrolysis of cellobiose using different acid-functionalized Fe3O4 magnetic nanoparticles. IET Nanobiotechnol 14(1):40–46. doi:https://doi.org/10.1049/iet-nbt.2019.0181
Ingle AP, Philippini RR, da Silva SS (2020b) Pretreatment of sugarcane bagasse using two different acid-functionalized magnetic nanoparticles: a novel approach for high sugar recovery. Renew Energy 150:957–964. https://doi.org/10.1016/j.renene.2019.11.146
Jong ED, Gosselink RJA (2014) Lignocellulose-based chemical products. In: Gupta V, Tuohy M, Kubicek C, Saddler J, Xu F (eds) Bioenergy research: advances and applications. Elsevier, London, pp 277–313
Keskin T, Abubackar HN, Arslan K, Azbar N (2019) Biohydrogen production from solid wastes. In: Pandey A, Mohan SV, Chang JS, Hallenbeck P, Larroche C (eds) Biohydrogen: biomass, biofuels, biochemicals, 2nd edn. Elsevier, London, pp 321–346
Kucharska K, Rybarczyk P, Hołowacz I, Łukajtis R, Glinka M, Kamiński M (2018) Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules 23(11):2937. doi:https://doi.org/10.3390/molecules23112937
Kumar AK, Sharma S (2017) Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour Bioprocess 4:7. doi:https://doi.org/10.1186/s40643-017-0137-9
Lai DM, Deng L, Guo QX, Fu Y (2011) Hydrolysis of biomass by magnetic solid acid. Energy Environ Sci 4:3552–3557. https://doi.org/10.1039/C1EE01526E
Mileo PC, Mulinari DR, Baptista CARP, Rocha GJM, Goncalves AR (2011) Mechanical behaviour of polyurethane from castor oil reinforced sugarcane straw cellulose composites. Procedia Eng 10:2068–2073. https://doi.org/10.1016/j.proeng.2011.04.342
Pena L, Ikenberry M, Ware B, Hohn KL, Boyle D, Sun XS, Wang D (2011) Cellobiose hydrolysis using acid-functionalized nanoparticles. Biotechnol Bioprocess Eng 16:1214–1222. doi:https://doi.org/10.1007/s12257-011-0166-8
Pena L, Ikenberry M, Hohn KL, Wang D (2012) Acid-functionalized nanoparticles for pretreatment of wheat straw. J Biomater Nanobiotechnol 3:342–352. https://doi.org/10.4236/jbnb.2012.33032
Pena L, Xu F, Hohn KL, Li J, Wang D (2014) Propyl-sulfonic acid functionalized nanoparticles as catalyst for pretreatment of corn stover. J Biomater Nanobiotechnol 5:8–16. https://doi.org/10.4236/jbnb.2014.51002
Qi W, He C, Wang Q, Liu S, Yu Q, Wang W, Leksawasdi N, Wang C, Yuan Z (2018) Carbon-based solid acid pretreatment in corncob saccharification: specific xylose production and efficient enzymatic hydrolysis. ACS Sustain Chem Eng 6(3):3640–3648. https://doi.org/10.1021/acssuschemeng.7b03959
Rajkumari K, Kalita J, Das D, Rokhum L (2017) Magnetic Fe3O4@silica sulfuric acid nanoparticles promoted regioselective protection/deprotection of alcohols with dihydropyran under solvent-free conditions. RSC Adv 7:56559–56565. doi:https://doi.org/10.1039/c7ra12458a
Saad MBW, Oliveira LRM, Candido RG, Quintana G, Rocha GJM, Goncalves AR (2008) Preliminary studies on fungal treatment of sugarcane straw for organosolv pulping. Enzyme Microb Technol 43:220–225. https://doi.org/10.1016/j.enzmictec.2008.03.006
Santos FA, Queiro´z JH, Colodette JL, Fernandes AS, Guimara˜es VM, Rezende ST (2012) Potential of sugarcane straw for ethanol production. Quim Nova 35:1004–1010 (original article is in Portuguese)
Shuttleworth PS, De-bruyn M, Parker HL, Hunt AJ, Budarin VL, Matharu AS, Clark JH (2014) Applications of nanoparticles in biomass conversion to chemicals and fuels. Green Chem 16:573–584. https://doi.org/10.1039/C3GC41555D
Su TC, Fang Z, Zhang F, Luo J, Li XK (2015) Hydrolysis of selected tropical plant wastes catalyzed by a magnetic carbonaceous acid with microwave. Sci Rep 5:17538. doi:https://doi.org/10.1038/srep17538
Wang H, Covarrubias J, Prock H, Wu X, Wang D, Bossmann SH (2015) Acid functionalized magnetic nanoparticle as heterogeneous catalysts for biodiesel synthesis. J Phys Chem C 119:26020–26028. https://doi.org/10.1021/acs.jpcc.5b08743
Acknowledgments
API is highly thankful to Research Council for the State of Sao Paulo (Fundação de Amparo à Pesquisa do Estado de São Paulo—FAPESP), Brazil for providing financial assistance (Process No. 2016/22086-2) in the form of Post-Doctoral Fellowship. RRP is grateful to Brazilian National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq) for post-doctoral fellowship (Process No. 153169/2018-4). YCSM is also grateful to FAPESP for providing financial assistance for scientific initiation scholarship program (Process No. 2019/19695-5). SSS is thankful to CNPq (Process No. 303943/2017-3) and FAPESP (Process No. 2016/10636-8) for providing research grants.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ingle, A.P., Philippini, R.R., de Souza Melo, Y.C. et al. Acid-functionalized magnetic nanocatalysts mediated pretreatment of sugarcane straw: an eco-friendly and cost-effective approach. Cellulose 27, 7067–7078 (2020). https://doi.org/10.1007/s10570-020-03262-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03262-y