Abstract
The dependence on fossil fuel in recent years has accentuated the importance of renewable resources such as biomass. Lignocellulosic biomass consisting mainly of cellulose, hemicellulose, and lignin, can be potentially converted into biochemicals and biofuels. Ionic liquid (IL) has been proven as an efficient and green solvent for biomass dissolution and further processing. Nonetheless, conventionally employed IL treatment requires a longer time and higher temperature making the overall process cost-intensive and time-consuming. The aim of the current research work is to develop a direct probe sonication assisted IL method for rapid biomass dissolution without any external heating. The effect of several experimental parameters on biomass dissolution such as acoustic power (20–60%), sonication time (10–70 min), biomass particle size (500–125 µm), initial sample loading (5, 7.5 and 10%), volume of sample (3, 6 and 9 mL), and types of IL were investigated and optimized. The lignocellulosic biomass and regenerated biomaterials were characterized using Fourier transform infrared spectroscopy, thermogravimetric analysis, Fourier electron scanning electron microscope, and powder x-ray diffraction to study the effect of probe sonication assisted IL treatment. The ultrasonic cavitation had significantly shortened the time for complete lignocellulose dissolution and considerably altered the thermophysical properties of regenerated cellulose-rich materials. The acetate-based IL showed the best performance, able to fully dissolved bamboo biomass in just 40 min in the probe sonication. In conclusion, probe sonication is an efficient method at lab scale which might need further development in technology to make the process economical at the industrial level for lignocellulosic biomass treatment.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chemical industries heavily depend on fossil fuel for their resources. Therefore, to avoid a future shortage, the importance of sustainable fuels urges the substitution from finite to renewable resources. Among many available resources, lignocellulosic biomass, primarily consisting of cellulose, hemicellulose, and lignin, has attracted considerable attention as a potential alternative source for biofuels and valuable chemicals (An et al. 2015; da Silva et al. 2013; Dong et al. 2015).
At present, numerous studies have been performed on the conversion of lignocellulose to various useful chemicals such as 5-hydroxymethylfurfural (Sarwono et al. 2017) and levulinic acid (Khan et al. 2018a), lactic acid, sorbitol, malic acid, and mannitol. Lignocellulosic biomass composed of three major components cellulose (crystalline), hemicellulose (amorphous) and lignin (amorphous). Cellulose, hemicellulose, and lignin in lignocellulosic biomass are bonded to others by covalent and non-covalent forces. The complex structure and strong intra and intermolecular hydrogen bonding in lignocellulosic biomass components hinder their dissolution in water and common organic solvents which ultimately limits its conversion to other chemicals (Khan et al. 2016). Pretreatment of lignocellulosic biomass is required before conversion into chemicals. Pretreatment improves the hydrolysis of lignocellulosic biomass due to depolymerization and alteration of biomass structure. Acid and alkali pretreatments of biomass increase the rate of enzymatic saccharification (Muhammad et al. 2015b). However, acid and alkali pretreatments are toxic, non-environmentally friendly and cause corrosion.
In contrast, ionic liquids (ILs) emerged as potential alternative solvents for pretreatment of lignocellulosic biomass. ILs are molten salts containing bulky cations and organic/inorganic anions, with high thermal and chemical stability, high melting and boiling points as well as recyclability and reusability (Khan et al. 2018b). IL as an alternative solvent used for dissolution of cellulose and lignocellulosic biomass (Graenacher 1934; Swatloski et al. 2002). Meanwhile, Man et al. focused on green amino acid-based ILs for biomass dissolution. Among various ILs tested, 1-ethyl-3-methylimidazolium glycinate ([Emim][Gly]) was able to dissolve biomass at 120 °C in 8 h (Muhammad et al. 2011). However, IL pretreated biomass using conventional methods required higher temperatures. Despite the good performance of conventional methods, they are time-consuming and require high temperature making the overall process inefficient.
Ultrasonic irradiation is the newly developed technology used for lignocellulosic biomass pretreatment as it shortens pretreatment time and temperature. Ultrasonic waves produce a cavitation bubble in the heterogeneous system of biomass/IL mixture, which leads to disruption of the biomass cell wall and increasing the surface area. This will eventually result in a more efficient lignocellulose dissolution and conversion to chemicals (Bussemaker and Zhang 2013). Sri Bala et al. demonstrated the application of ultrasonic waves in alkaline pretreatment which disrupted cellulose chain in a shorter time (SriBala et al. 2016). Ninomiya et al. reported a higher percentage of cellulose saccharification of kenaf powder in IL was obtained from 47 to 86% in 15 min when ultrasonic waves were applied (Ninomiya et al. 2012). To the best of our knowledge, the probe sonication has not been evaluated for lignocellulosic biomass dissolution, in comparison to bath sonication. Probe sonication and bath sonication are two different techniques of ultrasonic irradiation. In bath sonication process, the cavitation produced is uncontrollable throughout the bath and the intensity is lower to have effectiveness in lignocellulosic biomass deconstruction. In contrast,, the produced cavitation in probe sonication is localized at specific region and having higher intensity (Sarwono et al. 2017).
In this study, the probe ultrasonic assisted dissolution of bamboo was investigated in ILs. The bamboo biomass was selected for the current research work because of its fast-growing rate. Unlike wood, bamboo can be harvested within 3–5 years instead of 15 to 20 years. Conventional methods of ILs based treatment require longer dissolution time as well as higher temperatures in the process. Therefore, we evaluate for the first time the effect of probe sonication on ILs based dissolution. Three ionic liquids i.e.[Emim][OAc], [Emim][Cl] and [Emim][HSO4], known for a deconstruction of lignocellulosic materials were used. In addition, the acidity and basicity of these ionic liquids are underlying factors to be taken into consideration for probe sonication assisted ionic liquid treatment. The hydrogen bond basicities (β) of the selected ionic liquids are [Emim][OAc] (β = 1.06), [Emim][Cl] (β = 0.79) and [Emim][HSO4 (β = 0.61). The effect of process parameters such as acoustic power, sonication time, biomass particle size, initial biomass loading and volume of sample on the dissolution process were investigated and optimized. Furthermore, the effect of water on probe sonication-based treatment was also studied. The cellulose-rich materials (CRM) and lignin-rich materials (LRM) were separated by adding the water and acetone mixture into biomass/IL mixture. The untreated lignocellulosic biomass and cellulose-rich materials were characterized using various analytical techniques such Fourier transform infrared (FTIR), Fourier electron scanning electron microscope (FESEM) and thermogravimetric analysis (TGA) for detail thermophysical properties.
Methodology
Materials
Chemicals of analytical grade were used for the pretreatment of biomass. 1-ethyl-3-methylimidazolium acetate [Emim][OAc] (Manchester Organics, 97%), 1-ethyl-3-methylimidazolium chloride [Emim][Cl] (Merck, 98%), 1-ethyl-3-methylimidazolium hydrogensulfate [Emim][HSO4] (Merck, 98%), Dimethylsulfoxide (DMSO) (Merck, 99.9%), acetone (Merck, 99.8%), acetonitrile (Merck, 99.5%), hydrochloric acid (HCl) (Merck, 37%) and sodium hydroxide (R&M Chemicals, 97%). All chemicals were used without further purification. The bamboo biomass (Gigantochloa scortcheninii, a native plant of Malaysia known as “buluh semantan”) was obtained from the bamboo processing industry in Seri Iskandar, Perak Malaysia. The bamboo was grounded into powder and sieved into different particle sizes (500–125 µm) by Retsch Test Sieve (model AS 200).
Biomass dissolution and regeneration of cellulose rich materials (CRM)
In a typical experiment, bamboo biomass powder (5 wt%) having particle size 500 µm was charged into 16 mL vial containing 3 mL of IL. The experiments were performed using Ultrasonic Vibra Cell (130 kW, 50 Hz). The sonicator probe of a 6 mm diameter was inserted into the mixture to a point 1/3 of the total height of the sample surface (Sarwono et al. 2017). The mixture was sonicated at defined acoustic power, sonication time, biomass particle size, initial biomass loading and volume of sample to optimize the dissolution process. To monitor the presence of undissolved biomass materials in a mixture, one or two drops of the mixture were periodically taken with a pipette and examined by microscope (Dino-Lite Digital Microscope) at 30 × magnification. The effect of water content of ILs (5–20%, v/v) on biomass dissolution was also investigated. To achieve complete dissolution, IL/biomass mixture was added into 3 × volumes of DMSO with rapid stirring at 250 rpm and further centrifuged at 4000 rpm for 15 min. The residues formed were collected, filtered and washed few times with deionized water to remove the excess IL and DSMO. The precipitate was dried overnight at 80 °C in the oven and later weighed. The percentage of biomass dissolved in ILs was calculated according to Eq. 1:
where Wo is the initial weight of bamboo biomass and Wp is the undissolved materials.
Recovery process
The regeneration process of dissolved materials was performed according to Muhamad et al. (Man et al. 2011) with slight modification. The resultant slurry of biomass /IL was poured into a beaker containing 3 × volume of acetone: water (9:1 v/v) under vigorous agitation. The mixture was stirred at 300 rpm at room temperature for 30 min. The residue formed was filtered using a vacuum filter, thoroughly washed for few times with acetone/water to remove excess IL and subsequently dried in the oven overnight at 60 °C for further characterization. The filtrate containing IL, acetone, water, and lignin was subjected to reduced pressure to regenerate lignin. To enhance the precipitation of lignin regenerated materials (LRM), 0.5 M HCl (pH 2.0) was added to the filtrate. The LRM was collected and repeatedly washed with distilled water to ensure the removal of traces ionic liquids.
Recovery of ionic liquid
NaOH (0.5 M) solution was added into filtrate containing IL, acidified water and degraded carbohydrate to neutralize the pH. Excess water was removed, and acetonitrile was then added into the solution to dissolve IL, leaving sodium chloride as precipitates. The acetonitrile was further removed under reduced pressure for recovery of [Emim][OAc].
Characterization of biomass and regenerated materials
Fourier transform infrared spectroscopy (FTIR)
The functional groups for fresh biomass and IL pretreated biomass samples were determined using FTIR-ATR diamond crystal. All spectra were recorded using the FTIR spectrometer (Thermo Nicolet iS5 by Thermo Fisher Scientific, Waltham, Massachusetts, USA) in the range of 4000 to 400 cm−1 under 64 scanning.
Thermogravimetric analysis (TGA)
The thermal analysis for all samples was performed using a thermogravimetric analyzer TGA (STA 6000 Perkin Elmer). The sample was weighed approximately 5.0 mg in crucible pans and placed in the sample holder under 20 mL/min nitrogen flow. The analysis was performed in temperature range from 50 to 800 °C with a heating rate 10 °C /min.
Fourier electron scanning electron microscope (FESEM)
The morphology of samples was observed by FESEM, in a microscopy Zeiss Supra 55 VP with an acceleration volt of 5.00 kV under nitrogen atmosphere. The samples were dried overnight and coated with gold. The image zoom was 1.00 K x, 5.00 K x, and 10.0 K x.
Powder X-ray diffraction (PXRD) analysis
Untreated and probe sonication assisted ionic liquid treated samples of bamboo biomass were analyzed using powder X-ray diffraction (PXRD), model Bruker D8 Advance horizontal X-ray diffractometer equipped with Cu anode scanned within 5–35.00° 2θ with a step mode with a step of 0.01° and a rate of 1°/min).
Results and discussion
Dissolution of biomass
The influence of selected parameters such as ultrasonic time, biomass particle size, initial biomass loading and volume of biomass/IL mixture on biomass dissolution was studied in the following detail.
Ultrasonic acoustic power
In this study, the ultrasonic acoustic power was varied within range of 20–60% with 10% intervals shown in Fig. 1. A higher percentage of biomass dissolution was observed with the increase of acoustic power from 20 to 60%. The percent dissolution of bamboo biomass was calculated as 25.9%, 27%, 99.5% for 20%, 30% and 40% acoustic power, respectively. Figure 1 shows that the maximum dissolution was achieved within 50 min at 40% acoustic power. Therefore, low acoustic power of 40% was preferred over 50% and 60% to avoid the damaged of fragile tips of probe sonicator.
To get more insight into dissolution, a rise in temperature during biomass dissolution through probe sonication was also monitored. The maximum temperature reached about 86 °C, 114 °C and 125 °C with sonication at 20%, 30% and 40% acoustic power respectively for 40 min treatment shown in Fig. 2. High temperature is reported to be favorable for biomass dissolution (Carneiro et al. 2017). Miyafuji (2015) observed that 85% (wt/wt%) of Japanese beech wood residue remained after reaction at 90 °C for 24 h while 80% (wt/wt) of biomass dissolution was achieved at 110 °C for 24 h.
Period for sonication
Reaction time is an important and key parameter from an energy point of view. To study the effect of time on biomass dissolution, biomass with particle size 500 µm was sonicated using 40% acoustic power irradiation for 10, 20, 30, 40 and 50 min. Figure 3 shows the microscopic images of biomass/IL mixture taken at different time intervals of sonication. The absence of biomass particles under microscope marks complete dissolution of biomass. As the sonication period increased, the presence of undissolved particles in biomass/IL solution was reduced. A clear solution was obtained in 50 min of probe sonication indicating that biomass was completely dissolved.
The infusion of ultrasonic technology in biomass dissolution shortens dissolution time in comparison to a conventional heating method which typically requires 3–24 h (Man et al. 2011; Socha et al. 2014). The cavitation produced by ultrasonic tip creates a bubble, inside of which the temperature and pressure reach extremely high (~ 5000 K and ~ 50 MPa) (Kobayashi et al. 2012). The cavitation produced has both physical and chemical effects (Hart and Henglein 1985). The physical effect is related to the mass transfer enhancement due to the generation of intense local convection. In case of chemical effect, the bubble, which is due to high temperature and pressure, it collapses adiabatically causing the release of radicals. This proves the destruction of biomass structure that allows rapid IL penetration into biomass. Hence, probe sonication results in complete dissolution of biomass within 50 min as shown in Fig. 4. The probe sonication assisted ionic liquid treatment was also compared with conventional heating. Three heating temperatures 120, 110 and 80 °C was selected for treatment (5% bamboo biomass was loaded in [Emim][OAc] IL with magnetic stirring at 300 rpm). The dissolution attained at 120 °C and 110 °C was 12 h and 17 h respectively while at 80 °C, the mixture was partially dissolved for 24 h. Thus, probe sonication is more effective in dissolution as compared to the conventional heating.
Particle size of biomass
Biomass samples having different particle sizes 500–125 µm were added into a different vial containing IL and were probe sonicated to reach complete dissolution. Figure 5 shows how the particle size affects the dissolution time. It was observed that the increase of biomass particle size from 125 µm to 500 µm required longer dissolution time 40 to 50 min. The smaller size of biomass particles provides larger surface area. This allows the IL molecule to penetrate more into biomass chain structure thus results in higher rate of dissolution (Brandt et al. 2013; Darji et al. 2013). In contrast, the big biomass particle size of 500 µm hinders the diffusion of IL and consequently leads to slow down the dissolution process (Muhammad et al. 2015b).
Ability of selected ILs to completely dissolve biomass
To study the effect of the anion of ILs on biomass dissolution, three ILs containing same cation ([Emim]+) with different anions such as OAc−, Cl− and HSO4− were evaluated for dissolution of biomass having particle size of 125 µm using 40% amplitude of sonication. Among three anions tested, [OAc]− dissolved the biomass completely in a shorter time as compared to the other two ILs containing Cl− and HSO4−. This difference in the efficiency of ILs in dissolving the biomass might be due to differences in their viscosity and hydrogen bond basicity (β). Viscosity measured for [Emim][OAc] and [Emim][Cl] at 80 °C were 0.017 Pa s and 0.065 Pa s, respectively (Fendt et al. 2010). The viscosity value measured at 80 °C for [Emim][HSO4] was 0.105 Pa s (Poulimenou et al. 2014). In general, sonication prefers lower viscosity environment to facilitate maximum transfer of cavitation bubbles through liquid medium (Santos et al. 2009; Wu et al. 2013). The low dissolution efficiency of [Emim][HSO4] might be due to its high viscosity as well as lower value of hydrogen bond basicity (β = 0.61). The high viscosity of [Emim][HSO4] minimizes the bubbles transfer in heterogeneous solutions, affecting the cavitation produced by ultrasonic waves which ultimately disturb the dissolution process. In addition to viscosity, Muhammad et al. investigated the effect of α and β value in Kamlet-Taft parameters on the ability of ILs to dissolve biomass (Muhammad et al. 2015b). The β value represents the ability of anion as H-bond acceptor. Among the tested ILs, the IL containing OAc− anion (Emim][OAc]) has higher β (1.06) value than to Cl− anion [Emim][Cl] of β = 0.79, therefore efficiently dissolves the biomass. Complete dissolution was achieved within 40 min for Emim][OAc], while for [Emim][Cl], it prolongs to 2 h. The anion of IL with higher β value weakens the hydrogen bonding in lignocellulosic structure by interacting with hydroxyl group present in biomass thus the ionic liquid consequently causes swelling and dissolution of biomass (Brandt et al. 2010).
Initial loading of biomass sample into IL
To study the effect of biomass loading on dissolution process, various amount (5, 7.5 and 10 wt%) of biomass having a particle size of 125 µm was added into [Emim][OAc] and sonicated. Figures 6 and 7 show the effect of biomass loading on percentage dissolution. Increasing the amount of biomass loading into IL reduced the percentage dissolution as graphically presented in Fig. 6.
Figure 7 reveals the microscopic image of biomass/IL mixture after dissolution at the respective time. Undissolved particles were observed for both 7.5 and 10% biomass loading (b and c) even after passing the optimum dissolution time. The decrement in biomass dissolution at higher loading (7.5 and 10%) was attributed to the saturation point of IL dissolution capability. Adding excess biomass into IL also increases the viscosity, therefore, hinders the mixing and mass transfer. The viscosity of the heterogeneous mixture is one of the limiting factors in ultrasonic assisted dissolution (Luo et al. 2013, 2014). High viscosity of biomass/IL mixture prevents the cavitation bubbles, produced by ultrasonic tip, from being transferred evenly to rupture the biomass structure. Therefore, the low dissolution percentage for biomass loading at 7.5 and 10% was obtained.
Effect of sample volume
Three sample volumes (3, 6 and 9 mL) were evaluated by charging in 16 mL sample vial. The mixture (typically, 3 mL of ionic liquid, 5% (g/mL) of bamboo powder having the particle size of 125 µm) was sonicated at a specified amplitude with a probe diameter of 6 mm for complete dissolution. Figures 8 and 9 show the effect of IL volume on biomass dissolution. The result in Fig. 8 demonstrated that the dissolution of biomass decreases with the increase of IL volume from 3 to 9 mL. In the biomass deconstruction process, the cavitation transfer prefers low volume matrix in order to maximize the utilization of direct probe sonication. Lavilla and Bendicho (2017) stated that low volume of solution allows the maximum bubbles generated from ultrasonic tip during sonication thus maximum penetration of IL molecules inside biomass surface can be achieved.
The clear solution in 3 mL of IL confirms the effectiveness of ultrasonic cavitation in a low volume of IL as shown in Fig. 9. In contrast to 3 mL of IL, there might be formation of area known as ‘dead zone’ when volume increased to 6 and 9 mL. The dead zone is the region where the collapse bubbles are unable to reach the solutes thus do not contribute to the dissolution as shown in Fig. 9. The loading of 3 mL IL results in 99.4% dissolution while in the case of 6 mL and 9 mL, the dissolution calculated is 89 and 73% respectively.
Effect of water content on the dissolution process
The presence of water is undesirable for the dissolution of biomass in ionic liquids (Muhammad et al. 2015b). Water molecules compete with IL for its interaction with lignocellulosic biomass through hydrogen bonding and hence reduce the ability of IL to interact with lignocellulosic constituents causing a reduction in dissolution of biomass. Contrarily, the addition of water decreases the IL viscosity which favored the generation of cavitation bubbles in probe sonication for destruction of lignocellulosic structure. In addition to viscosity reduction, water also decreases the overall cost of pretreatment process and improves the recyclability of IL (Pang et al. 2016; Parthasarathi et al. 2015). Figure 10 shows the presence of undissolved biomass in the solution with (85:15 wt%) and (80:20 wt%) IL: water ratios.
Figure 11 shows that 80% and 60% of biomass were dissolved in the mixture of IL: water (95:5 wt%) and IL: water (90:10 wt%) respectively. The presence of water in the mixture of IL also influences the sonication time. In the absence of water, complete dissolution was observed at 40 min while for IL: water (95:5 wt%) ratio, it is prolonged to 50 min.
Dissolution and fractionation of biomass in IL
The dissolution and fractionation of biomass (particle size 125 µm) in IL [Emim][OAc] were carried out with 5 wt% loading of biomass and 40% acoustic power of probe sonication. The dissolution was assessed through the Dino-Lite microscope after 40 min of treatment. The mixture obtained is highly viscous due to the presence of aromatic lignin compounds and extractive from biomass (Zavrel et al. 2009). The fractionate materials of biomass were easily separated and regenerated by the addition of anti-solvent such as water, ethanol, and acetone (Lan et al. 2011; Shill et al. 2011; Singh et al. 2009). The treated sample containing biomass and IL were introduced into the mixture of acetone: water (9:1 v/v) to precipitate cellulose-rich materials (CRM). The lignin and some polysaccharides were solubilized in the acetone water mixture. The lignin-rich material (LRM) was obtained from the mixture by evaporating the acetone followed by addition of HCl to adjust the acidic pH. The yield obtained for CRM and LRM was 69.3% and 14.9% respectively of total biomass. The lignin content of bamboo biomass is 26.45% therefore 56.3% (14.9%) of total lignin was recovered in this treatment. Sonication causes the formation of the microbubbles, which release energy in the form of shockwave and causes turbulence (Rehman et al. 2013). This turbulence enhances the mixing between biomass solid phase and IL liquid phase and alters the structure of bamboo biomass. The cavitation destroys the cell wall structure, increases the surface area of bamboo biomass and facilitates IL molecule to penetrate the structure. The breakdown of lignin-carbohydrate bond which is caused by sonication allows the fractionation of biomass constituents (Li et al. 2012; Sul’man et al. 2011). The characterization of CRM along with untreated biomass was performed to investigate the effect of both ionic liquid and probe sonication.
Infrared spectroscopy
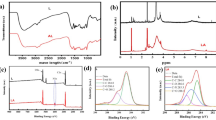
The pretreatment process of biomass alters the biomass structure as well as the surface functional groups. Figure 12 shows the FTIR spectra of untreated biomass (A), regenerated cellulosic materials (B) and commercial microcrystalline cellulose (C). The OH broad stretching was observed in all three spectra around 3320 cm−1. The C–H stretching absorption bands are observed at 2850 cm, while C=O stretching present in hemicellulose at 1,735 cm−1, is not detected in MCC spectra (C). Three characteristic peaks at 1598, 1506 and 1460 cm−1 assigned to lignin have been removed by comparing spectra of untreated (A) and cellulosic rich regenerated material (B) which confirmed the removal of lignin (Muhammad et al. 2013). The other peaks in the region between 1320 to 890 cm−1 related to carbohydrates are present in all spectra (João 2013).
Morphology of CRM
Figure 13 shows the FESEM images of untreated and ILs pretreated biomass taken under different magnification (100×, 1.00K× and 5.00K×). Untreated bamboo biomass sample has a compact and rigid surface showing the intact structure of the cell wall. The pores used for ventilation were also present. However, after IL pretreatment, considerable changes were observed in the morphology. The compactness of the surface was diminished and resulted in rough agglomerated texture. This change in texture of bamboo biomass was due to the removal of hemicellulose and lignin that holds the biomass components (Corredor 2008; Sun et al. 2016). The fibril structure of biomass was also diminished due to disruption of linkage present in lignocellulose material after IL pretreatment.
Thermal stability
To investigate the effect of IL pretreatment on the thermal stability of biomass, TGA analysis of biomass samples before and after IL pretreatment was carried out and shown in Fig. 14. TGA result showed that the untreated biomass has higher thermal stability as compared to IL pretreated sample. In the first stage of decomposition from 50 to 150 °C, the weight loss is due to the evaporation of surface moisture and volatile organic compounds. The second region (150 to 380–440 °C) is an active pyrolysis region where the major thermal degradation of hemicellulose and cellulose is taking place. The third region (above 410 to 1000 °C), is concerned with lignin degradation (Khan et al. 2016). In this region, the degradation of lignin starts and reduction in weight loss are observed with the increase of temperature until 1000 °C. This thermal decomposition of lignin in wide range of temperatures is due to heterogeneity in its components. After IL pretreatment, the thermal stability of biomass decreases which is due to the decrease in lignin content. A significant change in DTG curve was observed for untreated and IL treated biomass. The temperature at which maximum weight loss happened (Tmax) moves towards left side (lower temperature) after IL pretreatment. The Tmax for untreated sample is 325 °C, which moves towards 262 °C after IL treatment. This due to change in surface morphology after pretreatment with IL. The second peak in region from 490–550 °C which is assigned to thermal decomposition of lignin is completely finished after IL pretreatment. This is due to the removal of lignin from biomass samples after IL pretreatment (Khan et al. 2016). Similarly, the onset temperature (To) for untreated and IL pretreated biomass is 277.37 °C and 233.42 °C, respectively. The onset temperature represents the temperature where sample starts its degradation. DTG curve showed that there was strong peak present at temperature range of 200–390 °C. A shoulder also appears in untreated biomass DTG curve ranging at 381–580 °C which is attributed to temperature pyrolysis of lignin. The absence of lignin in IL treated sample is due to removal of lignin (Muhammad et al. 2015a; Zhang et al. 2014).
X-ray diffraction
The effect of probe sonication assisted ionic liquid treatment on bamboo biomass has been shown in Fig. 15. During probe sonication assisted ionic liquid treatment, the biomass including the crystalline cellulose component dissolves thereby disorientation in the arrangement of its chain structure occurred. However, during separation and regeneration with anti-solvent, the cellulose chains rearranged in other crystalline phases. In Fig. 15, the untreated bamboo patterns (A) show the characteristic peaks with high intensities at 2-theta values of 22.0 and 15.6 assigned to cellulose I. While for regenerated material (B), there is only one peak of lower intensity at 2-theta value of 20.3 that is assigned to cellulose II (Muhammad et al. 2011). Thus, during probe sonication assisted IL treatment causes the change in crystallinity of cellulose I to cellulose II (close to amorphous). A similar finding was reported by other groups while using the conventional heating for IL treatment of lignocellulosic biomass (Iqbal et al. 2019; Sun et al. 2009).
Recyclability of IL
The recyclability of ionic liquid renders it a feasible solvent for various engineering processes. Herein the ionic liquid was recycled four times with a yield of more than 95% as shown in Fig. 16. However, the amber color of [Emim][OAc] turned to dark color due to the degradation of lignocellulosic materials, introduction of various solvents and water during dissolution and regeneration process. It has been observed during recycling, the dissolution capacity of ionic liquid is slightly decreased (results have been not shown).
Conclusion
In conclusion, the probe ultrasonic technology was found to be an efficient and time effective method for biomass dissolution as compared to conventional heating. In this work, the complete dissolution of bamboo biomass in probe sonication assisted IL was achieved within 40 min at 40% ultrasonic acoustic power as compared to conventional heating, 12 h at 120 °C. It was observed that the biomass particle sizes, biomass initial loading, viscosity of IL and volume of IL in vial influenced the dissolution time. The presence of excess water in IL above 5 wt% led to the reduction of IL's ability to dissolve biomass. The pretreatment process resulted in fractionation of 69.33% of CRM and 14.99% of LRM. In FTIR analysis of lignocellulosic biomass, the characteristic peaks assigned to lignin disappeared after treatment which proved that lignin was efficiently separated during probe sonication/IL treatment and regeneration. The thermal stability of the bamboo biomass after treatment was decreased from 277 to 233 °C. Probe sonication/IL treatment diminished the compactness of the materials resulting in rough agglomerated texture of regenerated samples as observed in FESEM analysis. Moreover, cellulose I can be mercerized to convert it to II without dissolution, only swelling.
References
An Y-X, Zong M-H, Wu H, Li N (2015) Pretreatment of lignocellulosic biomass with renewable cholinium ionic liquids: Biomass fractionation, enzymatic digestion and ionic liquid reuse. Bioresour Technol 192(2015):165–171
Brandt A, Gräsvik J, Hallett JP, Welton T (2013) Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem 15(3):550–583
Brandt A, Hallett JP, Leak DJ, Murphy RJ, Welton T (2010) The effect of the ionic liquid anion in the pretreatment of pine wood chips. Green Chem 12(4):672–679
Bussemaker MJ, Zhang D (2013) Effect of ultrasound on lignocellulosic biomass as a pretreatment for biorefinery and biofuel applications. Ind Eng Chem Res 52(10):3563–3580
Carneiro AP, Rodríguez O, Macedo EA (2017) Dissolution and fractionation of nut shells in ionic liquids. Bioresour Technol 227:188–196
Corredor DY (2008) Pretreatment and enzymatic hydrolysis of lignocellulosic biomass. ProQuest
da Silva SPM, da Costa Lopes AM, Roseiro LB, Bogel-Łukasik R (2013) Novel pre-treatment and fractionation method for lignocellulosic biomass using ionic liquids. RSC Adv 3(36):16040–16050
Darji D, Alias Y, Som FM (2013) Dissolution of biomass from rubberwood with 1-butyl-3-methyl Imidazolium acetate ionic liquid. J Rubber Res 16:169–178
Dong S-J, Zhang B-X, Gao Y-F, Xiao-Mei H (2015) An efficient process for pretreatment of lignocelluloses in functional ionic liquids. Int J Polym Sci 2015:1–6
Fendt S, Padmanabhan S, Blanch HW, Prausnitz JM (2010) Viscosities of acetate or chloride-based ionic liquids and some of their mixtures with water or other common solvents. J Chem Eng Data 56(1):31–34
Graenacher C (1934) Cellulose solution. US Patent, No. 1943176
Hart EJ, Henglein A (1985) Free radical and free atom reactions in the sonolysis of aqueous iodide and formate solutions. J Phys Chem 89(20):4342–4347
Iqbal J, Muhammad N, Rahim A, Khan AS, Ullah Z, Gonfa G, Ahmad P (2019) COSMO-RS predictions, hydrogen bond basicity values and experimental evaluation of amino acid-based ionic liquids for lignocellulosic biomass dissolution. J Mol Liq 273:215–221
João KAG (2013) Pre-treatment of different types of lignocellulosic biomass using ionic liquids. Faculdade de Ciências e Tecnologia
Khan AS, Man Z, Bustam MA, Kait CF, Ullah Z, Nasrullah A, Khan MI, Gonfa G, Ahmad P, Muhammad N (2016) Kinetics and thermodynamic parameters of ionic liquid pretreated rubber wood biomass. J Mol Liq 223:754–762
Khan AS, Man Z, Bustam MA, Kait CF, Nasrullah A, Ullah Z, Sarwono A, Ahamd P, Muhammad N (2018a) Dicationic ionic liquids as sustainable approach for direct conversion of cellulose to levulinic acid. J Clean Prod 170:591–600
Khan AS, Nasrullah A, Ullah Z, Bhat AH, Ghanem OB, Muhammad N, Rashid MU, Man Z (2018b) Thermophysical properties and ecotoxicity of new nitrile functionalised protic ionic liquids. J Mol Liq 249:583–590
Kobayashi H, Ohta H, Fukuoka A (2012) Conversion of lignocellulose into renewable chemicals by heterogeneous catalysis. Catal Sci Technol 2(5):869–883
Lan W, Liu C-F, Sun R-C (2011) Fractionation of bagasse into cellulose, hemicelluloses, and lignin with ionic liquid treatment followed by alkaline extraction. J Agric Food Chem 59(16):8691–8701
Lavilla I, Bendicho C (2017) Fundamentals of ultrasound-assisted extraction. In: Water extraction of bioactive compounds. Elsevier, pp 291–316
Li M-F, Sun S-N, Xu F, Sun R-C (2012) Ultrasound-enhanced extraction of lignin from bamboo (Neosinocalamus affinis): characterization of the ethanol-soluble fractions. Ultrason Sonochem 19(2):243–249
Luo J, Cai M, Gu T (2013) Pretreatment of lignocellulosic biomass using green ionic liquids, Green biomass pretreatment for biofuels production. Springer, Berlin, pp 127–153
Luo J, Fang Z, Smith RL (2014) Ultrasound-enhanced conversion of biomass to biofuels. Prog Energy Combust Sci 41:56–93
Man Z, Muhammad N, Bustam MA, Abdul Mutalib M (2011) Dissolution of biomass using amino acid based ionic liquids
Miyafuji H (2015) Application of ionic liquids for effective use of woody biomass. J Wood Sci 61(2015):343–350
Muhammad N, Man Z, Bustam MA, Mutalib MI, Wilfred CD, Rafiq S (2011) Dissolution and delignification of bamboo biomass using the amino acid-based ionic liquid. Appl Biochem Biotechnol 165(3–4):998–1009
Muhammad N, Man Z, Bustam MA, Mutalib MA, Rafiq S (2013) Investigations of novel nitrile-based ionic liquids as pre-treatment solvent for extraction of lignin from bamboo biomass. J Ind Eng Chem 19(1):207–214
Muhammad N, Gao Y, Khan MI, Khan Z, Rahim A, Iqbal F, Khan AS, Iqbal J (2015a) Effect of ionic liquid on thermo-physical properties of bamboo biomass. Wood Sci Technol 49(5):897–913
Muhammad N, Man Z, Mutalib MA, Bustam MA, Wilfred CD, Khan AS, Ullah Z, Gonfa G, Nasrullah A (2015b) Dissolution and separation of wood biopolymers using ionic liquids. ChemBioEng Rev 2(4):257–278
Ninomiya K, Kamide K, Takahashi K, Shimizu N (2012) Enhanced enzymatic saccharification of kenaf powder after ultrasonic pretreatment in ionic liquids at room temperature. Bioresour Technol 103(1):259–265
Pang Z, Dong C, Pan X (2016) Enhanced deconstruction and dissolution of lignocellulosic biomass in ionic liquid at high water content by lithium chloride. Cellulose 23(1):323–338
Parthasarathi R, Balamurugan K, Shi J, Subramanian V, Simmons BA, Singh S (2015) Theoretical insights into the role of water in the dissolution of cellulose using IL/water mixed solvent systems. J Phys Chem B 119(45):14339–14349
Poulimenou N, Giannopoulou I, Panias D (2014) Preliminary investigation of ionic liquids utilization in primary aluminum production. In: Proceedings of the international conference on mining, material and metallurgical engineering
Rehman MSU, Kim I, Chisti Y, Han J-I (2013) Use of ultrasound in the production of bioethanol from lignocellulosic biomass. EEST Part A: Energ Sci Res 30(2):1391–1410
Santos HM, Lodeiro C, Capelo-Martínez JL (2009) The power of ultrasound. WILEYVCH verlag GmbH & Co., KGaA, Weinheim
Sarwono A, Man Z, Muhammad N, Khan AS, Hamzah WSW, Rahim AHA, Ullah Z, Wilfred CD (2017) A new approach of probe sonication assisted ionic liquid conversion of glucose, cellulose and biomass into 5-hydroxymethylfurfural. Ultrason Sonochem 37:310–319
Shill K, Padmanabhan S, Xin Q, Prausnitz JM, Clark DS, Blanch HW (2011) Ionic liquid pretreatment of cellulosic biomass: enzymatic hydrolysis and ionic liquid recycle. Biotechnol Bioeng 108(3):511–520
Singh S, Simmons BA, Vogel KP (2009) Visualization of biomass solubilization and cellulose regeneration during ionic liquid pretreatment of switchgrass. Biotechnol Bioeng 104(1):68–75
Socha AM, Parthasarathi R, Shi J, Pattathil S, Whyte D, Bergeron M, George A, Tran K, Stavila V, Venkatachalam S (2014) Efficient biomass pretreatment using ionic liquids derived from lignin and hemicellulose. Proc Natl Acad Sci 111(35):E3587–E3595
SriBala G, Chennuru R, Mahapatra S, Vinu R (2016) Effect of alkaline ultrasonic pretreatment on crystalline morphology and enzymatic hydrolysis of cellulose. Cellulose 23(3):1725–1740
Sul’man E, Sul’man M, Prutenskaya E (2011) Effect of ultrasonic pretreatment on the composition of lignocellulosic material in biotechnological processes. Catal Ind 3(1):28–33
Sun N, Rahman M, Qin Y, Maxim ML, Rodríguez H, Rogers RD (2009) Complete dissolution and partial delignification of wood in the ionic liquid 1-ethyl-3-methylimidazolium acetate. Green Chem 11(5):646–655
Sun X, Sun X, Zhang F (2016) Combined pretreatment of lignocellulosic biomass by solid base (calcined Na2SiO3) and ionic liquid for enhanced enzymatic saccharification. RSC Adv 6(101):99455–99466
Swatloski RP, Spear SK, Holbrey JD, Rogers RD (2002) Dissolution of cellose with ionic liquids. J Am Chem Soc 124(18):4974–4975
Wu TY, Guo N, Teh CY, Hay JXW (2013) Theory and fundamentals of ultrasound. Advances in ultrasound technology for environmental remediation. Springer, Berlin, pp 5–12
Zavrel M, Bross D, Funke M, Büchs J, Spiess AC (2009) High-throughput screening for ionic liquids dissolving (ligno-) cellulose. Bioresourc Technol 100(9):2580–2587
Zhang J, Feng L, Wang D, Zhang R, Liu G, Cheng G (2014) Thermogravimetric analysis of lignocellulosic biomass with ionic liquid pretreatment. Bioresour Technol 153:379–382
Acknowledgments
This work has been supported by Petroleum Research Fund (PRF) and Centre of Research in Ionic Liquids (CORIL), Universiti Teknologi PETRONAS, Tronoh, Perak, Malaysia for providing funding and facilities for this research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rahim, A.H.A., Man, Z., Sarwono, A. et al. Probe sonication assisted ionic liquid treatment for rapid dissolution of lignocellulosic biomass. Cellulose 27, 2135–2148 (2020). https://doi.org/10.1007/s10570-019-02914-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02914-y