Abstract
This review describes the recent advances in the production and application of cellulose nanomaterials. Cellulose nanomaterials (CNMs), especially cellulose nanocrystals and cellulose nanofibers, can be produced using different preparation processes resulting in materials with unique structures and physicochemical properties that are exploited in different fields such as, biomedical, sensors, in wastewater treatment, paper and board/packaging industry. These materials possess attractive properties such as large surface area, high tensile strength and stiffness, surface tailor-ability via hydroxyl groups and are renewable. This has been a driving force to produce these materials in industrial scale with several companies producing CNMs at tons-per-day scale. The recent developments in their production rate and their applications in various fields such as medical sector, environmental protection, energy harvesting/storage are comprehensively discussed in this review. We emphasize on the current trends and future remarks based on the production and applications of cellulose nanomaterials.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For the past decades, there has been unprecedented interest in the utilization of sustainable natural resources, as a result of their potential to manufacture numerous high-value products with low environmental impact. In this context, cellulose nanomaterials (CNMs) are considered as a potential candidate with regard to its abundant availability from different resources (Nechyporchuk et al. 2016; Reid et al. 2016). This is confirmed by the number of publications based on this subject for the past decade, i.e. between 2008 to date (see Fig. 1). These results were obtained by using descriptors like cellulose nanofibrils (CNF), microfibrillated cellulose (MFC), micro crystalline cellulose (MCC), cellulose nanocrystals (CNCs) and nanowhiskers (CNWs), in the Web of Science search engine (Fig. 1). The exponential research growth from 131 outputs in 2008 to more than 1400 in 2017 clearly indicates that cellulose nanomaterials with their unique valuable features has a potential to replace most of the synthetic nanomaterials in various fields in the near future. This is also justified by patent applications in the field of CNMs which include: composite materials (38%), non-woven absorbent webs (18%), paper and boards (16%), food products (13%), paper and boards coatings (8%), cosmetics and toiletries (3%), and filter materials (4%) (Blanco et al. 2018; Charreau et al. 2013; Durán et al. 2012; Sharma et al. 2018). CNMs possess attractive properties such as renewability, biodegradability, non-toxicity, large surface area, high aspect ratio, adaptable surface chemistry, and excellent mechanical properties (De France et al. 2017; Plackett et al. 2014). These properties enabled their application in polymer composites as reinforcing agent, and hybrid materials (e.g. aerogels) to afford lightweight products towards, packaging materials, sorbents materials, wound dressing materials, and tissue engineering (De France et al. 2017; Dufresne 2017, 2018; Plackett et al. 2014). Moreover, the biological inertness, surface tailor-ability and high water binding of CNMs renders their application as additives for fabrication of multifunctional materials as in oil drilling muds, cosmetics, rheology modifier and drug-delivery systems (De France et al. 2017; Dimic-Misic et al. 2013b; Plackett et al. 2014).
Illustration of the annual number of scientific publications since 2008, using the search terms nanofibrils (CNF), microfibrillated cellulose (MFC), micro crystalline cellulose (MCC), cellulose nanocrystals (CNCs) and nanowhiskers (CNWs). Data analysis completed using Web of Science search system on 22 August 2018
Depending on the source, different isolation procedures have been employed to produce cellulose nanomaterials owing to its hierarchical structure and semicrystalline nature (Blanco et al. 2018, Plackett et al. 2014). CNMs can be isolated from various sources which include algal cellulose, bacterial cellulose, bast fibers, cotton linters, microcrystalline cellulose, tunicates, wood pulp and agricultural wastes, as discussed in the next sections. Cellulose nanomaterials include all cellulose-based particles (i.e. at least one dimension in tens of nanometers) having various shapes, sizes, surface chemistries and properties (Chinga-Carrasco 2013; Foster et al. 2018; Plackett et al. 2014). CNMs are often classified based on the surface chemistry, isolation processing technique and cellulose source; i.e. cellulose nanocrystals (CNCs), cellulose nanofibrils (CNFs), tunicates-CNCs (t-CNCs), algal cellulose (AC) and bacterial cellulose (BC). Since the cellulose resources have different cellulose biosynthesis processes which influence cellulose chain stacking, the resulting CNMs have different degrees of crystallinity, polymorphs (cellulose I-IV), particles aspect ratios, viz. lengths and widths coupled with cross-section morphologies (Dufresne 2017, 2018; Foster et al. 2018, Jin et al. 2016; Oksman et al. 2016). These properties are crucial since they dictate the overall properties of the obtained CNMs, such as mechanical properties, optical clarity etc. CNCs also referred to as nanowhiskers, whiskers, nanocrystalline cellulose (NCC) are elongated crystalline rod- or needle shaped nanometric particles having width of 5–20 nm, lengths of 50–350 nm and aspect ratio of 5–30. CNFs also referred to as nanofibrillated cellulose (NFC), cellulose microfibrils (CF) or microfibrillated cellulose (MFC) are broadly flexible interconnected web-like fibrils networks composed of crystalline and amorphous regions with length greater than 1 µm, width of 5–100 nm and aspect ratio of 10–100, are often produced through mechanical treatment. In order to avoid confusion for all cellulose nanomaterials, standard definitions according to the TAPPI (TAPPIWI3021) will be used throughout this article to identify each material, and if the standard definition is not available commonly used names will be used.
Since plants are major industrial sources of cellulose nanomaterials, the primary focus of this review will be based on the cellulose nanomaterials extracted from plants, viz. lignocellulosic and non-lignocellulosic biomass. In case of lignocellulosic materials, cellulose is found to be embedded within hemicelluloses (35–50%) and lignin (10–25% dry weight). Therefore, the source is subjected to pulping, bleaching and pretreatments in order to facilitate the refinement steps with regard to intended CNMs (Maloney 2015). The main goal of pretreatment step(s) is to facilitate the reaction between the cellulose-containing materials with subsequent refinement treatments (George and Sabapathi 2015; Kumar et al. 2018). In the case of CNC, the purified cellulose through the dissolution of other constituents is subjected to chemical hydrolysis in order to digest the amorphous regions and produce highly intact crystalline spindle- or rod-like particles. The surface chemistry, charge and particle aspect ratio are directly dependent on the hydrolysis conditions. On the other hand, the pretreatment for CNFs are carried out to reduce high energy usage during their production by high pressure and/or shearing forces from mechanical fibrillation. The surface chemistry, charge, and cross-section and degree of branching of flexible interconnected web-like fibrils networks are depended on the pretreatments and mechanical shear process. For instance, the use of 2,2,6,6-tetramethylpiperidine 1-oxyl radical (TEMPO) oxidation result in oxidation of primary OH groups to carboxyl groups (Fraschini et al. 2017; Maloney 2015). Despite much effort that has been dedicated into the isolation of CNMs from various resources, their promising potential usefulness is inhibited by limited and below expectation scale-up production. The economic, sustainable and/or eco-friendly production of cellulose nanomaterials in order to realize their full potential in various fields remains a major challenge. Since the isolation processes differs as well as their resources, the resulting CNMs have different properties. This calls for innovative ideas to enhance the current isolation processing techniques in order to control the properties of the CNMs, and enhance production rate with low environmental impact. With that in mind, this review deals with recent scientific and technological advances in the CNMs production. The main aim is to highlight the recent published works based on the production of cellulose nanomaterials for the past decade, viz. 2008 to date, while comparing their strengths and limitations especially with regard to their commercial realization. We also highlighted on possible cellulose sources that can be utilized in order to address the anticipated future CNMs demands. Finally, this review presented the applications of CNMs in addressing the problems facing the current generation.
Cellulose and its sources
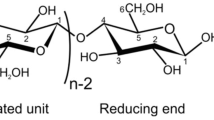
Cellulose was first isolated from wood using nitric acid by Anselme Payen in 1838. Cellulose, the most abundant available natural polymer on earth, consists of β-1,4-linked anhydroglucopyranoside units (Fig. 2) (Anwar et al. 2014; Naidu et al. 2017). Depending on the source, β-1,4-linked anhydroglucopyranoside units can reach values up to 1000. Cellulose chains are packed together to form more complex structure of highly crystalline microfibrils which are held together by hydrogen bonds and van der Waals forces. Individual cellulose chains are oriented in a parallel alignment and tightly bound to form elementary fibres that are further organized together to form larger fibrillar structures, viz., microfibrils. The diameter of each microfibril ranges from 2 to 20 nm depending on the source. The microfibrils comprised of crystalline domains which are linked along with disordered amorphous domains e.g. twists and kinks.
Diagrammatic representation of the framework of cellulose-containing source; cellulose; hemicellulose and lignin. Reprinted from Anwar et al. (2014) as distributed by creative common licences
Plant cellulose In lignocellulosic biomass, cellulose is found to be embedded within hemicelluloses and lignin as shown in Fig. 2. In this case, the percentage of lignin, hemicellulose and cellulose may vary depending on the growth conditions, source and cultivation process as depicted in Table 1. Hemicellulose as the second major component of plant sources accounts for 15–30% of the cell wall (Naidu et al. 2017). Hemicellulose and cellulose binds tightly with non-covalent attractions around microfibril bundles. As heterogeneous polymers, hemicellulose is mainly comprised of glucuronoxylan, glucomannan and other small amounts of polysaccharides (Fig. 2). Accounting for 10–25%, lignin is the smallest fraction of the biomass by weight (Naidu et al. 2017). It is considered as by-product or as a residue in bioethanol production. Lignin is a complex crosslinked polymer composed mainly of phenyl-propane units mostly linked by ether bonds (Fig. 2). It acts as glue filling gap between and around the hemicellulose and cellulose complexion. It is comprised of phenyl-propane, methoxy groups and non-carbohydrate polyphenolic substance which binds cell walls components together.
Microcrystalline cellulose (MCC) Commercialized in 1962 under the name Avicel, MCC is isolated from plants sources via hydrolysis process to reach level-off degree of polymerization of 200–300, followed by neutralization (Moon et al. 2011; Plackett et al. 2014; Trache et al. 2016). The neutralized suspension is washed, filtered and then spray-dried. It is industrial produced from wood and cotton but other sources such as agricultural waste, and bacterial cellulose have been investigated as potential sources (de Oliveira et al. 2011; Keshk and Haija 2011; Sainorudin et al. 2018). The latter is to avoid the costs and the use of harsh chemicals during pre-treatment since these sources have less or no lignin. MCC is highly crystalline (55–80%) and cheap material (~ 4 USD/kg) which found its way in pharmaceutical and food as binder and filler, as well as in cosmetic, and polymeric reinforcement. For a detailed isolation techniques and sources for MCC the readers are referred to review by Trache et al. (2016).
Bacterial cellulose Bacterial cellulose is synthesized by different bacteria such as Acetobacter, Rhizobium, Agrobacterium, Achromobacter, Azotobacter, Salmonella and Sarcina through aqueous culture media (Castro et al. 2012; Keshk 2014; Mohite and Patil 2014). BC appears as a nanoscale network-like structure having diameters ranging between 20 and 100 nm. It is recognized that properties of BC (e.g. crystallinity, dimensions etc.) depend on the culturing conditions and/or bacteria employed (Castro et al. 2012; Revin et al. 2018). The advantage of BC is the fact that it does not contain lignin, pectin, hemicellulose and organic residues associated with plant cell wall. BC features attractive attributes, such as high crystallinity (80–90%) with degree of polymerization of 4000–10,000 anhydroglucose units, excellent mechanical properties (strength of 100–160 GPa) and water holding capacity (up to 100 times its weight). It has been employed in different applications such as biomedical, tissue engineering, and fuel cells, batteries, and as a reinforcing agent in polymer reinforced composites. However, the biosynthesis of cellulose using bacteria is relatively expensive with regards to low productivity of the current used strains and the utilization of expensive culture medium as well as prolonged production periods makes it unsuitable for industrialization (Revin et al. 2018). There are interesting detailed reviews based on the production (i.e. focusing on the cutting overall production cost), characterization and applications of bacterial cellulose (Azeredo et al. 2019; Hussain et al. 2019; Wang et al. 2019).
Tunicates and algal cellulose (AC) Cellulose can be synthesized by different microorganisms such as algae and fungi (Zhang et al. 2017a, b; Keshk 2014). Cellulose microfibrils can be obtained in small quantities within the cell wall of brown algae (Phaeophyta), most green algae (Chlorophyta), red (Rhodophyta), blue-green (Cynophyta) and golden algae (Ochrophyta) (El Achaby et al. 2018; Chen et al. 2016b). Cellulose microfibrils structure vary depending on the algae specie as a result of difference in biosynthesis process (Moon et al. 2011). Interestingly, algae grow faster than terrestrial counterparts and have low content of physicochemical barriers (e.g. low lignin content) making easy for cellulose accessibility without the use of severe chemical treatments. Furthermore, algae is usually obtained in large quantities as waste from agar production, hence it is considered as alternative cellulose source for the production of cellulose nanomaterials to meet future demands. On the other hand, cellulose can be extracted from mantles of sessile sea creatures, viz., tunicates. In this case, mantle consists of tunic cellulose which aggregates to form microfibrils composed of a nearly pure cellulose Iβ allomorph which varies depending on the species (Zhao et al. 2015; Zhang et al. 2017a, b).
History and evolution of CNMs
Ever since the first report on the isolation of cellulose by Anselme Payen from wood in 1838, numerous discoveries have been made on its structural features, production and extraction (Rajinipriya et al. 2018; Payen 1838). The first report based on cellulose colloidal suspension obtained through controlled sulphuric acid (H2SO4) was communicated by Ranby in (1949). This work was a follow up on the study conducted by Nickerson and Habrle who recognized that hydrolysis of cellulosic fibers with aqueous hydrochloric acid (HCℓ) and H2SO4 at boiling temperature led to amorphous regions being attacked first (Nickerson and Habrle 1947). For better understanding of the history behind cellulose nanomaterials, we have listed a comprehensive, year-wise development of CNMs in Table 2 (Rånby 1951; Rånby and Ribi 1950; Mukherjee et al. 1952; Mukherjee and Woods 1953; Battista 1950; Battista et al. 1956; Marchessault et al. 1959; Turbak et al. 1983; Herrick et al. 1983; Revol et al. 1992; Favier et al. 1995; Kulpinski 2005; Crotogino 2012). After the first pilot plant in 2011 for cellulose nanomaterials production, research has dealt mainly on the aspects, such as enhancing the production rate using methods having low environmental impact, studying abundant available sources to reassure continuous supply of resources for up-scaling production, functionalization towards reinforcing application, and possible new applications of CNMs (Rajinipriya et al. 2018).
Cellulose nanomaterials
Cellulose nanomaterials can generally be defined as cellulose composed of crystalline and amorphous regions having diameters below 100 nm and the lengths reaching few microns. A wide variety of cellulose resources including wood (soft or hard wood), seed (cotton), bast (jute, flax, hemp), leaf (sisal), straw (rice, wheat), fruit (coir), tunicates, algae, fungi and bacteria have been utilized for the production of cellulose nanomaterials (Anwar et al. 2014; Blanco et al. 2018). Most of the industries and/or institutions which are involved in production of CNMs for commercial purposes use wood pulp as source of cellulose (Rajinipriya et al. 2018). The non-woody resources are, however, gaining much attention as alternative to wood because of their short growth period and easy delignification when compared to wood because wood has high content of lignin (Rajinipriya et al. 2018). Moreover, for wood sources the cellulose fibrils are located in the primary cells, whereas in non-woody species it is located in secondary cells which makes it necessary for different pretreatments steps for cellulose nanomaterials extraction. Numerous cellulose-containing agricultural wastes (e.g. cotton stalk, pineapple leaf, rice straw, flax, hemp, soy pods, rice husk, garlic straw, potato peel, tomato peel, grape skin, carrot pulp, corncobs etc.) are also considered as a viable source owing to their unique properties such as availability, low cost and the fact that they are rich source of carbohydrates (Rajinipriya et al. 2018). These materials are usually left in the fields or burnt which add to the current environmental crisis (Mtibe et al. 2015). The use of such materials to produce value-added products is of significance not only towards creating a positive environmental impact but also to create a close loop cycle economy benefiting farmers. On the other hand, it can be argued that geopolitics affect their prices which may influence their utilization for industrial production of CNMs. Beside these limitations, CNMs have been employed in different fields such as biomedical, construction (cement/concrete), coating, drilling fluids, filtration (barrier), drug delivery, polymer composites, and personal care (Dufresne 2017, 2018; Foster et al. 2018; Jin et al. 2016; Oksman et al. 2016).
Classification of CNMs
Cellulose nanomaterials are often broadly classified according to the production process and/or sources, as tabulated in Table 3. In this regard, the materials having different sizes, aspect ratio, degree of crystallinity, surface chemistry and polymorphs (cellulose I-IV) are produced. These differences result from biosynthesis processes of each source result in different chain stacking hence the overall properties. Since plants are the most used sources for the production the main sections in this review are dedicated to the preparation of different cellulose nanomaterials mainly from plant sources, hence for other CNMs’ sources the readers will be directed to the relevant reviews for more information.
Electrospun nanofibers (ENMs)
Besides being manmade, electrospun nanofibers qualifies as one of the cellulose nanomaterials because of the resulting fibres have diameters in the nanoscale. In this case, electrospinning technique is employed to afford the manufacturing of the nanofibrous material featuring distinctive properties, such as large surface-to-volume ratio, tuneable porosity and high permeability (Mokhena et al. 2015). In this process, the pure cellulose or derivative is dissolved in a suitable solvent and subjected to high voltage to form electrified jet which moves towards to an oppositely charged collector. Reaching a collector, the jet solidifies to form non-woven cellulose webs or in case of cellulose’ derivatives post-treatment is conducted to produce pure cellulose (regenerated cellulose) (Viswanathan et al. 2006; Ma and Ramakrishna 2008). For instance, Ma and Ramakrishna produced electrospun pure cellulose nanofibers by electrospinning cellulose acetate using acetone/DMF/trifluoroethanol (3:1:1) as solvent (Ma and Ramakrishna 2008). The obtained non-woven cellulose acetate nanofibers were then thermally treated at 210 °C for an hour followed by deacetylation using 0.1M NaOH (in H2O/ethanol, 2:1) for 24 h in order to obtain pure cellulose nanofibers. The morphology of electrospun cellulose nanofibers is shown in Fig. 3a (Viswanathan et al. 2006). The fibers exhibited highly branched structure and the diameters were ranging from few nanometers to several microns.
Micrographs of CNMs: a electrospun nanofibers (Reprinted from (Viswanathan et al. 2006). Copyright 2006 American Society); b CNCs from Maize stalk; c bacterial cellulose (BC) (Reprinted from Revin et al. (2018). Copyright 2017 Elsevier); d tunicate cellulose (t-CNCs) (Reprinted from Zhao et al. (2015). Copyright 2015 Elsevier); and e CNFs extracted from wood (Reprinted from Wang and Drzal (2012). Copyright 2012 American Society)
Cellulose nanocrystals (CNCs)
Cellulose nanocrystals (CNCs) are elongated crystalline rod- or needle shaped nanometric particles (see Fig. 3b) having width of 5–20 nm, lengths of 50–350 nm and aspect ratio of 5–30, as well as bending strength of about 10 GPa, coupled with tensile strength of up to 7.5 GPa and Young’s modulus of ~ 150 GPa (Dufresne 2017, 2018; Foster et al. 2018, Jin et al. 2016; Oksman et al. 2016; Sharma et al. 2018). The extraction of CNCs can be categorized into two main steps: (1) pretreatment steps and (2) refinement. The former is generally employed to remove other constituents than cellulose fibers (as discussed in detail in "Pretreatments step" section). The refinement step involves the hydrolysis of purified cellulose into nanocrystals components as shown in Fig. 3b. Besides resources of pure cellulose sources such as CNFs, bleached wood pulp and micro crystalline cellulose (MCC), different cellulose-containing sources (e.g. agricultural by-products, wood and non-wood) are first subjected to different pretreatment processes which reduces time by allowing chemical accessibility in the second/refinement step (see Fig. 4). The obtained purified cellulose consists of well-ordered crystalline and amorphous regions which vary with regard to the source. The amorphous domains are more susceptible to refinement treatments (i.e. chemical acid hydrolysis, enzymatic hydrolysis, mechanical refining, ionic liquids, subcritical water hydrolysis, oxidation or a combination of these processes) promoting the isolation of well-ordered crystalline domains (CNCs) (Fig. 3b) (Kumar et al. 2018; Wang et al. 2013a; Mtibe et al. 2015; Tang et al. 2015b).
Schematic presentation of recent processes to achieve cellulose nanomaterials. Reprinted from Kumar et al. (2018) distributed under creative commons license
Tunicates CNCs (t-CNC)
Mantle of sessile creatures (tunicates) have been used for the isolation of ribbon-like shaped cellulose particles with high crystallinity (85–100%). The resulting particle are mainly composed of Iβ allomorph which vary depending on the specie under investigation (Zhao et al. 2015; Zhang et al. 2017a, b). The morphology of the resulting t-CNCs are shown in Fig. 3d. Similar to CNCs, t-CNC are extracted using acid hydrolysis process.
Algal and bacterial cellulose
Algal cellulose nanomaterials can be isolated from the cell walls of the algae species to afford particles with high aspect ratio (El Chaby et al. 2018; Chen et al. 2016b). Depending on the refining treatment i.e. chemical hydrolysis or mechano-chemical process and the specie various particles having different dimensions can be produced. Besides plants being the main source of cellulose, cellulose can be produced as extracellular product by different bacteria such as Acetobacter, Rhizobium, Agrobaterium etc. It is extracted as high purity (> 99%) ribbon-like fibrils having diameters of 20–100 nm and the lengths reaching few microns (Fig. 3c). These dimensions and crystallography (Iα/Iβ) are directly dependent on the culturing conditions i.e. stirring, temperature and additives as well as bacteria under investigation (Sharma et al. 2018).
Cellulose nanofibrils (CNFs) and cellulose microfibrillated cellulose (MFC)
Cellulose nanofibrils (CNFs) are generally defined as cellulose nanomaterials consist of fibrils with dimension in the nanometric scale primarily obtained from by means of mechano-chemical treatments. CNF consists of aggregated nanofibrils with diameters of 5–30 nm and up to several microns long (Fig. 3e). Although wood is the most used resource for CNF production, other non-woody resources (e.g. algal, agricultural crops, water plants, and their by-products) are considered as alternatives because of their benefits such as short growth periods and easy delignification. This can be related to less or no lignin of these resources when compared to wood. Pretreatments alleviated the high energy consumption associated with CNFs defibrillation processes from 30,000 to less than 2000 kWh/ton, thus unlocking large-scale production (Desmaisons et al. 2017; Kumar Mishra et al. 2018). The combination of both enzymatic or chemical pretreatments and mechanical treatments are applied for both the pilot scale and industrial scale production (Fig. 4). These treatments are applied according to the fibre sources, production conditions and specific quality requirements. Similarly, MFC are generally obtained through mechanical means of purified cellulose from different sources (see Table 1). The resulting particles are composed of amorphous and crystalline; and diameters of 10–100 nm and lengths reaching values greater than micron. MFC have quite similar properties as CNFs, hence they are often used interchangeably in literature. It is recognized, however, the difference is the extent of the defibrillation process which result in their dimensions, gelation and optical clarity. In this case of CNFs have smaller diameters. In this review, to avoid confusion CNFs will be used to represent both MFC and CNF in the next sections.
Isolation of CNMs
Pretreatments step
Different cellulose-containing sources are first subjected to different pretreatment processes which reduce time by allowing chemical accessibility in the second step (Fig. 4). It was observed that pretreatment increases the surface area which makes the fibres to be more susceptible to refinement step(s). The difference in chemical composition depends on the environmental growth, maturity and cellulose resource-type. Figure 4 shows different extraction processes of cellulose nanomaterials and the most commonly adopted pretreatment. Pretreatment is generally employed to remove other constituents (e.g. lignin, hemicellulose/extractives/contaminants for lignocellulosic resources; proteins for tunicates) than cellulose fibers. In the case bacterial cellulose, pretreatment involves the culturing processes followed by cellulose recovery through purification to remove medium components other than pure cellulose fibers (Revin et al. 2018).
In case of lignocellulosic biomass, as main focus of this review, the content of each component viz. lignin, hemicelluloses, extractives and contaminants of cellulose-containing sources varies from one plant to the next; hence, the adopted pretreatment has to be carefully considered for isolation of pure cellulose. Basically, the raw materials are washed with water and then subjected to alkali pretreatment using sodium hydroxide (NaOH) or potassium hydroxide (KOH) to remove water-soluble constituents and other components (hemicellulose, lignin, pectin and waxes) than cellulose, i.e. pulping. The main concern from environmental point is usage of water, toxic chemicals and energy. Yet other processes employed to remove these components (i.e. hemicellulose, lignin, pectin and waxes) include organic solvents, soxhlet, or mineral acids. These treatments are recognized as a step to remove non-cellulosic components and induce defibrillation of the fibre bundles. The resulting material is then introduced to bleaching step or delignification using different bleaching agents such as sodium chlorite (NaCℓO2), chlorine dioxide (CℓO2), hydrogen peroxide (H2O2), peroxyacids, oxygen or ozone. The bleaching step can be single or multiple stages with regard to the intended cellulose material and its application. In this step, it was observed the size of the fibres reduced by a further defibrillation of the fibres into individual fibres. The environmental regulations are, however, very stringent towards the use of chlorinated reagents which opened doors for the utilization of chlorine oxide-based in Elemental Chlorine-Free (ECF) and Total chlorine-free (TCF) sequences (Robles et al. 2018b).
Additional processes such as Kraft or sulphite process were reported in the literature in order to produce pure cellulose. These pretreatments are mostly used in industrial scale, with market dominance of 96% belonging to Kraft. Kraft process removes mostly lignin using a mixture of NaOH and sodium sulphide in the digester. The digester operates at about 175 °C for 2–5 h. Sodium sulphide addition facilitates ether cleavage and controls undesirable condensation reactions. This process is recognized by high yield of strong fibres. Although sulphite is recognized with low yields of weaker fibers, it removes more lignin thus the resulting fibres are more suitable for high-quality paper or cellulose. In this regard, the fibres are treated with magnesium bisulphate and excess sulphur dioxide at 175 °C for 6–12 h. The disadvantage of these processes is that the generated sulphite derivatives may link to cellulose and cause environmental problems with regard to their disposal (Robles et al. 2018b; Ferreira et al. 2018).
Due to strict environmental regulations, organosolv has emerged as an alternative process owing to its unique features (Ferreira et al. 2018; Robles et al. 2018b). This includes the extraction of lignin using environmentally benign solvents which are recovered after purification process by distillation. Organosolv involves heating the raw material in the presence of water, solvent and catalyst for about 2 h (Ferreira et al. 2018; Robles et al. 2018b). Besides various solvents being employed for this process (e.g. mono- and polyalcohols, aldehydes, ketones, thio compounds, organic acids and bases, and dimethyl sulphoxide), triethyl glycol is the most suitable solvent. Triethyl glycol, however, is limited by high boiling point which makes it difficult to recover. Ethanol is the most preferred solvent due to its low cost and low toxicity. Numerous catalysts such as HCℓ, H2SO4, ferric chloride, ammonia, aluminium salts, and urea are often employed. It is worth mentioning that these processes cannot remove all lignin; and thus high quality (pure) cellulose is obtained by subsequent bleaching which has been already stated previously (Ferreira et al. 2018).
Enzymatic hydrolysis, 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO)-mediated, and carboxymethylation are often considered as secondary pretreatment usually implemented after pulping and bleaching, since they can afford to produce the CNMs as it will be discussed in the next sections (see Fig. 4). Another secondary pretreatment method which was first used by Wågberg Decher et al. (2008) is carboxymethylation process (Hubbe et al. 2017; Im et al. 2018, 2019; Lourenço et al. 2019; Wågberg Decher et al. 2008; Wei et al. 2019). Carboxymethylation involves the etherification of the cellulose hydroxyl group using monochloroacetic acid (MCA) under alkaline conditions. In this case, the pulp is solvent exchange to ethanol followed by carboxymethylation process in alcohol mixture i.e. isopropanol/methanol or isopropanol/ethanol under alkaline conditions. The solvent exchange and the limitation to recycle the employed chemicals makes this method expensive. Similarly, acetylation involves esterification of the cellulose hydroxyl group into an acetoxy group, hence weakens hydrogen bonding between fibrils.
Refinement step
Beside cellulose nanocrystals being commonly produced by classic mineral acid hydrolysis, recent developments indicate that the CNCs can be produced through integrated production routes such that the cellulose loss is minimized to nearly zero (Chen et al. 2016a; Lv et al. 2019; Wang et al. 2012a, b; Wang et al. 2013c). In this regard, the hydrolysed materials during isolation of CNCs leave behind solid cellulose residue that can be recovered and used to produce CNFs with sugars that can be used for other purposes such as chemical production and energy production. The integration of such techniques are of interest from ecological and economic viewpoints especially for industrial-scale application. For instance, Lv et al. (2019) employed formic acid hydrolysis to produce CNCs and CNFs by varying acid concentration, reaction temperature and duration. It was reported that the high concentration of acid result in shorter time for reaction, while high temperatures adversely affect the yield. It was demonstrated that all products from acid hydrolysis can be recovered to be used for other purposes since the formic acid is eco-friendlier with cellulose solid residue being used to produce CNF by subsequent mechanical treatment. In most cases, the pretreatments have been employed prior to the key refinement treatments, such as high-pressure homogenization, microfluidization, micro-grinding, high-intensity sonication, melt extrusion, and steam explosion to facilitate production process and improve the quality of the CNF (Nechyporchuk et al. 2016). TEMPO is the most used pretreatment for CNFs production. In general, it can be argued that there is no single technique that can produce CNFs from the source, however, a combination of the processing techniques can improve the properties of the resulting CNFs (Abe and Yano 2009; Moser et al. 2015; Siqueira et al. 2016).
Acid hydrolysis treatment
Beside acid hydrolysis being the most commonly employed technique to extract CNCs from different sources, it has been used to improve the quality of the purified cellulose (e.g. MCC). In this regard, the pretreated cellulose-containing source is subjected to a given concentration of desired acid for a certain period at favourable temperatures to avoid degradation of cellulose particles. The dimension and/or structure of the CNMs depend on the cellulose source, as well as on the conditions of the acid hydrolysis process such as time, temperature, acid concentration, and the nature of acid (Csiszár and Nagy 2017; Li et al. 2018b). For instance, diluted acid i.e. 1–10 wt% are commonly used for pretreatment since it hydrolysis hemicellulose to its monomeric units, hence exposes cellulose. In the case of concentrated acids solutions (30–70 wt%), the cellulose-containing sources requires less temperatures and shorter time to affords the isolation of highly crystalline cellulose particles (Bian et al. 2017a, b; Bian et al. 2018).
Sulphuric and hydrochloric acid are the commonly used acids to afford the extraction of CNCs, but other acids such as hydrobromic (Sucaldito and Camacho 2017; Sadeghifar et al. 2011), phosphoric (Tang et al. 2015b; Camarero Espinosa et al. 2013), and phosphotungstic acid (Lu et al. 2016; Liu et al. 2014; Budhi et al. 2018), were also reported for such purposes as summarized in Table 4. Recently, organic acids (e.g. maleic (Filson and Dawson-Andoh 2009), formic (Du et al. 2016a; Li et al. 2015) and oxalic), were also reported as alternative acids to overcome some shortcomings of mineral acids. This is as a result of possibility of recycling and reusing these acids for extraction purposes or/and to recover extracted sugars. The amorphous regions in cellulose are susceptible to acid attack which promotes hydrolytic cleavage of the glycosidic bonds and releasing individual crystallites. This is as a result of the difference in kinetics of hydrolysis between amorphous and crystalline regions which lead to selective cleavage in the amorphous region along cellulose chains, thus leaving behind elongated crystalline particles.
Despite the easy manipulation of the mineral acids to obtain desired morphology and/or dimensions, there are shortcomings that still need to be addressed. These include equipment corrosion, large quantities of water usage and generation of large amount of waste with fairly low yields. Some reports proposed the substitution of the strong liquid acid with solids acids in order to overcome some of these shortcomings, especially environmental impact and sustainability (Tang et al. 2011). In this regard, cation exchange resin-catalyzed hydrolysis was employed and similar crystalline rod-like particles as for sulphuric acid were obtained, however, with only 50% yield (Tang et al. 2011). This strategy has several advantages compared to classic acid hydrolysis such as recovery/regeneration of solid acid, easy handling, and less corrosive. The low yield and long hydrolysis time are some of key issues that require more research to afford the up-scaling of this hydrolysis process. Some reports demonstrated that the use of phosphotungstic acid for CNMs isolation is one of the rational solutions to solve the low yield (Liu et al. 2014; Budhi et al. 2018). It was shown that a yield of more than 60% can be achieved and the acid can be recovered and reused to extract CNMs. The major concerns associated with this procedure include long hydrolysis time, washings using ethanol (which may be costly than water), CNMs flocculation after standing for more than 12 h, as well as the use of diethyl ether to recover both acid and CNMs. The use of mechanochemical activation during phosphotungstic acid hydrolysis was found to increase yield to 88% which demonstrates the possibility of industrial production (Lu et al. 2016). Mechanochemical activation was carried out by ball milling in the presence of phosphotungstic acid followed by heating at 90 °C for 4.5–5.5 h as compared to 35 h in (Liu et al. 2014; Budhi et al. 2018). Thermal stability of the resulting CNCs was also improved as compared to sulphuric acid hydrolysis with a dramatic increase of maximum degradation temperature from 338 (i.e. for raw material) to 348 °C. Beside the abovementioned issues associated with phosphotungstic acid hydrolysis, this procedure has the capability to go beyond pilot scale with regard to recovery and reuse of acid, as well as high yields coupled with thermally stable CNCs.
Phosphoric acid is also known to produce highly thermal stable CNCs with yield reaching values of 70–80% depending on the hydrolysis conditions (Camarero Espinosa et al. 2013). However, economic chemical recovery is yet to be demonstrated especially considering up-scaling to industrial production. Recent reports on utilization of alternatives to mineral acids have been published (Jia et al. 2017b; Chen et al. 2016a; Wang et al. 2017). Organic acids (e.g. oxalic, maleic and p-toulenesulphonic acids) received much interest because they are readily available and are produced from natural resources (biomass) (Jia et al. 2017b). It was observed that organic acid hydrolysis produced thermally stable carboxylated CNCs with reasonably high yields. In addition, organic acids can be recovered and reused through classic and commercially proven crystallization process at low temperatures for sustainable and economic manufacturing. It was demonstrated in these studies that this procedure has a potential of achieving commercial success at low cost and sustainable manufacturing. Jia et al. (2017a) compared three acid hydrolysis preparation processes, i.e. disk-milled oxalic acid hydrolysis (D-OCNCs), oxalic acid hydrolysis (OCNCs) and sulphuric acid hydrolysis (S-CNCs). It was demonstrated that using oxalic acid hydrolysis, thermally stable carboxylated CNCs can be obtained with a high yield by including disk milling as pretreatment. The yield reached a value of 36.9% which was sevenfold higher than oxalic acid alone (OCNCs, 4.4%) and for sulphuric acid hydrolysis it was 30%. The maximum thermal degradation temperature of the D-OCNCs was 298 °C, while for OCNCs and S-CNCs were 307 °C and 218 °C respectively.
The utilization of HCℓ vapour in order to avoid high water consumption was demonstrated by Kontturi et al. (2016). They reported that the produced CNCs had high crystallinity with degree of polymerisation (DP) being reduced to the levelled off DP (LODP) of about 170 (i.e. LODP corresponding to classic CNCs extraction processes). The yield reached a value of 97.4% as compared to 20–50% with regard to liquid/solid processes. The closest yield of about 94% using hydrochloric acid under hydrothermal was demonstrated by Yu et al. (2013). It was reported this isolation process afforded the production of highly crystalline (88.6%), and thermally stable (maximum degradation temperature of 363.9 °C) cellulose nanomaterials within acceptable reaction time (3 h), temperatures 110 °C, and mild acid concentration (60 mL g−1). The diameters were between 16 and 20 nm with lengths ranging between 255 and 290 nm, i.e. aspect ratio of 13–16. The concern about this process include prolong reaction time (3 h vs. l h of less for H2SO4), the recovery of the by-products and high temperature involved. Despite the fact that acid hydrolysis result in nanocrystals which are elongated rod-like particles, spherical particles were also produced by a mixture of acids followed by intense sonication (Yu et al. 2017; Wang et al. 2007b; Zhang et al. 2007; Azrina et al. 2017). Similarly, enzymatic hydrolysis with ultrasonication was recently reported as an alternative green method to produce spherical particles with diameters less than 100 nm (Meyabadi et al. 2014). While sulphuric acid hydrolysis process is the most used method to extract CNCs, microbial and catalytic ionic liquid hydrolysis were also reported for such purpose (Trache et al. 2017; Xie et al. 2018).
Enzymatic hydrolysis treatment
Enzymatic hydrolysis is recognized as either secondary pretreatment or refining step. In the former, the pre-treated cellulose pulp is introduced into monocomponent or multicomponent of enzymes to promote the fibrillation process (Teixeira et al. 2015). Despite the fact that, enzymatic hydrolysis produces less stable CNMs as compared to acid hydrolysis, this process is eco-friendlier and results in isolation of highly crystalline particles (Teixeira et al. 2015). The principle of this process is similar to that of conventional acid hydrolysis treatment, but herein enzymes are responsible for breaking the glycosidic bonds of the amorphous domains to produce CNMs (Lynd et al. 2002). Cellulases is a composite of active components, viz.: (1) endoglucanases, (2) exoglucanases, and (3) cellobiohydrolases (Filson et al. 2009). These enzymes act synergistically in hydrolysis process. Endoglucanase seldom attacks amorphous domains, whereas exoglucanases attacks cellulose chains from either the reducing or non-reducing ends. Cellobiohydrolases mainly acts from the C1 or the C4 ends using a protein in each case, into cellubiose units (Filson et al. 2009). Filson et al. (2009) prepared cylindrical CNCs with widths between 30 and 80 nm and lengths between 100 and 1.8 µm by hydrolysing recycled pulp using endoglucase enzyme. Endoglucanase hydrolytic treatment led to the maximum yield of 38.2% for microwave heating and 29% for conventional heating at 50 °C in water. Teixeira et al. (2015) isolated both CNFs and CNCs from different cellulose resources using enzymes from wet-disk milling-treatment. It was found that highly crystalline CNFs and CNCs were obtained with different dimesnions depending on the cellulose source, hence the presence of other components than cellulose. The CNMs had diameters ranging between 500 and 3000 nm and diameter of 6–20 nm depending on the source. Even though this method seems promising towards the industrial scale production with regard to its eco-friendliness, low yields and prolonged hydrolysis treatment still need further demonstrations to improve its efficiency. In addition, the enzyme activity depends on variables such as the temperature, and incubation time, which makes it difficult to control the extent of hydrolysis. Nonetheless, recent study by Bauli et al. (2019) demonstrated that enzymatic hydrolysis can be employed for both pre-treated (i.e. bleached) and untreated wood waste to afford highly crystalline CNMs. It was reported that although the yield was too small (~ 3%) for untreated waste as compared to pre-treated waste (~ 12%), the life cycle impact assessment (LCIA) indicated that this route is favourable to produce CNMs.
Oxidation treatment
The oxidation of cellulose-containing source with strong oxidizing agent [(e.g. sodium periodate, ammonium persulphate (APS), TEMPO)] has been also reported to promote the isolation of homogeneous rod-like CNMs (Zhang et al. 2016; Lam et al. 2012). 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO)-mediated oxidation is also considered as a reliable and efficient pretreatment method to facilitate the isolation of CNMs (Pääkkönen et al. 2015; Wu et al. 2017). It is the most utilized pretreatment for isolation of CNFs (Isogai et al. 2011; Moser et al. 2015). It relies on selectively oxidizing primary alcohols (C6-primary hydroxyls) to carboxylate groups such that adhesion between cellulose fibrils via hydrogen bonding is weakened. This results in the reduction of energy required in the CNFs production since the fibrils can be easily broken-down into nanofibrils. The obtained nanofibrils have vast of carboxylate groups which can be suitable for further functionalization and degree of polymerization (DP) is often maintained depending on the refining step. The disadvantage of this process is the amount of water used, expensiveness, corrosiveness and toxicity of the chemical used. In addition, the toxic chemicals such as C6-aldehydes and C2/C3-ketones are produced during oxidation process (Fan et al. 2019).
APS, however, has a promising potential owing to its unique features: low toxicity, high water solubility and low cost (Cheng et al. 2014; Jiang et al. 2017). Cheng et al. (2014) showed that spherical shape particles of 29–96 nm were obtained through APS as oxidizing agent with the yield of 22 to 75% depending on the experimental conditions. In comparison with TEMPO ultrasonic assisted process (TO-CNC), APS single step extraction process resulted in CNCs (AO-CNC) with higher crystallinity, and better thermal stability, however, at low yields (Zhang et al. 2016). The increase in oxidant content in both cases led to a great deal of carboxyl groups introduced on the surface of the CNCs. It was indicated that one-pot APS oxidation is simpler and can easily be adopted in large scale production, however, the yield is lower when compared to TEMPO ultrasonic assisted process. Overall, the oxidation using strong oxidant agents display high yield as compared to classic methods (mineral acid hydrolysis). On the other hand, it consumes lot of oxidants, reaction time is longer, and the oxidants are expensive which can affect the price of resulting carboxylated CNMs. The comparison between the mineral acids and this process is required in terms of the cost from the beginning to final product. Recent study by Fan, et al. (2019) demonstrated that CNMs can be produced using eco-friendly oxidant consisted of Fe2+ hydrogen peroxide (H2O2). In this regard, the mixture of the MCC and H2O2/ferrous sulphate tetrahydrate (FeSO4.4H2O) was sonicated for 10 min followed by thermal treatment (60 °C) while stirring for 2–8 h. It was shown that at this temperature Fe2+ effectively catalyzes H2O2 to produce H+, OH. radical; and H2O2 with the former penetrating the cellulose amorphous domains and H+ protonate β 1-4 glycosidic bond while OH. attacks hydroxyl groups producing carboxylated CNMs. The obtained highly crystalline (84–89%) carboxylated CNMs (0.7–2.2 mol g−1) with widths of 19–23 nm and lengths of 92–141 nm as well as high thermal stability (maximum thermal decomposition temperature of 325–342) depending on the reaction time.
Ionic liquids (Ils)
Ionic liquids (Ils) serve as a suitable solution for extraction of CNC because they are recyclable and addresses the environmental concerns of other extraction processes (Mao et al. 2013, 2015). Moreover, Ils are chemically, and thermally stable, non-flammable, and possess low vapour pressure. Ils are consists of anion and cations which are of significant importance to solubilize lignin during cellulose particles isolation. The interruption of the Ils ion with intra- and intermolecular hydrogen bonding promotes the cleavage of the crystalline particles from cellulose biomass. Laborie and coworkers (Mao et al. 2015) managed to produce highly crystalline CNCs (76 ± 2.0%) with a yield of about 76% from MCC using two-step 1-butyl-3-methylimidazolium hydrogen sulphate ([BMIM]HSO4) mediated hydrolysis (viz. 24 h swelling at ordinary temperature and 12 h hydrolysis at 100 °C). Abushammala et al. (2015) were the first to report on the use of 1-ethyl-3-methylimidazolium acetate([EMIM][Oac]) to extract CNCs from wood. It was shown that despite the method involving two-steps 95% of ionic liquid could be recovered. The authors found that highly crystalline (~ 74%) acetylated rodlike particles with width of 2–5 nm and lengths of 75–125 nm could be obtained by treating wood twice with [EMIM][Oac]. The yield reached a value of 20% of the original wood mass (or 60% of the produced pulp).
Homogenization
In 1983, Turbak et al. (1983) and Herrick et al. (1983) were first to isolate CNFs with diameters less than 100 nm from wood pulp. Since then homogenizers were used to extract CNFs from a wide variety of raw materials. In this process, cellulose suspension is passed through a small gap between two valve seats and high pressure is applied. Subsequently, the high shear forces generated between the gaps causes fibrillation of cellulose. High energy consumption and clogging are major issues associated with this process. Nonetheless, different pretreatments were adopted to mitigate energy consumption as discussed in previous section (Wei et al. 2017). In the case of clogging, reducing fibre size before homogenization was found to be a suitable solution to avoid machinery clogging. Overall, homogenization process is capable for the large scale production of CNFs. Two acids, viz. formic and hydrochloric acid were used for pretreatment followed by high-pressure homogenization to afford CNFs having widths of 5–20 nm and length of 300–1200 nm. In addition, the as-prepared CNFs from formic acid and HCℓ pretreatment exhibited good thermal stability (maximum thermal decomposition temperature of ~ 330 °C and 328 °C, respectively) (Du et al. 2016b).
Microfluidization
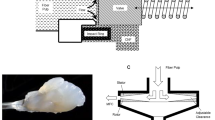
In 2004, Zimmermann and coworkers were the first to isolate CNFs from sulphite pulp using microfluidization process to obtain CNFs having diameters of 20–100 nm and lengths of several tens of micrometers (Zimmermann et al. 2004). Degree of depolymerization (DP) decreased from 1381 to 643 after the treatment. This process involves passing sample slurry through a Z- or Y- shaped chamber having channel size of 200–400 µm as shown in Fig. 5 (Xie et al. 2018). CNFs are isolated by applying high pressure through the intensifier pump to delaminate fibers as a result of shear forces from their collision and the channels (Spence et al. 2011; Ferrer et al. 2012; Chiappone et al. 2011). There are two major issues associated with this technique which limits its progress, viz. high energy consumption and clogging. Pretreatment of the cellulose-containing source reduces energy consumption; whereas clogging can be resolved by reverse flow through the chamber since this technique has no in-line moving parts.

Adapted from Xie et al. (2018) distributed under creative common license)
Mechanical processes and their working principles for CNFs production: a homogenizer, b microfluidizer and c grinding.
Grinding
In grinding, CNFs are isolated by passing sample slurry through supermasscolloider grinder (Fig. 5) (Xie et al. 2018). In this case, the fibers are ground by passing the slurry between a static and a rotating stone which produces high shearing forces to cleave the cell wall structure and cellulosic bonds (Mtibe et al. 2015; 2012a, b; Siqueira et al. 2016). The gap between the discs can be adjusted to obtain the desired structure based on the type raw material. This process renders some advantages such as high efficiency, large capacity, low energy consumption and less prone to clogging which demonstrate its applicability in industrial production. However, fiber damage due to strong mechanical forces results in CNFs having low crystallinity, thermal stability and physical strength makes this process undesirable for production of CNFs where those properties are of significance. Elsewhere in literature, it was demonstrated that the damage of the CNFs can be avoided by performing one-time grinding treatment (Abe and Yano 2009). In this case, CNFs were isolated from different raw materials (i.e. wood, rice straw and potato tuber) using supermasscolloider (model MKCA6-3; Masuko Sangyo Co., Ltd., Saitama, Japan) grinder at 1500 rpm with clearance gauge of -6 (corresponding to 0.6 mm shift) from zero position. It is worth mentioning that, a series of chemical pretreatments were performed prior mechanical treatment as shown in Fig. 6. The resulting fibers had diameters below 60 nm regardless of the raw material. The diameter range distribution varied among the raw materials viz. 12–20 nm for wood, 12–35 nm for rice straw and 12–55 nm for potato tuber due to the difference in patterns of aggregation among sources. Moreover, the tensile properties and crystallinity were similar for all samples; however, the crystallinity was higher than that of purified cellulose.

Adapted from Abe and Yano (2009). Copyright © 2009, Springer Science Business Media B.V
Experimental procedure for the preparation of cellulose nanofibers from each plant source.
Ball milling
In this process, cellulose-containing source is placed in hollow cylindrical container partly filled with balls (e.g. ceramic, metal or zirconia) and the rotation of the containers causes high energy collision between the balls and fibers, which result in defibrillation (Zhang et al. 2018; Baheti et al. 2012). Figure 7 represents schematic presentation of ball milling process (Zhang et al. 2015). The effect of milling conditions viz. ball-to-cellulose mass ratio, milling time, ball size and alkaline pretreatment on the resulting CNFs was studied by Zhang, Tsuzuki, and Wang (Zhang et al. 2015). It was found that selection of ball size is essential to facilitate defibrillation of nanofibers. Using small balls does not have sufficient impact energy, whereas large balls have high impact energy causing damage to the fibres to form particles. It was demonstrated that milling time and ball-to-pulp mass ratio are responsible for number of collisions events, thus careful consideration is needed to maximize CNFs’ extraction. On the other hand, it was reported that alkali pre-treatment weakened the hydrogen bonding between the fibers thereby facilitating the extraction of CNFs. It was demonstrated that careful control of milling conditions can result in CNFs with high dimensional homogeneity and average fiber diameters less than 100 nm through ball milling. The effects of nature of milling, i.e. dry or wet was studied by Baheti et al. (Baheti et al. 2012). It was reported that dry milling causes fibres to stick to milling media which makes it difficult to produce CNFs with a narrow size distribution, whereas wet milling afforded the production of CNFs with diameters below 500 nm with narrow size distribution after 3 h. Although this method results in high production rate, the control on the CNFs size is very limited which makes it more difficult for industrial production where reliable and consistent data is needed. Moreover, prolonged milling time is required to obtain CNFs with narrow distribution diameters which lead to their contamination. Besides being commonly being used for CNFs production, ball milling has been employed for the isolation of CNCs as reported by Mohd Amid, et al. 2015. MCC dispersed in deionized waster was first introduced to sonication followed by ball milling to yield elongated rod-like particles (57–76%) having diameters of 15–18 nm and lengths of 317–320 nm depending on the milling time. The authors further introduced the mild acid (1 wt% phosphoric acid) in order to improve the thermal stability and dispersion of the particles in aqueous medium. This resulted in highly crystalline (88–90%) particles with high aspect ratio of 33 (i.e. width of ~ 8 and length of about 230 nm, respectively). In addition, the yield reached 71%.
Cryocrushing
Another strategy to isolate CNFs common in literature is cryocrushing. This strategy involves freezing of swollen cellulosic fibres in liquid nitrogen and then crushed by mechanical grinding (Dufresne et al. 1997). The impact of high shearing forces causes the release of the exerted pressure of ice crystals on the cell wall to break and release cell fragments, thereby forming CNFs. Different cellulose-containing sources (e.g. wheat straw (Alemdar and Sain 2008), soy hulls (Alemdar and Sain 2008) and hemp fibers (Wang et al. 2007)) were reported in literature to produce CNFs using this method. It was observed that extraction of CNFs utilizing this method can produce CNFs at high yields having diameters of 50–100 nm (Alemdar and Sain 2008). The involvement of different pretreatments and post treatments makes this process not suitable for industrial upscaling. Wang et al. (2007a) utilized NaOH pretreatment (12% w/w, 2 h), acid hydrolysis (1M HCℓ, 80 °C, 1.5 h) and alkali treatment (2% w/w NaOH, 80 °C, 2 h) before cryocrushing in liquid nitrogen followed by high pressure defibrillation to obtain cellulose nanofibers having a width in the range of 30–100 nm. This clearly demonstrates that this process is time consuming, and lot of chemicals and energy are required to be able to obtain such CNFs, thus undesirable for industrial up-scaling. More passes (viz. 10–15) are required to produce CNFs with narrower diameters and the overall yield is only above 20% with crystallinity reaching values of 55% (Sain and Bhatnagar 2003, 2008).
Ultrasonication
Ultrasonication employs ultrasonic waves to facilitate the isolation of CNFs. In this regard, the high frequency ultrasound generated by a transducer results in the formation, growth, and collision of gas bubbles in aqueous solution. The hydrodynamic forces of the ultrasound disrupts the relatively weak interfaces (e.g. van der Waals forces) to liberate CNFs. Figure 7 shows the exact picture of isolation of cellulose nanofibers using ultrasonication (Chen et al. 2011b). The morphology of the CNFs were found to be dependent on the sonication time, power output and cellulose source-type. Zhu and Yadama (2018) isolated CNFs from Douglas Fir using ultrasonic processor, Brason 450 sonifierat 20 kHz at different amplitudes viz. 40 and 60%. The residues from hot water extraction (HWE) of Douglas Fir was used as raw material. It was found that by increasing ultrasonication time and/or amplitude resulted in formation of narrower fibril bundles, thus improving the defibrillation process. This was attributed to the mechanism of acoustic cavitation. The longer ultrasonication time and amplitude increases the possibility of fibers reacting with the micro-bubbles generated by the ultrasonication process. It was demonstrated that the inclusion of HWE in pretreatment(s) can reduce costs related to the removal of hemicellulose by alkali treatment. Using different output power of the ultrasonication, Chen et al. (2011b) found that high output of 1000 W or above is required to individualize nanofibers (i.e. having widths of 5–20 nm) from chemical purified wood. Despite prolonged time and changes in amplitude to control the resulting morphology of the CNFs, aggregated CNFs with wide distribution are usually obtained through ultrasonication method. Numerous reports on the use of ultrasonication to isolate CNFs using different pretreatments to facilitate the extraction process and control the structure and/or morphology were conducted (Chen et al. 2011b; Cheng et al. 2007). On the other hand, different sources were also used as raw material to produce CNFs through this method, i.e. wood (Zhao et al. 2007), bamboo (Zhao et al. 2007; Chen et al. 2011a), banana peel (Khawas and Deka 2016), regenerated cellulose fiber (Cheng et al. 2009, 2010), pure cellulose fiber (Cheng et al. 2009, 2010), microcrystalline cellulose (Cheng et al. 2007, 2009, 2010), cotton (Zhao et al. 2007), ramie (Zhao et al. 2007) and hemp fibers (Zhao et al. 2007). Despite being mostly used for CNFs isolation, there are few studies that indicated the capability of this technique for the production of CNCs (Mohd Amin et al. 2015). CNCs were produced from MCC dispersed in deionized water using sonication treatment for about 50 min at an output of 500 W, frequency of 20 kHz and amplitude of 20% by Mohd Amain, et al. 2015. They obtained rice-like crystalline particles with a width of ~ 11 nm and lengths of 165 ± 23 nm i.e. average aspect ratio 15. In addition, the yield was fairly low viz. 8–10% with the onset degradation temperature reaching 250 °C and crystallinity of 90%.
Extrusion
The extruder consists of feeding zone (where material is introduced), the kneading and heating zones in which high shears, temperatures and pressure are achieved (Mokhena et al. 2018b). The processor controls the morphology and structure of the end-product by varying the parameters, such as screw speed, screw configuration, screw length-to-diameter ratio (L/D), temperature, feed rate and die shape/size (Mokhena et al. 2018b). The study on the use of twin screw extruder for defibrillation of needle-leaf bleached Kraft pulp to produce CNFs was conducted by Ho, et al. (Ho et al. 2015). They found that after 1 to 3 passes microsized fibers were still visible, however, more homogeneous cellulose material was obtained after reaching 10 passes. The higher number of passes led to more defibrillation effect; however, at the expense of degree of polymerization, thermal stability and crystallinity. This was ascribed to the degradation of the cellulose material at higher passes. Moreover, the damage and/or degradation of the fibers resulted in poor mechanical properties at higher passes. In this treatment, the cellulosic fibers are fibrillated by two co-rotating screws exerting high shearing forces on the solid cellulose (Ho et al. 2015; Cobut et al. 2014). The advantage of this treatment includes high solid fibre contents which can be disintegrated to produce high quality CNFs viz. 33–45 wt% solid content. The damage and/or degradation of the fibers at high passes limit the size of the CNFs that can be obtained from this process. It can be argued that optimization of this process can result in CNFs having different sizes which can afford other applications, especially in nanocomposites. The solid form of CNFs offers advantages considering their transportation, and storage when compared to aqueous suspensions. On the other hand, re-dispersion in solvents for other industrial applications where suspensions are required has to be taken into account when using this technique.
Steam explosion
This process is known as thermomechanical treatment which was first introduced by Mason in 1927 to defibrillate wood into fiber for board production (Deepa et al. 2011). In this method, the raw material is subjected to pressurized steam followed by sudden decompression resulting in substantial break down of the lignocellulosic structure, hydrolysis of hemicellulose and depolymerization of the lignin and defibrillation. Steam penetrates the sample by diffusion, and then high shear forces generated from pressure causes cleavage of the glycosidic and hydrogen bonds releasing cellulose nanofibers. CNFs produced using this process were reported in literature (Deepa et al. 2011; Cherian et al. 2010; Abraham et al. 2011). Steam explosion method is recognized by its attractive properties such as low environmental impact, low energy consumption, less equipment corrosion and the use of less hazardous chemicals (Cherian et al. 2008; Yang et al. 2018). However, the non-uniformity and poor quality of the resulting CNFs still need to be resolved (Yang et al. 2018). In addition, the high temperatures (100–250 °C) used during the treatment also need demonstration. Yang et al. (2018) combined H2O2 bleaching with steam explosion, high speed blending, and ultrasonic treatment to overcome the non-uniform size of the resulting CNFs. They reported that the combination of these methods resulted in CNFs having average diameter of 22 nm and maximum degradation temperature of 346.4 °C due to the fact that each treatment played important role in the defibrillation of cellulose which made the whole extraction more energy efficient.
Other methods for CNMs isolation
As water is the cheapest reagent with low corrosion, the method which involves this kind of liquid is of essence from ecological and economical viewpoints. Subcritical water hydrolysis was also reported as a green method to extract CNCs (Novo et al. 2015, 2016). Curvelo and co-workers (Novo et al. 2015) data, showed that using supercritical water hydrolysis at 120 °C and 20.3 MPa for 60 min resulted in formation of highly crystalline rod-like shaped CNCs (79%) with a yield of 21.9%. The resulting CNC had length of 242 ± 98 nm and width of 55 ± 20 nm; and showed thermal degradation onset 15 °C higher than native cellulose. It was demonstrated that the overall cost price is 0.02 USD/kg as compared to 1.54 USD/kg through conventional methods (i.e. sulphuric acid hydrolysis). The low yield and the need for complex reactors, however, as compared to conventional mineral acid hydrolysis makes this method not feasible for up-scaling (Novo et al. 2016). Transition metal catalytic hydrolysis process was also reported as feasible method to increase the yield (Yahya et al. 2015). Chen et al. (2017) reported that a maximum yield of 83.6 ± 0.6% of CNC was achieved using transition metal catalytic hydrolysis process. The conditions of the process were as follows: temperature, time, concentration and solid–liquid ratio were − 80 °C, 1.5 h, and 0.8 M Cr(NO3)3 and 1:30, respectively. In addition, the CNC exhibited crystalline index and thermal degradation onset of ~ 87% and 344 °C, respectively. The sedimentation of the suspension after few hours standing and stability of the transition metals still need to be resolved before up-scaling this method. Abd Hamid et al. (2016) also demonstrated that it is possible to reduce time by utilizing tungsphosphoric acid as a catalyst for production of cellulose nanofibrils through sonication treatment. It was reported that 225 W sonication power over 10 min resulted in rod-like particles having diameters ranging between 15 and 35 nm; and the bimodal distribution lengths 150–300 nm to 350–450 nm. This was attributed to the synergistic effect between the protonation of glycosidic bonds by the acid and sonication energy. The high yield of 85% under the reported conditions clearly indicate such method has a promising potential for industrial upscaling.
Laboratory to industry
The extraction of CNCs using organic acid is of interest with regard to the recyclability of the acids, however, the prolonged extraction process is the limiting barrier towards its industrialization realization. It can be argued that despite the fact that sulphuric acid hydrolysis have limitations (i.e. large water usage, equipment corrosion, and generation of large quantities of waste) this technique is still the most effective, simple and requires shorter time than other processes. Moreover, the current industrial CNCs’ producers using sulphuric hydrolysis are producing material with comparable dimensions and properties as demonstrated by Reid, Villalobos and Cranston (Reid et al. 2016). Up to now, several companies and/or institutions have already started on the production of large quantities of cellulose nanocrystals; with four of them have production capacities beyond pilot scale as depicted in Table 5. CelluForce, the world’s largest CNCs producer, uses conventional 64 wt% sulphuric acid hydrolysis on bleached Kraft pulp. The process was scaled up at FPInnovations, Pointe Claire, QC, Canada. CelluForce owns 20% of all CNCs active patents. CelluForce, however, claims that they do recycle the chemicals used during extraction process and converts sugars into energy. The extracted CNCs are then separated, cleaned and spray-dried to produce powder in order to reduce the costs associated with storage and transportation.
Nevertheless, the one-pot functionalization (oxidation) of the CNCs extraction process using different treatments and/or combination of extraction methods have potential for industrial scalability. For example, carboxylated CNCs were extracted by one-pot preparation using mild reaction conditions (50 °C, 1 wt% sulphuric acid medium) with potassium permanganate (KMnO4) and oxalic acid (OA, H2C2O4) as the oxidizing and reducing agent, respectively (Zhou et al. 2018). A high yield of 68% with carboxylate content of 1.58 mmol g−1 was achieved. Rod-like particles with an average diameter of 10–22 nm and lengths of 150–300 nm and crystallinity of 89.2% were obtained. Yet the prolonged reaction durations of up to 8 h (as compared to sulphuric acid hydrolysis reaction) associated with this method and recyclability of the used materials still need to be addressed.
In the case of CNFs, the production revolves around chemical or enzymatic pretreatment of the cellulose-containing source followed by intensive mechanical disintegration, viz. homogenization, grinding or refining, microfluidization, ultrasonication, cryocrushing in liquid nitrogen, ball milling and extrusion. Despite most of these techniques being already adopted in industrial scale production, different energy requirement, sources, shear mechanisms and intensities result in CNFs having different morphologies and properties which still need more research for quality and consistency purposes (Qing et al. 2013) Besides TEMPO being most commercial used pretreatment to produce carboxylated cellulose nanomaterials, it can be argued that enzymatic pretreatment is of interest with regard to recovery of both sugars (for fuels and chemicals production) during CNMs production. Moreover, it is eco-friendly compared to other reported chemical pretreatments, thus it is one of the most promising pretreatments for industrial CNMs’ scaling up production. Amongst all CNFs extraction processes, extrusion approach seems to be more practical with regard to industrialization and availability of this technique in different industries; however, the degradation of the fibers and variations morphologies of obtained CNFs need to be resolved. It can be concluded the combination of the pretreatments and mechanical treatments such as multiple processing approach results in different CNFs properties and morphologies, and therefore the chosen approach has to be carefully considered with regard to desired properties and the cost involved as well as environmental impact. There are about seventeen entities that are producing CNFs on different scales, viz. commercial, precommercial, and pilot scales (http://www.mktintell.com/files/JCMiller_Pres.pdf (accessed 07-13-2018) as listed in Table 5. The largest producers of CNFs are Paperlogic (USA, 2000 kg day−1), University of Maine (USA, 1000 kg day−1) Borregaard (Norway, 500 kg day−1) and American Process (USA, 500 kg day−1). The most common mechanical extraction techniques used in commercial scale include refining, grinding and homogenization in combination with enzymatic or TEMPO-mediated oxidation pretreatments. With that in mind, there is no single technique/equipment developed for CNFs isolation, up to date. Since a combination of various processes has been a major route to produce CNFs, the energy efficiency and environmental impact of these processes still remain an important issue. On the other hand, the optimal conditions especially with regard to desired application and cost of CNFs still need to be addressed. Over the past decades, there has been more research on the utilization of sustainable natural resources to produce cellulose nanomaterials. Thus, in future we foresee the emergence of new producers and innovative commercial applications being realized.
Applications of CNMs
Cellulose nanomaterials are produced using different preparation processes which results in materials with unique structures and physicochemical properties that are exploited in different fields, such as in the biomedical sector, sensors, and wastewater treatment. In the early stages, applications in CNMs as reinforcing agent in polymer composites enlightened research of CNMs. Over the years, applications were also extended to many other fields (Fig. 8) based on the distinctive structures and physicochemical properties of CNMs.
Composites
There are several challenges that need to be addressed to achieve full reinforcement potential of cellulose nanomaterials (John et al. 2013; Ng et al. 2017; Oksman et al. 2016). These include irreversible agglomeration upon drying and low compatibility with hydrophobic polymeric matrices (John et al. 2013; Ng et al. 2017; Oksman et al. 2016). For the past decade, a lot of attention has been dedicated in finding suitable modifications to overcome those issues and to add specific functions without losing the valuable properties of cellulose nanomaterials (Dufresne 2017, 2018; Espino-Pérez et al. 2014; Lin et al. 2012; Oksman et al. 2016). The surface functionalization of CNMs prior to mechanical disintegration serves as a more suitable route to modify CNMs. This can be achieved by pretreatment which is usually used to facilitate the isolation process of the CNFs such TEMPO-mediated oxidation, carboxymethylation, cationization, etc. the time consumption and use of different organic solvents makes these processes undesirable which opened door for systems which involves one-step hydrophobization of the CNMs (Espino-Pérez et al. 2014). Nonetheless, the surface modification of CNMs can be classified into: (1) adsorption of molecules on the CNMs (cationic interaction (Fortunati et al. 2012; Hasani et al. 2008; Kim et al. 2009; Bondeson and Oksman 2007; Salajková et al. 2012)); (2) covalent grafting of single molecules (silylation (Khanjanzadeh et al. 2018; Robles et al. 2018a), acetylation (Berlioz et al. 2009; De Menezes et al. 2009), etc.); and (3) covalent grafting of polymeric chains (by radical (Kan et al. 2013; Tehrani and Neysi 2013; Morandi et al. 2009), or ring opening polymerization (Carlmark et al. 2012; Goffin et al. 2011; Labet and Thielemans 2011). The use of surfactants (anionic and nonionic) coating were also found to facilitate the dispersion of the CNMs in the polymeric matrix (Kim et al. 2009; Bondeson and Oksman 2007). The mechanical properties were also improved due the enhanced dispersion and compatibility with the hydrophobic matrices. It was demonstrated, however, that the concentration of the surfactant and CNMs plays major role in the resulting mechanical properties. It is worth mentioning that, surface modification of CNMs, especially CNCs, has to be carefully applied such that it does not act against the interwhisker hydrogen network which is believed to be responsible for their high reinforcing effect as reported by Favier, et al. (Favier et al. 1995). For instance, de Menezes, et al. reported that the surface modification of the CNCs facilitated their dispersion, however, there was no significant improvement to the mechanical properties of the resulting nanocomposites (De Menezes et al. 2009). This was attributed to the chemical grafting decreasing the possibility of interwhiskers interaction which are believed to be the basis of the reinforcing effect for CNCs reinforced nanocomposites.
Despite the progress that has been made in cellulose nanomaterials reinforced composites, the translation of extraordinary mechanical (tensile strength and Young’s modulus of the CNMs) from nanoscale building blocks to the macroscale bulk is still a challenge. Nonetheless, recent reports by Söderberg, et al. (Mittal et al. 2018) demonstrated that it is possible to realize full reinforcement potential of CNFs by using a combination of electrostatic and hydrodynamic interactions to maximize the fibril alignment. CNFs were obtained through TEMPO oxidation followed by homogenization in order to introduce carboxylic groups to facilitate electrical repulsion between the fibers to afford their fixation in the colloidal gel. The resulting fibrils exhibited a modulus of 86 GPa and strength of 1.57 GPa which exceeded natural and commercial bio-based materials. The alignment of the fibrils was also demonstrated by Kim et al. (2019) using magnetic and electric field in order to fabricate microfibrils via wet spinning process. The resulting microfibrils exhibited excellent mechanical properties with Young modulus of ~ 19 GPa, tensile strength of 290 MPa and toughness of 12 mJ m−3 which were attributed to strong hydrogen bonding between the aligned fibrils. Lu et al. also demonstrated that the alignment of the CNFs in the regenerated silk fibroin using dry-spinning through a microfluidic chip led to breaking strength up to 686 MPa and tensile strength of 487 MPa as well as elongation at break of 16% (Lu et al. 2019). These studies demonstrated that the translation of the mechanical properties of the cellulose nanomaterials will be soon recognized, which will unlock their implementation in load-bearing applications. Nevertheless, research is being carried out on incorporation of CNMs in polymer for use in automotive components such as seat foams, door panels, console substrates, and wire harness (https://paper360.tappi.org/2018/06/18/ford-goes-further-with-renewable-nanomaterials/ (accessed on 22 May 2019)). Further research is also being carried out on using the produced composite materials in exterior and under-the-hood applications. The objective is to reduce weight, thus fuel consumption in order to meet the new 2025 Corporate Average Fuel Economy (CAFÉ) standards of 54.5 mile per gallon (mpg). However, the challenges that hurdles CNMs success in automotive are as mentioned earlier, namely, high moisture absorption and incompatibility with commonly used polymers in automotive such as acrylonitrile butadiene styrene (ABS), polystyrene, polyethylene, polypropylene (PP), polyvinyl chloride (PVC), polyurethane (PU) etc. Provided that the research has been dedicated on finding a suitable surface modification of CNMs without compromising their valuable properties, the application of CNMs-based composites in different fields will be realized in the near future.
Medical applications
The recent developments in the extraction process for cellulose nanomaterials indicated that nanomaterials exhibiting different functional groups, dimensions, and crystallinity can be produced and processed into different shapes and/or structures. The ease of functionalization shows the potential of CNMs in medical applications such as organ repair or replacement, drug delivery, medical implants, dental fills, medical diagnostics and instrumentation. Table 7 lists the applications of CNMs in different biomedical sectors.
Three dimensional (3D) printing of cellulose nanomaterials and cellulose nanomaterial-based composites has revealed that different functionalized shapes towards tissue/organ repair or regeneration can be fabricated as attested by a number of patents and press releases published in the context of 3D-printing (McAlpine 2016; Markstedt et al. 2015). 3D printing also referred to as rapid prototyping, additive layer manufacturing, additive fabrication, or solid-free-form fabrication, begins from the design of the three dimensional (3D) model which is then transferred to the 3D printer. The model is sliced into several layers which are interpreted by the printer to allow printing of the desired object starting from the base. In the case of medical application, the image obtained from computed tomography (CT) and magnetic resonance imaging (MRI) can be translated into a design of a 3D model, which can be transferred to the printer. It is recognized that cellulose materials plays a major role in providing mechanical properties and environment for cell growth, proliferation, differentiation, and motility. 3D-printed composite materials composed of alginate and CNFs (i.e. obtained from TEMPO-mediated oxidation followed by homogenization) crosslinked with CaCℓ2 displayed mechanical properties comparable to native tubular tissues, such as porcine caroid arteries, rat abdominal aorta, and spinal cord (Torres-Rendon et al. 2016). This demonstrated the possibility of their replacement (porcine caroid arteries, rat abdominal aorta, and spinal cord) and regeneration with cellulose nanomaterials based composites. The study on alginate/CNFs bio-ink using human nasoseptal chondrocytes (hNC) showed cell viability from 73 to 86% after 1 and 7 days of 3D culture (Markstedt et al. 2015). This clearly justifies the suitability of cellulose nanomaterials-based bio-ink for 3D printing with living cells for printing living tissues or organs. It is worth mentioning that the concentration of CNMs and the matrix play a major role in the cell attachment and proliferation. Two bio-inks viz. CNF with alginate (CNF/A) and hyaluronic acid (CNF/HA) for cartilage tissue engineering were studied by Nguyen, et al. (Nguyen et al. 2017). CNF was obtained by mechanical treatment followed by enzymatic hydrolysis. CaCℓ2 was used for crosslinking alginate based structures, whereas H2O2 was employed for CNF/HA. They observed that 3D bioprinted CNF/A (60/40 wt%) performed best and was capable of supporting the growth and differentiation into chondrocytes of human-derived induced pluriponent stem cells.
Another area of interest for cellulose nanomaterials is wound dressing. Wound dressing is the most important step to facilitate the healing of affected part and it has to offer protection against secondary infections due to microorganisms (Rees et al. 2015). Also open pore structure is essential for wound breathing (while maintaining sufficient moisture) and to carry/release antibacterial drugs. Rees et al. (2015) prepared 3D printable CNFs through homogenization after pre-treatment with a combination of carboxymethylation and periodate oxidation using TEMPO oxidized CNFs as substrate. During printing CaCℓ2 was used as crosslinker to stabilize the constructs followed by freeze-drying. The constructs formed tracks with an open porosity which can be exploited for carrying and releasing antimicrobial drugs. It was found that the assessed cellulose nanomaterials did not support bacterial growth which is advantageous with regard to wound dressing. Leppiniemi et al. (2017) reported that CNF/alginate/glycerine composite was suitable for 3D-printing towards wound dressing. It was demonstrated that collapsing of printing paste can be avoided by adding CNFs as additives which improves shape fidelity and stability of printed patterns. The presence of voids within the printed structure provided room for swelling in moist and wet conditions which is desirable in wound healing Table 6 (Li et al. 2018a; Ávila et al. 2016; Jiang et al. 2018; Markstedt et al. 2015; Müller et al. 2017).
Provided attractive attributes such as reactive surface with abundant surface hydroxyl groups for desired surface modifications, large surface area for large amount of drug loading, biocompatibility and environmental biodegradability CNMs are promising carriers for drug delivery systems (De France et al. 2017; Plackett et al. 2014). Ndong Ntoutoume et al. (2016) exploited the surface negative charges of the CNMs to attach cationic (β-cyclodextrin (CD)) via electrostatic coupling to afford an ionic complex network such that the anticancer drug, i.e. curcumin I, can be inserted within CD hydrophobic cavities towards anticancer drug delivery. It was found that the presence of the CNMs improved the cell uptake hence enhanced the anti-proliferate efficacy. Since the production route affect the drug carrier/release properties, CNMs with gel-like nature and biological inertness are found to be desirable for production of various materials, such as nanofibers, films, hydrogels, aerogels and spray dried particles having suitable mechanical properties and malleable porosity to afford desired drug-carrier and releasing properties (De France et al. 2017; Villanova et al. 2011; Lin et al. 2011; Kolakovic et al. 2012; Ooi et al. 2016). For instance, Valo et al. fabricated CNFs aerogels via freeze-drying for oral drug delivery systems (Valo et al. 2013). The drug particles were well dispersed and immobilized tightly within the microporous structured aerogels resulting sustained drug release over time. Kolavic et al. reported that the spray-dried CNMs afforded microparticles that are capable of sustained drug-release due to CNM fibril network for over 2 months (Kolakovic et al. 2012).
Application in CNMs in chemistry
Catalyst
The welcomed paradigm shift towards the use of renewable materials as stabilizing and reducing agents in the synthesis of nanoparticles (NPs) opened doors for exploration of CNMs for such purposes (Chen et al. 2015; Gupta et al. 2017; Tang et al. 2015a). Beside the control over the structural properties (size, morphology, and available facets) of the nanocatalysts, there has been challenges associated with their recovery after being utilized. In order to fine-tune the structural properties of the resulting nanocatalysts, the use colloidal stabilizers and supporting substrates for their recovery based on polymers, carbon materials, silica, metal frameworks and metal oxides have been the most promising solutions to realize their full catalytic activities.
Recently, CNMs with tailorable surface functionality and high surface area have been employed as a novel platform to grow nanocatalysts as supporting substrate as well as colloidal stabilizers. In this case hybrid materials can be synthesized with CNMs not only act as supporting substrate but enhancing the colloidal stability of the nanocatalysts thereby avoiding their irreversible aggregation. In addition, the enhanced colloidal stability using these materials avoid the “poisoning” and deactivation of the resulting nanoparticles, which is often associated with commonly used capping agents (e.g. polymeric ligands). NiFe2O4 were introduced onto the surface of CNFs using a hydrothermal method to afford the catalytic efficiency of 98% dye degradation after 120 min (Gupta et al. 2017). Most recently, Au and Ag nanoparticles were successfully deposited onto CNCs using green solid-state synthetic processing route by milling freeze dried CNC in the presence of their metal salts and ascorbic acid as a reducing agent, and enhancements on the catalytic reduction of 4-nitrophenol to aminophenol was achieved (Eisa et al. 2018). The success deposition of the metal nanoparticles (i.e. hexagonal Ag and spherical Au particle) was ascribed to the availability of CNCs hydroxyl groups promoting their interaction nanoparticles via hydrogen bonding. Tang, et al. 2015 reported that CNCs served as an ideal “green” carrier which facilitated the dispersion stability of the nanoparticles as justified by their stability for a week without sedimentation when compared to neat particles (Tang et al. 2015a). This demonstrated that CNC can be employed as a facile and green carrier for fabricating stable catalysts for industrial applications.
In an interesting study, the catalyst was immobilized directly on the surface of the CNCs rather than the nanoparticles to afford eco-friendlier product (Liu et al. 2015a). In this regard, dirhodium-CNCs catalyst was prepared by exploiting ligand exchange between CNCs’ carboxyl groups and rhodium trifluoroacetate dimer (Rh2(OOCCF3)4) to induce covalent bonding. The catalyst exhibited high yield of about 75–80% in 180 min for model cyclopropanation reaction of styrene at ambient conditions without leaching after 3. Ellebracht and Jones followed the same protocol to functionalize CNMs for acid–base organocatalysis (Ellebracht and Jones 2018). In this case, it was demonstrated that the functionalized CNMs can achieved by mild dilution with HCℓ followed by TEMPO oxidation and functionalization via amide coupling to afford bifuntional organocatalyst with acid (sulphate esters, carboxylic acids) and base (primary amines) functionality for aldol condensation reaction.
Cells and batteries
Research has escalated in the exploitation of utilizing renewable materials as a replacement of synthetic materials for energy storage from economic and ecological viewpoints. CNMs have been used as separators or templates due to their attractive attributes such low-cost, good optical properties, fairly low coefficient of thermal expansion (CTE), abundant availability and their mechanical robustness (Table 7). Wang et al. (2018) reported on nanocellulose structured paper-based lithium metal batteries using CNFs as separator, and as substrate for positive and negative electrodes (Wang et al. (2018)). CNMs rendered porous structure to accommodate the deposited lithium and oxidation reactions to afford structural stability (viz., no volume changes) during charging/discharging cycles. This resulted in enhancements of the lifespan and sustainability of the battery achieving 85% retention capacity after 100 cycles at a rate of 1.27 mA cm−2. PEC for high performance lithium-sulphur batteries based on CNF composite was conducted by Nair et al. (Nair et al. 2016). It was reported that the presence of the CNFs improved the armophicity of the crosslinked PEC network, thereby, ensuring its shape retention during organic liquid electrolyte (with only 42% volume change after 180% electrolyte uptake) as well as enhancement of the pore structure to avoid polysulphide shuttle through the polymer matrix. It was also found that the PEC retained its physical integrity upon bending test (viz., withstanding a bending radius up to 3 mm). It is a prerequisite that the PEC must be able to withstand at least less than 2% deformation under 1000 Psi (6.89 MPa) as issued by US Advanced Battery consortium, hence the reinforcing capabilities of the CNMs in the preparation of the PEC enhanced their resulting mechanical properties as demonstrated in these studies (Pan et al. 2016; Pan et al. 2018). Pan et al. (2016) reported that the presence of CNMs improved the deformation stability, i.e. 0.11% at 1000 Psi hence qualified for battery separators application. In addition, the as-prepared PEC exhibited good thermal stability up to 150 °C and electrochemically stable in the potential values 0–5.0 V with excellent discharge capacity retention of 99.5% after 50 discharge/charging cycles. The presence of the CNMs does not only contribute to the structural stability of the resulting PEC, but it also provides evenly distributed pores facilitating in homogenous current distribution, thereby enhancing the overall performance of the battery (Pan et al. 2019). In addition, the thermal stability of the resulting separator increases, hence no shrinkage was observed for PEC at high temperature greater than 200 °C with the hydrophilic-nature of CNMs improving the electrolyte wettability (Pan et al. 2018, 2019).
Supercapacitors, also known as electrochemical capacitors or ultracapacitors are recognized as other favourable form of energy storage with high power density, low maintenance cost and long durability (Dutta et al. 2017; Mochane et al. 2018). Over the past years, carbon based materials have been used in supercapacitors (e.g. electrode) because of their beneficial properties such as high conductivity, relatively low cost, and industrial production processes which make them easily accessible. The use of natural materials as precursors to synthesize carbon materials serve as alternative route to produce cheaper pure carbon materials (Silva et al. 2015; Zu et al. 2016). Silva et al. (Silva et al. 2015) used CNCs as a precursor for needle-shaped carbon which to be used as hard template for supporting metal oxide nanoparticles synthesis. TEM images revealed that needle-shaped carbon nanostructures were successfully coated with Co3O4 nanoparticles having diameters of 8–10 nm. A specific capacitance of 90.8 Fg−1 was achieved due to the amount of carbon present which provided the material with large electrochemical active surface area. Despite being a carbon source via carbonization, cellulose has been exploited to provide mechanical stability, and high porosity for ion electrolyte reservoir in order to avoid leakage (Dutta et al. 2017). It was reported that it can act as a good electrolyte reservoir which promotes electrolyte accessibility. Reaching a specific capacitance of 51 Fg−1 (i.e. 318% higher than pure polypyrrole (PPy)) confirmed that cellulose can act as good electrolyte reservoir to enhance the ion transfer (De Adhikari et al. 2015). Further improvement can be attained by combining CNF/polypyrrole (PPy)/graphene with specific capacitance reaching a value of 243 Fg−1 which is 273% higher compared to PPy/graphene composite. The flexible electrodes can also be manufactured using cellulose nanomaterials as substrate. Flexible electrodes render opportunity to afford their application as flexible energy storage for portable electronic devices, namely electronic papers, stretchable integrated circuits and wearable for personal multimedia, computing, or medical devices (Mochane et al. 2018). A flexible and stable electrode based on a hybrid of graphene oxide/polypyrrole/cellulose was recently reported by Wan and co-workers (Wan et al. 2017). The hybrid exhibited high areal capacitance of 2.20 F cm−2 at discharge current of 2 mA cm−2 with good cyclic stability (i.e. retention of 89.5% after cycling for 5000 times) due to cellulose acting as substrate for absorbing electrolyte and electrolyte reservoir for efficient ion transport.
Over the past years, energy harvesting received tremendous interest from research communities. CNMs were incorporated as part of photovoltaics as reported in a number of studies due to their mechanical robustness and optical clarity (Luo et al. 2014; Nogi et al. 2015; Fang et al. 2014; Zhou et al. 2014). Nogi, et al. (2015) fabricated transparent and flexible nanofiber paper containing silver nanowires with excellent electrical conductivity by dipping in silver nanowires. It was reported that the as-prepared nanofiber/silver hybrid exhibited high optical transparency (i.e. 91% at 600 nm) and excellent mechanical strength of 1.6–3.0 GPa. In addition, the hybrid exposure to a pressure of 2 MPa resulted in a low sheet resistance (43 Ω/square) with optical transparency of 92.8% (similar to commonly transparent conductive substrates e.g. indium tin oxide glass) because of the high thermal stability (for preparation using heat) and mechanical robustness of the CNMs. The hybrid displayed achieved a power conversion efficiency of 3.2% and short current density of 9.58 mA/cm2, which were maintained under folding and after holding. CNCs as substrate for freestanding solar cells was reported by Zhou et al. (2013). In this case, a semi-transparent silver layer was deposited on the CNCs film to eliminate roughness associated with CNFs-based devices. It was shown in this study that the cell was recyclable as it disintegrates within 30 min after being immersed in distilled water and had energy conversion of 2.7%.
Cellulose nanomaterials have shown a huge potential as an important component in energy harvesting devices, which exploits energy from different mechanical motions, viz. vibration, rotation, liner sliding, finger typing, wind, waves etc. Triboelectric nanogenerators (TENG) are new technology inventions for harvesting ambient mechanical energy on the basis of the triboelectric effect and for conversion to electricity. This technique has proven to be cost-effective, simple and robust for self-powered devices and systems (Peng et al. 2017). Peng et al. (2017) fabricated triboelectric nanogenerator from polydimethylsiloxane (PDMS)/CNC flakes (CNCFs) composite (Peng et al. 2017). The presence of CNMs with high dielectric surface (~ 5.2) improved flexibility and the triboelectric performance of the prepared composite generator. It was found that an extra electric field was generated by positively charged CNMs hence improved charge transfer and surface charge density. The output power of 1.65 mW (0.76 W cm−2) was a tenfold power increase when compared to triboelectric generator made of pure PDMS films and the energy conversion efficiency under applied compression force of 40 N reached 17% with an external resistance of 10 MΩ; capable of lighting up 100 light emitting diodes (LEDs).
Applications of CNMs for environmental protection
Removal of toxic wastes
Water pollution is one of critical issues of the 21st century. Different pollutants (copper, cadmium, nickel, lead, chromium, etc.), often, are discharged by industries into river streams and marine water leading to health hazards and water pollution. A number of processes (e.g. chemical precipitation, flocculation, and electrolysis) have been employed to remove these pollutants from water bodies, however they cannot eliminate all these contaminants (Mautner et al. 2019). The use of renewable materials from industrial waste for the removal of toxic wastes from water bodies which does not generate harmful by-products is of essence with regard to low cost and abundant availability. CNMs have potential as adsorbents of different pollutants with regard to the large surface area, availability of vast hydroxyl groups and their easy functionalization as tabulated in Table 8 (Kardam et al. 2014; Liu et al. 2015b; Mautner et al. 2019). Kardam et al. (2014) exploited the large surface area and available hydroxyl groups of CNMs for removal of Cd(II), Pb(II) and Ni(II) and recorded adsorption capacity of 9.70, 9.42, and 8.85 mg/g, respectively. The modification of the CNMs in order to improve the adsorption capacity and efficiency was also reported as summarized in Table 8.
In the case of dyes, surface modification can also be applied to inprove adsorption capacity of the CNMs-based adsorbents. For example, maleic anhydride grafted CNCs (M-CNCs) were prepared by Qioa et al. to determine the adsorption kinetics of M-CNCs on four different cationic dyes, namely, crystal violet (CV), methylene blue (MB), malachite green (MG) and basic fuchsin (BF) (Qiao et al. 2015). The order of adsorption capacity was found to be MG (150 mg/g) < CV (224 mg/g) < MB (243 mg/g) < BF (350 mg/g). It was also reported that pure CNCs exhibited adsorption capacity of 185.19 for CV dye. The M-CNC maintained high desorption rate (more than 80%) after adsorption–desorption four cycles. Jin and coworkers oxidized CNCs by sodium periodate to produce dialdehyde functionalized CNC (D-CNC) (Jin et al. 2015b). D-CNC was then used as a crosslinker to react with amphoteric polyvinylamine (PVAm) to produce microgels for anionic dyes adsorption (i.e. congo red 4BS, acid red GR and reactive light yellow K-4G) (Jin et al. 2015b). The maximum adsorption values of 869.1, 1469.7, and 1250 mg/g were reported for congo red 4BS, acid red GR and reactive light yellow K-4G respectively. The adsorption mechanism fitted well with Sips model and followed pseudo second order kinetics, indicating a chemisorption nature. Despite the improvement of dyes removal by CNMs because of their large surface-to-area, their functionalization, generally enhanced their adsorption capacity as depicted in Table 8 (Jin et al. 2015a; Batmaz et al. 2014; Pei et al. 2013).
CNMs have been employed to prepare aerogels for oil-in-water purification via vacuum-drying, freeze-drying process or supercritical CO2 drying of hydrogels. In this regard, the ultra-porous CNMs aerogels (porosity > 98%) with low density, mechanically robust, high sorption rate and capacity were produced by varying the concentration of the CNMs as well as the drying process conditions (Cervin et al. 2012; Korhonen et al. 2011). It is, however, recognized that the sorbent surface wettability plays a major role on their sorption behaviour since it determines the wetting/dewetting properties when it comes into contact with the liquids (Cervin et al. 2012; Gao et al. 2018; Gong et al. 2019; Korhonen et al. 2011). Therefore, the hydrophilic-nature of CNMs is often modified to afford highly hydrophobic product (water contact angle > 150°) for oil/water separation. For instance, Cervin et al. fabricated aerogels from NFC obtained via carboxymethylation process followed by homogenization to afford diameter ranging between 5 and 20 nm. The resulting suspension was freeze-dried, then vacuum dried and finally modified with octyltrichlorosilane through vapour phase deposition to produced highly hydrophobic aerogels (water contact angle of 150°). The as-prepared aerogels were highly buoyancy and exhibited high sorption capacity, i.e. 45 times its own weight. Elsewhere, a nanoscopic layer (~ 7 nm) of titanium dioxide (TiO2) was coated onto cellulose nanofibers to afford highly hydrophobic/oleophilic aerogels (Korhonen et al. 2011). The surface wettability properties were found to improve the buoyancy of the aerogel and absorption rate, i.e. 1–3 s oil absorption depending on oil-viscosity. In addition, the aerogel was capable absorbing 20–40 g/g depending on oil-type. Similarly, Gao et al. (2018) used mussel adhesive chemistry to functionalize CNMs by using polydopamine as bridge to coat with hydrophobic octadecylamine molecules for oily wastewater purification. The resulting light weight sorbent exhibited excellent buoyance and oil/water separation selectivity reaching a maximum absorption capacity ranging from 83 to 176 g/g. Recent study based on hydrophobic aerogel composed of CNCs and poly(vinyl alcohol) (PVA) chemically modified via thermal vapour deposition of methyltrichlorosilane (Gong et al. 2019). The as-prepared ultralight aerogel exhibited three-dimensional interconnected structure with high porosity of about 97.7%, low density (22.5 to 36.1 mg cm−3), excellent buoyancy and good hydrophobicity (95.1–144.5°). The presence of the CNCs improved the compressive modulus of the aerogel up to 208 kPa and aerogel was able to maintain the compressive strength and large strain (20–60%) after 50 cyclic compressions. In addition, the sorbent displayed oil absorption capacity from 21.2 to 32.7 times its own weight.
Several authors also reported on the utilization of CNMs as barrier/selective layer in filtration membranes (Ma et al. 2011a, b; Cao et al. 2013; Wang et al. 2014; Ma et al. 2014; Karim et al. 2016; Mokhena et al. 2018a; Goetz et al. 2018). Among these studies, Ma et al. (2011b) prepared filtration membrane by casting CNCs on top of electrospun polyacrylonitrile (PAN) scaffold giving rise to a smooth porous selective layer membrane (see Fig. 9). In comparison with commercially available ultrafiltration (UF) membranes (viz. PAN10 and PAN400), pure water permeability of prepared thin film nanofibrous composite (TFNC) membrane was 18 times higher than that of PAN10 and 1.7 times higher than that of PAN400. The stable flux permeation in oil/water separation for TFNC was observed, but higher than that of PAN10 (11 times) while retaining high rejection ratio (>99.6%) after 48 h. PAN400 exhibited lower rejection ratio of 98.2% and permeation flux 2.5 times lower than that of TFNC. This was attributed to the hydrophilic nature of the CNCs. Further analysis using MS2 bacteriophage as model virus, TFNC membrane displayed log reduction value (LRV) greater than 3.7 when MS2 was slightly positively and 1.6 ± 0.2 when negatively charged at pH 7.2 which were superior to many commercial membrane (e.g. GS0.20 from Millipore, ~ 1.0 LRV under the same conditions). A study by Mokhena and Luyt (Mokhena et al. 2018a) also showed that the oil removal of 98.4% from water (i.e. ~ 6 mg/L of oil concentration) was obtained by using CNCs as a barrier layer coated on top of electrospun alginate nanofibers. Despite contributing to the overall performance in filtration, CNMs plays a major role in enhancing mechanical properties of the filtration membrane such that it can withstand high pressures associated with filtration processes. Cao et al. (2013) reported increase in tensile strength of the membrane by casting CNCs in both layers of the membrane due to strong hydrogen bonding of CNC network formed on both surfaces. The membrane displayed oil/water retention of 99.5% and high filtration efficiency of 7–40 nm particles. Microfiltration (MF) fabricated by infusing CNMs into PAN nanofibrous resulted in a complete removal of Escherichia coli (by size exclusion), LRV of 4 for MS2 virus removal and adsorption capability of 100 mg Cr(IV) or 260 mg Pb(II) per gram of cellulose nanofiber, while maintaining a high permeation rate of 1300 L/m2h/psi (Wang et al. 2013b). Multi-layered CNMs membrane prepared using vacuum-filtration resulted in a ~ 100% removal of Ag+, Cu2+ and Fe3+/Fe2+ (from mirror industry effluents) (Karim et al. 2016). Elsewhere, MF prepared from CNCs as functional entities in chitosan matrix via freeze-drying process proved success in removing 98%, 84% and 70% of Victoria Blue 2B, Methyl Violet 2B and Rhodamine 6G after 24 h in contact, respectively (Karim et al. 2014). A very recent report based on the antifouling effect of ultrafiltration membranes consisted of CNCs (obtained from sulphuric acid) was conducted by Zhang and coworkers (Lv et al. 2018). They observed an increase in pure water flux and bovine serum albumin (BSA) rejection respectively from 9.8 to 206.9 L/m2h2 and 83.3 to 88.2% with an increase in CNCs’ content. The flux recovery ratio also increased with an increase in CNCs’ content. This was attributed to the presence of hydroxyl groups on CNC protruding laterally along the molecules and to being readily available for hydrogen bonds to form ordered hydration layer on the membrane surface, thereby effectively reducing contaminant clogging and hydrophobic adsorption.
SEM images of a cross-sectioned view of TFNC membrane with cellulose nanofiber barrier layer; b top view of TFNC membrane; c magnified cellulose nanofiber barrier layer of TFNC membrane; d Permeation flux and rejection ratio of TFNC membrane containing cellulose nanofiber barrier layer (0.10 ± 0.02 μm thickness, prepared from 0.05 wt% aqueous suspensions) as a function of time; measurement carried out at a constant pressure of 30 psi and temperature of 37 °C based on ultrafiltration of oil/water emulsions. (The ultrafiltration performance of commercial PAN10 and PAN400 membranes was also included for comparison.). Reprinted from Ma et al. (2011b) Copyright 2011 American Chemical Society
Other applications
For the past years, different independent research institutes have been working on the development of alternative packaging materials to replace conventional packaging materials (petroleum-based materials) (Johansson et al. 2012). Recent studies have demonstrated that packaging from forest-based resources have a potential to replace classic packaging materials such as glass and plastics owing to their lightweightness (favourable for transportation), renewable nature and abundance. Recently, VTT, produced cellulose-based materials for packaging from enzymatic fibrilliated cellulose (HefCel) technology (https://www.vttresearch.com/media/news/innovation-and-competitiveness-from-nanocellulose (accessed on 19 May 2019)). The product has valuable properties such as efficient oxygen, grease and mineral oil barrier making it suitable for packaging fatty dry foods such as crisps, potato chips and muesli. The product is expected to be ready for commercialization by end of 2019. It is recognized that it is not only the shelf-life of the food which is essential for consumers and food industries, the appearance as well as the weight of the packaging material are of significant importance with regard to transportation costs (Johansson et al. 2012). Cellulose nanomaterials with high transparency and low density shows a great potential in packaging applications (Johansson et al. 2012). In comparison with classic packaging materials (e.g. petroleum-based plastics), the use of CNMs in packaging serve as a future for packaging industries with regard to their biodegradability, renewability and availability from limitless renewable resources. Different antimicrobial agents can also be added into the CNMs to improve their antibacterial efficacy in order to preserve the quality of food and shelf-life as demonstrated by Saini et al. (Saini et al. 2016). The authors demonstrated that grafting nisin onto CNFs improved antibacterial activity towards Bacillus subtilis and Staphylococcus aureus bacteria showing their outstanding potential in antibacterial active food packaging for products like cheese or canned vegetables. Researchers at VTT are also working on the incorporation of CNMs as an additive in water-based polyurethane (PU) varnishes and paints which improves the durability and also offers protection them from attrition caused by UV radiation (https://www.vttresearch.com/media/news/innovation-and-competitiveness-from-nanocellulose (accessed on 19 May 2019)). This was found to extend the life-span of the varnishes and paints, thus reduces the environmental burden and cost with regards to their frequent replacement. Nippon Paper Crecia Co., Ltd. also indicated that they are producing antibacterial and deodorant sheets, adult diapers and mild incontinence pad made from CNFs obtained from TEMPO-oxidation pretreatment followed by mechanical treatment. CNFs also shows a potential as partial replacement of co-binders in paper coatings not only to improve the overall appearance but to reduce the overall price of the product (Dimic-Misic et al. 2013a).
Current scenario and future work
According to Google Patent database, there are more than 550 granted patents up-to-date, which clearly indicate a significant interest in CNMs. In 2019, the global outlook for CNMs estimated a turnover of about 700 million USD by the year 2024. This could be associated with distinctive properties of CNMs which gained much interest from academic and industrial communities. The possibility of new applications rather than classic polymeric materials reinforcement indicates that there are plenty of opportunities for development of new products in the near future. The fact that CNMs are derived from renewable and sustainable sources broadens the possibility of more potential applications to be established, however, their price still need to be addressed for their competiveness in the market. Recent report by Chauve and Bras demonstrated that the estimation of the end-price for CNFs ranges from 7 per kg to 12 USD per kg (Chauve and Bras 2014). The estimation of energy cost in mega-watt per hour for CNF production as indicated by Jonnobi, et al. (Jonoobi et al. 2012) was 113 USD per ton which totalled to a cost of 113 USD per ton with regard to the fact that the source (i.e. sludge) was obtained at no cost. On the other hand, the manufacturing costs for CNCs were estimated to range from 3632 to 4420 USD per ton which depends on the source (Yang and Wyman 2008). This clearly indicates that the price of these materials can be affected by geopolitical issues. Nonetheless, few companies are actively employing CNMs in different applications such as packaging (as in coatings), construction (e.g. drilling fluids, concrete mixtures, insulators) and biomedical (e.g. adult diaper, antibacterial sheets etc.). This result from exploration of the presence of large number of hydroxyl groups that can also be tailored according to the intended application, low density, large surface area, gelation and low thermal expansion. These applications can be further extended to other applications as: filters, sensors, energy harvesting/storage, wastewater treatment, environmental protection and catalysis.
Currently, more research has been dedicated to finding alternatives for isolation of CNMs which are economically sustainable and environmentally benign, with high yield. This is confirmed by numerous comparative studies on the extraction strategies continuously reported in the literature (Qing et al. 2013; Jonoobi et al. 2012; Mtibe et al. 2015; Desmaisons et al. 2017; Reid et al. 2016). Few companies have already announced their activity in the large-scale production of cellulose nanomaterials; however, there is a missing link between the production and applications of these materials. Moreover, the difference between the grades produced by various commercial producers and quality still need to be addressed. Numerous studies have been devoted to elucidate on the advantages/disadvantages of commonly used CNMs characterization methods in order to address the issues associated with quality control during production (Reid et al. 2016; Chinga-Carrasco 2013; Moser et al. 2015; Foster et al. 2018; Desmaisons et al. 2017). The properties of CNMs such as morphology, degree of polymerization (DP), crystallinity, chemical composition, and rheological properties are important for the advancement in understanding process optimization, and utilization in material development; hence, standard measurement protocols are crucial for consistent, reliable and accurate material characterization as extensively discussed by Foster, et al. (Foster et al. 2018). The authors demonstrated that the measurements protocols that are necessary for consistent, reliable and accurate CNMs properties have been outpaced by exponential growth in interest/activity from commercialization and research in different fields. Furthermore, the current adopted characterization methods are still not feasible for simultaneous application during CNMs production process. Thus, in future new characterization methods that can be used simultaneously during production is of essence in order to control the quality and reliability of the resulting products. In addition, the developments of standards method to characterize CNMs will be of essence to further their competitiveness edge against other nanomaterials.
Although many companies reported that they recover and recycle all chemicals employed during the extraction process, more research is still underway in improving the extraction process towards less laborious process without harsh chemicals. In future, we foresee a single step process to extract cellulose nanomaterials from different sources being developed by researchers. Smart materials using modern techniques such as 3D-printing that can respond to external stimuli (e.g. pH, temperature, gas, UV (light) irradiation, electric field etc.) will be explored to improve our lives. It is envisaged that in future cellulose materials will replace most of synthetic materials in various instances such as biomedical applications especially in disease detection and prevention before its manifestation; in construction, textiles, sensors, wearable intelligent devices and intelligent energy systems. With discovery of new applications, more industries/governments will start to invest in cellulose nanomaterials which will then create a circular economy, especially in agricultural industries.
Conclusions
In this review, we presented a comparison between the CNMs isolation methods and their application in solving global issues. It can be concluded that apart from the conventional sulphuric acid hydrolysis other greener methods with higher yields were reported with the potential of being adopted in industrial scale; however, their implementation in order to produce CNMs with comparable properties still need to be demonstrated. Despite TEMPO-mediated oxidation and enzymatic hydrolysis being the most adopted pretreatments in industrial landscape, the development of industries that can produce CNMs at low cost while being able to extract and recover other bio-refinery products (i.e. lignin, sugars and hemicelluloses) is of significance from economic and ecological viewpoints. In future we foresee exponential growth of research and investments on the production of high quality CNMs from different sources (i.e. agricultural waste and algae) with new products being realized. The use of such by-products will facilitate the reduction of the price as well as environmental protection, while opening doors for new industries (viz., from energy, medical sectors to advanced smart electronic devices) by exploiting attractive attributes of the CNMs.
References
Abe K, Yano H (2009) Comparison of the characteristics of cellulose microfibril aggregates of wood, rice straw and potato tuber. Cellulose 16(6):1017
Abraham E, Deepa B, Pothan L, Jacob M, Thomas S, Cvelbar U, Anandjiwala R (2011) Extraction of nanocellulose fibrils from lignocellulosic fibres: a novel approach. Carbohydr Polym 86(4):1468–1475
Abushammala H, Krossing I, Laborie M-P (2015) Ionic liquid-mediated technology to produce cellulose nanocrystals directly from wood. Carbohydr Polym 134:609–616
Alemdar A, Sain M (2008) Isolation and characterization of nanofibers from agricultural residues–wheat straw and soy hulls. Biores Technol 99(6):1664–1671
Anwar Z, Gulfraz M, Irshad M (2014) Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: a brief review. J Radiat Res Appl Sci 7(2):163–173
Ávila HM, Schwarz S, Rotter N, Gatenholm P (2016) 3D bioprinting of human chondrocyte-laden nanocellulose hydrogels for patient-specific auricular cartilage regeneration. Bioprinting 1:22–35
Azeredo HMC, Barud H, Farinas CS, Vasconcellos VM, Claro AM (2019) Bacterial cellulose as a raw material for food and food packaging applications. Front Sustain Food Syst 3:1–14
Azrina ZZ, Beg MDH, Rosli M, Ramli R, Junadi N, Alam AM (2017) Spherical nanocrystalline cellulose (NCC) from oil palm empty fruit bunch pulp via ultrasound assisted hydrolysis. Carbohydr Polym 162:115–120
Baheti V, Abbasi R, Militky J (2012) Ball milling of jute fibre wastes to prepare nanocellulose. World J Eng 9(1):45–50
Batmaz R, Mohammed N, Zaman M, Minhas G, Berry RM, Tam KC (2014) Cellulose nanocrystals as promising adsorbents for the removal of cationic dyes. Cellulose 21(3):1655–1665
Battista OA (1950) Hydrolysis and crystallization of cellulose. Ind Eng Chem 42(3):502–507
Battista O, Coppick S, Howsmon J, Morehead F, Sisson WA (1956) Level-off degree of polymerization. Ind Eng Chem 48(2):333–335
Bauli CR, Rocha DB, de Oliveira SA, Rosa DS (2019) Cellulose nanostructures from wood waste with low input consumption. J Clean Prod 211:408–416
Berlioz S, Molina-Boisseau S, Nishiyama Y, Heux L (2009) Gas-phase surface esterification of cellulose microfibrils and whiskers. Biomacromolecules 10(8):2144–2151
Bian H, Chen L, Dai H, Zhu JY (2017a) Integrated production of lignin containing cellulose nanocrystals (LCNC) and nanofibrils (LCNF) using an easily recyclable di-carboxylic acid. Carbohydr Polym 167:167–176
Bian H, Chen L, Gleisner R, Dai H, Zhu JY (2017b) Producing wood-based nanomaterials by rapid fractionation of wood at 80 °C using a recyclable acid hydrotrope. Greeen Chem 19(14):3370–3379
Bian H, Gao Y, Yang Y, Fang G, Dai H (2018) Improving cellulose nanofibrillation of waste wheat straw using the combined methods of prewashing, p-toluenesulfonic acid hydrolysis, disk grinding, and endoglucanase post-treatment. Bioresour Technol 256:321–327
Blanco A, Monte MC, Campano C, Balea A, Merayo N, Negro C (2018) Nanocellulose for industrial use: cellulose nanofibers (CNF), cellulose nanocrystals (CNC), and bacterial cellulose (BC). In: Hussain CM (ed) Handbook of nanomaterials for industrial applications. Elsevier, Amsterdam, pp 74–126
Bolloli M, Antonelli C, Molméret Y, Alloin F, Iojoiu C, Sanchez J-Y (2016) Nanocomposite poly(vinyl fluride)/nanocrystalline cellulose porous membranes as separators fro lithium-ion batteries. Electrochim Acta 214:38–48
Bondeson D, Oksman K (2007) Dispersion and characteristics of surfactant modified cellulose whiskers nanocomposites. Compos Interfaces 14(7–9):617–630
Budhi Y, Fakhrudin M, Culsum N, Suendo V, Iskandar F (2018) Preparation of cellulose nanocrystals from empty fruit bunch of palm oil by using phosphotungstic acid. In: IOP conference series: earth and environmental science, IOP Publishing, vol 1, p 012063
Camarero Espinosa S, Kuhnt T, Foster EJ, Weder C (2013) Isolation of thermally stable cellulose nanocrystals by phosphoric acid hydrolysis. Biomacromolecules 14(4):1223–1230
Cao X, Huang M, Ding B, Yu J, Sun G (2013) Robust polyacrylonitrile nanofibrous membrane reinforced with jute cellulose nanowhiskers for water purification. Desalination 316:120–126
Carlmark A, Larsson E, Malmström E (2012) Grafting of cellulose by ring-opening polymerisation–a review. Eur Polym J 48(10):1646–1659
Castro C, Zuluaga R, Álvarez C, Putaux J-L, Caro G, Orlando JR, Mondraggon I, Gañán P (2012) Bacterial cellulose produced by a new acid-resistant strain of Gluconacetobacter genus. Carbohydr Polym 89(4):1033–1037
Cervin NT, Aulin C, Larsson PT, Wågberg L (2012) Ultra porous nanocellulose aerogels as separation medium for mixtures of oil/water liquids. Cellulose 19(2):401–410
Charreau H, Foresti ML, Vázquez (2013) Nanocellulose patents trends: a comprehensive review on patents on cellulose nanocrytals, microfibrillated and bacterial cellulose. Recent Pat Nanotechnol 7:56–80
Chauve G, Bras J (2014) Industrial point of view of nanocellulose materials and their possible applications. In: Handbook of green materials: 1 Bionanomaterials: separation processes, characterization and properties. World Scientific, pp 233–252
Chen W, Yu H, Liu Y (2011a) Preparation of millimeter-long cellulose I nanofibers with diameters of 30–80 nm from bamboo fibers. Carbohydr Polym 86(2):453–461
Chen W, Yu H, Liu Y, Chen P, Zhang M, Hai Y (2011b) Individualization of cellulose nanofibers from wood using high-intensity ultrasonication combined with chemical pretreatments. Carbohydr Polym 83(4):1804–1811
Chen L, Cao W, Quinlan PJ, Berry RM, Tam KC (2015) Sustainable catalysts from gold-loaded polyamidoamine dendrimer-cellulose nanocrystals. ACS Sustain Chem Eng 3(5):978–985
Chen L, Zhu J, Baez C, Kitin P, Elder T (2016a) Highly thermal-stable and functional cellulose nanocrystals and nanofibrils produced using fully recyclable organic acids. Green Chem 18(13):3835–3843
Chen YW, Lee HV, Juan JC, Phang S-M (2016b) Production of new cellulose nanomaterial from red algae marine biomass Gelidium elegans. Carbohydr Polym 151:1210–1219. https://doi.org/10.1016/j.carbpol.2016.06.083
Chen YW, Tan TH, Lee HV, Abd Hamid SB (2017) Easy fabrication of highly thermal-stable cellulose nanocrystals using Cr (NO3) 3 catalytic hydrolysis system: a feasibility study from macro-to nano-dimensions. Materials 10(1):42
Chen X-Q, Deng X-Y, Shen W-H, Jia M-Y (2018) Preparation and characterization of the spherical nanosized cellulose by the enzymatic hydrolysis of pulp fibers. Carbohydr Polym 181:879–884
Cheng Q, Wang S, Rials TG, Lee S-H (2007) Physical and mechanical properties of polyvinyl alcohol and polypropylene composite materials reinforced with fibril aggregates isolated from regenerated cellulose fibers. Cellulose 14(6):593–602
Cheng Q, Wang S, Rials TG (2009) Poly (vinyl alcohol) nanocomposites reinforced with cellulose fibrils isolated by high intensity ultrasonication. Compos A Appl Sci Manuf 40(2):218–224
Cheng Q, Wang S, Han Q (2010) Novel process for isolating fibrils from cellulose fibers by high-intensity ultrasonication. II. Fibril characterization. J Appl Polym Sci 115(5):2756–2762
Cheng M, Qin Z, Liu Y, Qin Y, Li T, Chen L, Zhu M (2014) Efficient extraction of carboxylated spherical cellulose nanocrystals with narrow distribution through hydrolysis of lyocell fibers by using ammonium persulfate as an oxidant. J Mater Chem A 2(1):251–258
Cherian BM, Pothan LA, Nguyen-Chung T, Mennig G, Kottaisamy M, Thomas S (2008) A Novel method for the synthesis of cellulose nanofibril whiskers from banana fibers and characterization. J Agric Food Chem 56(14):5617–5627. https://doi.org/10.1021/jf8003674
Cherian BM, Leão AL, de Souza SF, Thomas S, Pothan LA, Kottaisamy M (2010) Isolation of nanocellulose from pineapple leaf fibres by steam explosion. Carbohydr Polym 81(3):720–725
Chinga-Carrasco G (2013) Optical methods for the quantification of the fibrillation degree of bleached MFC materials. Micron 48:42–48
Cobut A, Sehaqui H, Berglund LA (2014) Cellulose nanocomposites by melt compounding of TEMPO-treated wood fibers in thermoplastic starch matrix. BioResources 9(2):3276–3289
Crotogino R (2012) NanoCellulose. In: International symposium on assessing the economic impact of nanotechnology, p 28
Csiszár E, Nagy S (2017) A comparative study on cellulose nanocrystals extracted from bleached cotton and flax and used for casting films with glycerol and sorbitol plasticisers. Carbohydr Polym 174:740–749
De Adhikari A, Oraon R, Tiwari S, Lee JH, Nayak G (2015) Effect of waste cellulose fibres on the charge storage capacity of polypyrrole and graphene/polypyrrole electrodes for supercapacitor application. RSC Adv 5(35):27347–27355
De France KJ, Hoare T, Cranston ED (2017) Review of hydrogels and aerogels containing nanocellulose. Chem Mater 29:4609–4631
De Menezes AJ, Siqueira G, Curvelo AA, Dufresne A (2009) Extrusion and characterization of functionalized cellulose whiskers reinforced polyethylene nanocomposites. Polymer 50(19):4552–4563
De Oliveira RL, da Silva BH, de Assunҫao RM, da Silva MC, Carvalho GO, Filho GR, Messaddeq Y, Ribeiro (2011) Synthesis and characterization of microcrstalline cellulose produced from bacterial cellulose. J Therm Anal Calorim 106(3):703–709
Deepa B, Abraham E, Cherian BM, Bismarck A, Blaker JJ, Pothan LA, Leao AL, De Souza SF, Kottaisamy M (2011) Structure, morphology and thermal characteristics of banana nano fibers obtained by steam explosion. Biores Technol 102(2):1988–1997
Desmaisons J, Boutonnet E, Rueff M, Dufresne A, Bras J (2017) A new quality index for benchmarking of different cellulose nanofibrils. Carbohydr Polym 174:318–329
Dimic-Misic K, Gane PAC, Paltakari J (2013a) Micro-and nanofibrillated cellulose as a rheology modifier additive in CMC-containing pigment-coating formulations. Ind Eng Chem Res 52(45):16066–16083
Dimic-Misic K, Puisto A, Gane P, Nieminen K, Alava M, Paltakari J, Maloney T (2013b) The role of MFC/NFC swelling in the rheological behavior and dewatering of high consistency furnishes. Cellulose 20(6):2847–2861
Dong Ntoutou GMA, Granet R, Mbakid JP, Brégier F, Léger DY, Fidanzi-Dugas C, Lequart V, Joly N, Liagre B, Chaleix V, Sol V (2016) Development of curcumin–cyclodextrin/cellulose nanocrystals complexes: new anticancer drug delivery systems. Biorgan Med Chem Lett 26(3):941–945
Du H, Liu C, Mu X, Gong W, Lv D, Hong Y, Si C, Li B (2016a) Preparation and characterization of thermally stable cellulose nanocrystals via a sustainable approach of FeCl3-catalyzed formic acid hydrolysis. Cellulose 23(4):2389–2407
Du H, Lui C, Zhang Y, Yu G, Si C, Li B (2016b) Preparation and characterization of functional cellulose nanofibrils via formic acid hydrolysis pretreatment and the followed high-pressure homogenization. Ind Crops Prod 94:736–745
Dufresne A (2017) Cellulose nanomaterial reinforced polymer nanocomposites. Curr Opin Colloid Interface Sci 29:1–8
Dufresne A (2018) Cellulose nanomaterials as green nanoreinforcements for polymer nanocomposites. Philos Trans R Soc A 376(2112):20170040
Dufresne A, Cavaillé JY, Vignon MR (1997) Mechanical behavior of sheets prepared from sugar beet cellulose microfibrils. J Appl Polym Sci 64(6):1185–1194
Durán N, Lemes AP, Seabra AB (2012) Review of cellulose nanocrystals patents: preparation, composites and general applications. Recent Pat Nanotechnol 6:16–28
Dutta S, Kim J, Ide Y, Kim JH, Hossain MSA, Bando Y, Yamauchi Y, Wu KC-W (2017) 3D network of cellulose-based energy storage devices and related emerging applications. Mater Horizons 4(4):522–545
Eisa WH, Abdelgawad AM, Rojas OJ (2018) Solid-state synthesis of metal nanoparticles supported on cellulose nanocrystals and their catalytic activity. ACS Sustain Chem Eng 6(3):3974–3983
El Achaby M, Kassab Z, Aboulkas A, Gaillard C, Barakat A (2018) Reuse of red algae waste for the production of cellulose nanocrystals and its application in polymer nanocomposites. Int J Biol Macromol 106:681–691. https://doi.org/10.1016/j.ijbiomac.2017.08.067
Ellebracht NC, Jones CW (2018) Amine-functionalization of cellulose nanocrystals for acid-base organocatalyis: surface chemistry, cross-linking, and solvent effects. Cellulose 25(11):6495–6512
Espino-Pérez E, Domenek S, Belgacem N, Cc Sillard, Bras J (2014) Green process for chemical functionalization of nanocellulose with carboxylic acids. Biomacromolecules 15(12):4551–4560
Fan X-M, Yu H-Y, Wang D-C, Mao Z-H, Yao J, Tam KC (2019) Facile and green synthesis of carboxylated cellulose nanocrystals as efficient adsorbents in wastewater treatments. ACS Sustain Chem Eng. https://doi.org/10.1021/acssuschemeng.9b05081
Fang Z, Zhu H, Yuan Y, Ha D, Zhu S, Preston C, Chen Q, Li Y, Han X, Lee S (2014) Novel nanostructured paper with ultrahigh transparency and ultrahigh haze for solar cells. Nano Lett 14(2):765–773
Favier V, Canova G, Cavaillé J, Chanzy H, Dufresne A, Gauthier C (1995) Nanocomposite materials from latex and cellulose whiskers. Polym Adv Technol 6(5):351–355
Ferreira F, Mariano M, Rabelo S, Gouveia R, Lona L (2018) Isolation and surface modification of cellulose nanocrystals from sugarcane bagasse waste: from a micro-to a nano-scale view. Appl Surf Sci 436:1113–1122
Ferrer A, Filpponen I, Rodríguez A, Laine J, Rojas OJ (2012) Valorization of residual empty palm fruit bunch fibers (EPFBF) by microfluidization: production of nanofibrillated cellulose and EPFBF nanopaper. Biores Technol 125:249–255
Filson PB, Dawson-Andoh BE (2009) Sono-chemical preparation of cellulose nanocrystals from lignocellulose derived materials. Biores Technol 100(7):2259–2264
Filson PB, Dawson-Andoh BE, Schwegler-Berry D (2009) Enzymatic-mediated production of cellulose nanocrystals from recycled pulp. Green Chem 11(11):1808–1814
Fortunati E, Peltzer M, Armentano I, Torre L, Jiménez A, Kenny J (2012) Effects of modified cellulose nanocrystals on the barrier and migration properties of PLA nano-biocomposites. Carbohydr Polym 90(2):948–956
Foster EJ, Moon RJ, Agarwal UP, Bortner MJ, Bras J, Camarero-Espinosa S, Chan KJ, Clift MJ, Cranston ED, Eichhorn SJ (2018) Current characterization methods for cellulose nanomaterials. Chem Soc Rev 47(8):2609–2679
Fraschini C, Chauve G, Bouchard J (2017) TEMPO-mediated surface oxidation of cellulose nanocrystals (CNCs). Cellulose 24(7):2775–2790
Gao R, Xiao S, Gan W, Liu Q, Amer H, Rosenau T, Li J, Lu Y (2018) Mussel adhesive-inspired design of superhydrophobic nanofibrillated cellulose aerogels fro oil/water separation. ACS Sustain Chem Eng 6(7):9047–9055
George J, Sabapathi S (2015) Cellulose nanocrystals: synthesis, functional properties, and applications. Nanotechnol Sci Appl 8:45
Goetz LA, Naseri N, Nair SS, Karim Z, Mathew AP (2018) All cellulose electrospun water purification membranes nanotextured using cellulose nanocrystals. Cellulose 25(5):3011–3023
Goffin A-L, Raquez J-M, Duquesne E, Siqueira G, Habibi Y, Dufresne A, Dubois P (2011) From interfacial ring-opening polymerization to melt processing of cellulose nanowhisker-filled polylactide-based nanocomposites. Biomacromol 12(7):2456–2465
Gong X, Wang Y, Zeng H, Betti M, Chen L (2019) Highly porous, hydrophobic, and compresssible cellulose nanocrystals/poly(vinyl alcohol) aerogels as recyclable absorbents for oil-water separation. ACS Sustain Chem Eng 7(13):11118–11128
Gupta K, Kaushik A, Tikoo K, Kumar V, Singhal S (2017) Enhanced catalytic activity of composites of NiFe2O4 and nano cellulose derived from waste biomass for the mitigation of organic pollutants. Arab J Chem. https://doi.org/10.1016/j.arabjc.2017.07.016(in Press)
Hamid SBA, Zain SK, Das R, Centi G (2016) Synergic effect of tungstophosphoric acid and sonication for rapid synthesis of crystalline nanocellulose. Carbohydr Polym 138:349–355
Hasani M, Cranston ED, Westman G, Gray DG (2008) Cationic surface functionalization of cellulose nanocrystals. Soft Matter 4(11):2238–2244
Herrick FW, Casebier RL, Hamilton JK, Sandberg KR (1983) Microfibrillated cellulose: morphology and accessibility. In: J Appl Polym Sci Appl Polym Symp, vol CONF-8205234-Vol 2. ITT Rayonier Inc., Shelton, WA
Ho TTT, Abe K, Zimmermann T, Yano H (2015) Nanofibrillation of pulp fibers by twin-screw extrusion. Cellulose 22(1):421–433
Hokkanen S, Repo E, Sillanpää M (2013) Removal of heavy metals from aqueous solutions by succinic anhydride modified mercerized nanocellulose. Chem Eng J 223:40–47
Hubbe MA, Tayeb P, Joyce M, Tyagi P, Dimic-Misic M, Pal L (2017) Rheology of nanocellulose-rich aqueous suspensions: a review. BioResources 12(4):9556–9661
Hussain Z, Sajjad W, Khan T, Wahid F (2019) Production of bacterial cellulose from industrial wastes: a review. Cellulose 26(5):2895–2911
Im W, Lee S, Abhari AR, Youn HJ, Lee HL (2018) Optimization of carboxymethylation reaction as a pretreatment for production of cellulose nanofibrils. Cellulose 25(7):3873–3883
Im W, Oh K, Abhari AR, Youn HJ, Lee HL (2019) Recycling of isopropanol fro cost-effective, environmentally friendly production of carboxylmethylated cellulose nanofibrils. Carbohydr Polym 208:365–371
Isogai A, Saito T, Fukuzumi H (2011) TEMPO-oxidized cellulose nanofibers. Nanoscale 3(1):71–85
Jia C, Bian H, Gao T, Jiang F, Kierzewski IM, Wang Y, Yao Y, Chen L, Shao Z, Zhu J (2017a) Thermally stable cellulose nanocrystals toward high-performance 2D and 3D nanostructures. ACS Appl Mater Interfaces 9(34):28922–28929
Jia C, Chen L, Shao Z, Agarwal UP, Hu L, Zhu J (2017b) Using a fully recyclable dicarboxylic acid for producing dispersible and thermally stable cellulose nanomaterials from different cellulosic sources. Cellulose 24(6):2483–2498
Jiang H, Wu Y, Han B, Zhang Y (2017) Effect of oxidation time on the properties of cellulose nanocrystals from hybrid poplar residues using the ammonium persulfate. Carbohydr Polym 174:291–298
Jiang Y, Zhou J, Yang Z, Liu D, Xv X, Zhao G, Shi H, Zhang Q (2018) Dialdehyde cellulose nanocrystal/gelatin hydrogel optimized for 3D printing applications. J Mater Sci 53(16):11883–11900
Jin L, Li W, Xu Q, Sun Q (2015a) Amino-functionalized nanocrystalline cellulose as an adsorbent for anionic dyes. Cellulose 22(4):2443–2456
Jin L, Sun Q, Xu Q, Xu Y (2015b) Adsorptive removal of anionic dyes from aqueous solutions using microgel based on nanocellulose and polyvinylamine. Biores Technol 197:348–355
Jin E, Guo J, Yang F, Zhu Y, Song J, Jin Y, Rojas OJ (2016) On the polymorphic and morphological changes of cellulose nanocrystals (CNC-I) upon mercerization and conversion to CNC-II. Carbohydr Polym 143:327–335
Johansson C, Bras J, Mondragon I, Nechita P, Plackett D, Simon P, Svetec DG, Virtanen S, Baschetti MG, Breen C (2012) Renewable fibers and bio-based materials for packaging applications–a review of recent developments. BioResources 7(2):2506–2552
John MJ, Anandjiwala R, Oksman K, Mathew AP (2013) Melt-spun polylactic acid fibers: effect of cellulose nanowhiskers on processing and properties. J Appl Polym Sci 127(1):274–281
Jonoobi M, Mathew AP, Oksman K (2012) Producing low-cost cellulose nanofiber from sludge as new source of raw materials. Ind Crops Prod 40:232–238
Kan KH, Li J, Wijesekera K, Cranston ED (2013) Polymer-grafted cellulose nanocrystals as pH-responsive reversible flocculants. Biomacromol 14(9):3130–3139
Kardam A, Raj KR, Srivastava S, Srivastava M (2014) Nanocellulose fibers for biosorption of cadmium, nickel, and lead ions from aqueous solution. Clean Technol Environ Policy 16(2):385–393
Karim Z, Mathew AP, Grahn M, Mouzon J, Oksman K (2014) Nanoporous membranes with cellulose nanocrystals as functional entity in chitosan: removal of dyes from water. Carbohydr Polym 112:668–676
Karim Z, Claudpierre S, Grahn M, Oksman K, Mathew AP (2016) Nanocellulose based functional membranes for water cleaning: tailoring of mechanical properties, porosity and metal ion capture. J Membr Sci 514:418–428
Keshk S (2014) Bacterial cellulose production and its industrial applications. J Bioprocess Biotech 4(150):2
Keshk SM, Haija MA (2011) A new method for producing microcrystalline cellulose ffrom Gluconacetobacter xylinus and kenaf. Carbohydr Polym 84(4):1301–1305
Khanjanzadeh H, Behrooz R, Bahramifar N, Gindl-Altmutter W, Bacher M, Edler M, Griesser T (2018) Surface chemical functionalization of cellulose nanocrystals by 3-aminopropyltriethoxysilane. Int J Biol Macromol 106:1288–1296
Khawas P, Deka SC (2016) Isolation and characterization of cellulose nanofibers from culinary banana peel using high-intensity ultrasonication combined with chemical treatment. Carbohydr Polym 137:608–616
Kim J, Montero G, Habibi Y, Hinestroza JP, Genzer J, Argyropoulos DS, Rojas OJ (2009) Dispersion of cellulose crystallites by nonionic surfactants in a hydrophobic polymer matrix. Polym Eng Sci 49(10):2054–2061
Kim CH, Kim JW, Zhai L, Kim J (2019) Strng and tough long cellulose fibers made by aligning cellulose nanofibers under magnetic and electric fields. Cellulose 26:5821–5829
Kolakovic R, Laaksonen T, Peltonen L, Laukkanen A, Hirvonen J (2012) Spray-dried nanofibrillar cellulose microparticles for sustained drug release. Int J Pharm 430(1–2):47–55
Kontturi E, Meriluoto A, Penttilä PA, Baccile N, Malho JM, Potthast A, Rosenau T, Ruokolainen J, Serimaa R, Laine J (2016) Degradation and crystallization of cellulose in hydrogen chloride vapor for high-yield isolation of cellulose nanocrystals. Angew Chem Int Ed 55(46):14455–14458
Korhonen JT, Kettunen M, Ras RHA, Ikkala O (2011) Hydrophobic nanocellulose aerogels as floating, sustainable, reusable, and recyclable oil absorbents. ACS Appl Mater Interfaces 3(6):1813–1816
Kulpinski P (2005) Cellulose nanofibers prepared by the N-methylmorpholine-N-oxide method. J Appl Polym Sci 98(4):1855–1859
Kumar R, Ha SK, Verma K, Tiwari SK (2018) Recent progress in some selected bio-nanomaterials and their engineering applications: an overview. J Sci Adv Mater Dev 3:263–288
Labet M, Thielemans W (2011) Improving the reproducibility of chemical reactions on the surface of cellulose nanocrystals: ROP of ε-caprolactone as a case study. Cellulose 18(3):607–617
Lam E, Leung AC, Liu Y, Majid E, Hrapovic S, Male KB, Luong JH (2012) Green strategy guided by Raman spectroscopy for the synthesis of ammonium carboxylated nanocrystalline cellulose and the recovery of byproducts. ACS Sustain Chem Eng 1(2):278–283
Leppiniemi J, Lahtinen P, Paajanen A, Mahlberg R, Metsä-Kortelainen S, Pinomaa T, Pajari H, Vikholm-Lundin I, Pursula P, Hytönen VP (2017) 3D-printable bioactivated nanocellulose–alginate hydrogels. ACS Appl Mater Interfaces 9(26):21959–21970
Li B, Xu W, Kronlund D, Määttänen A, Liu J, Smått J-H, Peltonen J, Willför S, Mu X, Xu C (2015) Cellulose nanocrystals prepared via formic acid hydrolysis followed by TEMPO-mediated oxidation. Carbohydr Polym 133:605–612
Li VC-F, Dunn CK, Zhang Z, Deng Y, Qi HJ (2017) Direct ink write (DIW) 3D printed cellulose nanocrystal aerogel structures. Sci Rep 7(1):8018
Li VC, Mulyadi A, Dunn CK, Deng Y, Qi HJ (2018a) Direct ink write 3D printed cellulose nanofiber aerogel structures with highly deformable, shape recoverable, and functionalizable properties. ACS Sustain Chem Eng 6(2):2011–2022
Li Y-Y, Wang B, Ma M-G, Wang B (2018b) The influence of pre-treatment time and sulfuric acid on cellulose nanocrystals. BioResources 13(2):3585–3602
Lin N, Huang J, Chang PR, Feng L, Yu J (2011) Effect of polysaccharide nanocrystals on structure, properties, and drug release kinetics of alginate-based microspheres. Colloids Surf B 85(2):270–279
Lin N, Huang J, Dufresne A (2012) Preparation, properties and applications of polysaccharide nanocrystals in advanced functional nanomaterials: a review. Nanoscale 4(11):3274–3294
Liu Y, Wang H, Yu G, Yu Q, Li B, Mu X (2014) A novel approach for the preparation of nanocrystalline cellulose by using phosphotungstic acid. Carbohydr Polym 110:415–422
Liu J, Plog A, Groszewicz P, Zhao L, Xu Y, Breitzke H, Stark A, Hoffmann R, Gutmann T, Zhang K (2015a) Design of a heterogeneous catalyst based on cellulose nanocrystals for cyclopropanation: synthesis and solid-state NMR characterization. Chem Eur J 21(35):12414–12420
Liu P, Borrell PF, Božič M, Kokol V, Oksman K, Mathew AP (2015b) Nanocelluloses and their phosphorylated derivatives for selective adsorption of Ag+, Cu2+ and Fe3+ from industrial effluents. J Hazard Mater 294:177–185
Liu K, Liu M, Cheng J, Dong S, Wang C, Wang Q, Zhou X, Sun H, Chen X, Cui G (2016) Novel cellulose/polyurethane composite gel polymer electrolyte for high performance lithium batteries. Electrochim Acta 215:261–266
Lourenço AN, Godinho D, Gamelas JAF, Sarmento P, Ferreira PJT (2019) Carboxymethylated cellulose nanofibrils in papermaking: influence on filler retention and paper properties. Cellulose 26(5):3489–3502
Lu Q, Lin W, Tang L, Wang S, Chen X, Huang B (2015) A mechanochemical approach to manufacturing bamboo cellulose nanocrystals. J Mater Sci 50(2):611–619
Lu Q, Cai Z, Lin F, Tang L, Wang S, Huang B (2016) Extraction of cellulose nanocrystals with a high yield of 88% by simultaneous mechanochemical activation and phosphotungstic acid hydrolysis. ACS SustainChem Eng 4(4):2165–2172
Lu L, Fan S, Niu Q, Peng Q, Geng L, Yang G, Shao H, Hsiao BS, Zhang Y (2019) Strong silk fibers containing cellulose nanofibers generated by a bioinspired microfluidic chip. ACS Sustain Chem Eng. https://doi.org/10.1021/acssuschemeng.9b02713
Luo Y, Zhang J, Li X, Liao C, Li X (2014) The cellulose nanofibers for optoelectronic conversion and energy storage. J Nanomater 2014:11
Lv J, Zhang G, Zhang H, Zhao C, Yang F (2018) Improvement of antifouling performances for modified PVDF ultrafiltration membrane with hydrophilic cellulose nanocrystal. Appl Surf Sci 440:1091–1100
Lv D, Du H, Che X, Wu M, Zhang Y, Liu C, Nie S, Zhang X, Li B (2019) Tailored and integrated production of functional cellulose nanocrystals and cellulose nanofibrils via sustainable formic acid hydrolysis: kinetic study and characterization. ACS Sustain Chem Eng 7(10):9449–9463
Lynd LR, Weimer PJ, Van Zyl WH, Pretorius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66(3):506–577
Ma Z, Ramakrishna S (2008) Electrospun regenerated cellulose nanofiber affinity membrane functionalized with protein A/G for IgG purification. J Membr Sci 319(1):23–28. https://doi.org/10.1016/j.memsci.2008.03.045
Ma H, Burger C, Hsiao BS, Chu B (2011a) Nanofibrous microfiltration membrane based on cellulose nanowhiskers. Biomacromol 13(1):180–186
Ma H, Burger C, Hsiao BS, Chu B (2011b) Ultrafine polysaccharide nanofibrous membranes for water purification. Biomacromol 12(4):970–976
Ma H, Burger C, Hsiao BS, Chu B (2014) Fabrication and characterization of cellulose nanofiber based thin-film nanofibrous composite membranes. J Membr Sci 454:272–282
Maloney TC (2015) Network swelling of TEMPO-oxidized nanocellulose. Holzforschung 69(2):207–213
Mao J, Osorio-Madrazo A, Laborie M-P (2013) Preparation of celllulose I nanowhiskers with a mildly acidic aqueous ionic liquid: reaction effciency and whiskers attributes. Cellulose 20(4):1829–1840
Mao J, Heck B, Reiter G, Laborie M-P (2015) Cellulose nanocrystals’ production in near theoretical yields by 1-butyl-3-methylimidazolium hydrogen sulfate ([Bmim] HSO4)-mediated hydrolysis. Carbohydr Polym 117:443–451
Mao J, Abushammala H, Pereira LB, Laborie M-P (2016) Swelling and hydrolysis kinetics of Kraft pulp fibers in aqueous 1-butyl-3-methylimidazolium hydrogen sulfate solutions. Carbohydr Polym 153:284–291
Marchessault R, Morehead F, Walter N (1959) Liquid crystal systems from fibrillar polysaccharides. Nature 184(4686):632–633
Markstedt K, Mantas A, Tournier I, Hc MÁ, Hägg D, Gatenholm P (2015) 3D bioprinting human chondrocytes with nanocellulose–alginate bioink for cartilage tissue engineering applications. Biomacromol 16(5):1489–1496
Mautner A, Kwaw Y, Weiland K, Mvubu M, Botha A, John MJ, Mtibe A, Siqueira G, Bismarck A (2019) Natural fibre-nanocellulose composite filters for the removal of heavy metal ions from water. Ind Crops Prod 133:325–332. https://doi.org/10.1016/j.indcrop.2019.03.032
McAlpine KJ (2016) 4D-printed structure changes shape when placed in water. Harvard Gazette 25
Meyabadi TF, Dadashian F, Sadeghi GMM, Asl HEZ (2014) Spherical cellulose nanoparticles preparation from waste cotton using a green method. Powder Technol 261:232–240
Mishra RK, Sabu A, Tiwari SK (2018) Materials chemistry and the futurist eco-friendly applications of nanocellulose: status and prospect. J Saudi Chem Soc 22:949–978
Mittal N, Ansari F, Gowda VK, Brouzet C, Chen P, Larsson PT, Roth SV, Lundell F, Wågberg L, Kotov NA, Söderberg DL (2018) Multiscale control of nanocellulose assembly: transferring remarkable nanoscale fibril mechanics to macroscale fibers. ACS Nano 12(7):6378–6388
Mochane MJ, Mokhena TC, Mokhothu TH, Mtibe A, Sadiku ER, Ray SS (2018) The importance of nanostructured materials for energy storage/conversion. In: Hussain CM (ed) Handbook of nanomaterials for industrial applications. Elsevier, Amsterdam, pp 768–792
Mohd Amin KN, Annamalai PK, Morrow IC, Martin D (2015) Production of cellulose nanocrystals via a scalable mechanical method. RCS Adv 5(70):57133–57140
Mohite BV, Patil SV (2014) A novel biomaterial: bacterial cellulose and its new era applications. Biotechnol Appl Biochem 61(2):101–110
Mokhena TC, Jacobs VN, Luyt AS (2015) A review on electrospun bio-based polymers for water treatment. Express Polym Lett 9(10):839–880
Mokhena TC, Jacobs NV, Luyt AS (2018a) Nanofibrous alginate membrane coated with cellulose nanowhiskers for water purification. Cellulose 25(1):417–427
Mokhena TC, Sefadi JS, Sadiku ER, John MJ, Mochane MJ, Mtibe A (2018b) Thermoplastic processing of PLA/cellulose nanomaterials composites. Polymers 10:1363. https://doi.org/10.3390/polym10121363
Moon RJ, Martini A, Nairn J, Simonsen J, Youngblood J (2011) Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev 40:3941–3994
Morandi G, Heath L, Thielemans W (2009) Cellulose nanocrystals grafted with polystyrene chains through surface-initiated atom transfer radical polymerization (SI-ATRP). Langmuir 25(14):8280–8286
Moser C, Lindström ME, Henriksson G (2015) Toward industrially feasible methods for following the process of manufacturing cellulose nanofibers. BioResources 10(2):2360–2375
Mtibe A, Linganiso LZ, Mathew AP, Oksman K, John MJ, Anandjiwala RD (2015) A comparative study on properties of micro and nanopapers produced from cellulose and cellulose nanofibres. Carbohydr Polym 118:1–8
Mukherjee S, Woods H (1953) X-ray and electron microscope studies of the degradation of cellulose by sulphuric acid. Biochem Biophys Acta 10:499–511
Mukherjee S, Sikorski J, Woods H (1952) Electron-microscopy of degraded cellulose fibres. Taylor & Francis, Routledge
Müller M, Öztürk E, Arlov Ø, Gatenholm P, Zenobi-Wong M (2017) Alginate sulfate–nanocellulose bioinks for cartilage bioprinting applications. Ann Biomed Eng 45(1):210–223
Naidu DS, Hlangothi SP, John MJ (2017) Bio-based products from xylan: a review. Carbohydr Polym 179:28–41
Nair JR, Bella F, Angulakshmi N, Stephan AM, Gerbaldi C (2016) Nanocellulose-laden composite polymer electrolytes for high performing lithium–sulphur batteries. Energy Storage Mater 3:69–76
Nechyporchuk O, Belgacem MN, Bras J (2016) Production of cellulose nanofibrils: a review of recent advances. Ind Crops Prod 93:2–25
Ng H-M, Sin LT, Bee S-T, Tee T-T, Rahmat A (2017) Review of nanocellulose polymer composite characteristics and challenges. Polym-Plast Technol Eng 56(7):687–731
Nguyen D, Hägg DA, Forsman A, Ekholm J, Nimkingratana P, Brantsing C, Kalogeropoulos T, Zaunz S, Concaro S, Brittberg M (2017) Cartilage tissue engineering by the 3D bioprinting of iPS cells in a nanocellulose/alginate bioink. Sci Rep 7(1):658
Nickerson R, Habrle J (1947) Cellulose intercrystalline structure. Ind Eng Chem 39(11):1507–1512
Nogi M, Karakawa M, Komoda N, Yagyu H, Nge TT (2015) Transparent conductive nanofiber paper for foldable solar cells. Sci Rep 5:17254
Novo LP, Bras J, García A, Belgacem N, Curvelo AA (2015) Subcritical water: a method for green production of cellulose nanocrystals. ACS Sustain Chem Eng 3(11):2839–2846
Novo LP, Bras J, García A, Belgacem N, da Silva Curvelo AA (2016) A study of the production of cellulose nanocrystals through subcritical water hydrolysis. Ind Crops Prod 93:88–95
Oksman K, Aitomäki Y, Mathew AP, Siqueira G, Zhou Q, Butylina S, Tanpichai S, Zhou X, Hooshmand S (2016) Review of the recent developments in cellulose nanocomposite processing. Compos A Appl Sci Manuf 83:2–18
Ooi SY, Ahmad I, Amin MCIM (2016) Cellulose nanocrystals extracted from rice husks as a reinforcing material in gelatin hydrogels for use in controlled drug delivery systems. Ind Crops Prod 93:227–234
Oun AA, Rhim J-W (2017) Characterization of carboxymethyl cellulose-based nanocomposite films reinforced with oxidized nanocellulose isolated using ammonium persulfate method. Carbohydr Polym 174:484–492
Pääkkönen T, Dimic-Misic K, Orelma H, Pönni R, Vuorinen T, Maloney T (2015) Effect of xylan in hardwood pulp on the reaction rate of TEMPO-mediated oxidation and the rheology of the final nanofibrillated cellulose gel. Cellulose 23(1):277–293
Pan R, Cheung O, Wang Z, Tammela P, Huo J, Lindh J, Edström K, Strømme M, Nyholm L (2016) Mesoporous Cladophora cellulose separators for lithium-ion batteries. J Power Sour 321:185–192
Pan R, Xu X, Sun R, Wang Z, Lindh J, Edström K, Strømme M, Nyholm L (2018) Nanocellulose modified polyethylene separators from lithium metal batteries. Small 14:1704371
Pan R, Sun R, Wang Z, Lindh J, Edström K, Strømme M, Nyholm L (2019) Sandwich-structured nano/micro fiber-based separators for lithium metal batteries. Nano Energy 55:316–326
Payen A (1838) Mémoire sur la composition du tissu propre des plantes et du ligneux. Comptes rendus 7:1052–1056
Pei A, Butchosa N, Berglund LA, Zhou Q (2013) Surface quaternized cellulose nanofibrils with high water absorbency and adsorption capacity for anionic dyes. Soft Matter 9(6):2047–2055
Peng J, Zhang H, Zheng Q, Clemons CM, Sabo RC, Gong S, Ma Z, Turng L-S (2017) A composite generator film impregnated with cellulose nanocrystals for enhanced triboelectric performance. Nanoscale 9(4):1428–1433
Plackett DV, Letchford K, Jackson JK, Burt HM (2014) A review of nanocellulose as a novel vehicle for drug delivery. Nord Pulp Pap Res J 29(1):105–118
Qiao H, Zhou Y, Yu F, Wang E, Min Y, Huang Q, Pang L, Ma T (2015) Effective removal of cationic dyes using carboxylate-functionalized cellulose nanocrystals. Chemosphere 141:297–303
Qing Y, Sabo R, Zhu J, Agarwal U, Cai Z, Wu Y (2013) A comparative study of cellulose nanofibrils disintegrated via multiple processing approaches. Carbohydr Polym 97(1):226–234
Rajinipriya M, Nagalakshmaiah M, Robert M, Elkoun S (2018) Importance of agricultural and industrial waste in the field of nanocellulose and recent industrial developments of wood based nanocellulose: a review. ACS Sustain Chem Eng 6(3):2807–2828
Ranby BG (1949) Aqueous colloidal solutions of cellulose micelles. vol 3. Munksgaard Int Publ Ltd 35 Norre Sogade, Po Box 2148, DK-1016 Copenhagen, Denmark
Rånby BG (1951) Fibrous macromolecular systems. Cellulose and muscle. The colloidal properties of cellulose micelles. Discuss Faraday Soc 11:158–164
Rånby B, Ribi E (1950) Über den feinbau der zellulose. Experientia 6(1):12–14
Rees A, Powell LC, Chinga-Carrasco G, Gethin DT, Syverud K, Hill KE, Thomas DW (2015) 3D bioprinting of carboxymethylated-periodate oxidized nanocellulose constructs for wound dressing applications. BioMed Res Int
Reid MS, Villalobos M, Cranston ED (2016) Benchmarking cellulose nanocrystals: from the laboratory to industrial production. Langmuir 33(7):1583–1598
Revin V, Liyaskina E, Nazarkina M, Bogatyreva A, Shchankin M (2018) Cost-effective production of bacterial cellulose using acidic food industry by-products. Braz J Microbiol 49:151–159
Revol J-F, Bradford H, Giasson J, Marchessault R, Gray D (1992) Helicoidal self-ordering of cellulose microfibrils in aqueous suspension. Int J Biol Macromol 14(3):170–172
Robles E, Csóka L, Labidi J (2018a) Effect of reaction conditions on the surface modification of cellulose nanofibrils with aminopropyl triethoxysilane. Coatings 8(4):139
Robles E, Fernandez-Rodriguez J, Barbosa AM, Gordobil O, Carreno NL, Labidi J (2018b) Production of cellulose nanoparticles from blue agave waste treated with environmentally friendly processes. CarbohydR Polym 183:294–302
Sadeghifar H, Filpponen I, Clarke SP, Brougham DF, Argyropoulos DS (2011) Production of cellulose nanocrystals using hydrobromic acid and click reactions on their surface. J Mater Sci 46(22):7344–7355
Sain M, Bhatnagar A (2003) Manufacturing of nanofibrils from natural fibres, agro based fibres and root fibres. CA Patent Appl 2
Sain MM, Bhatnagar A (2008) Manufacturing process of cellulose nanofibers from renewable feed stocks. Google Patents
Saini S, Sillard C, Belgacem MN, Bras J (2016) Nisin anchored cellulose nanofibers for long term antimicrobial active food packaging. RSC Adv 6(15):12422–12430
Sainorudin MH, Mohammad M, Kadir NHA, Abdullah NA, Yaakob Z (2018) Characterization of several microcrystalline cellulose (MCC)-based agricutural waste via X-ray diffraction method. Solid State Phenom 280:340–345
Salajková M, Berglund LA, Zhou Q (2012) Hydrophobic cellulose nanocrystals modified with quaternary ammonium salts. J Mater Chem 22(37):19798–19805
Salminen R, Reza M, Pääkkönen T, Peyre J, Kontturi E (2017) TEMPO-mediated oxidation of microcrystalline cellulose: limiting factors for cellulose nanocrystal yield. Cellulose 24(4):1657–1667
Satyamurthy P, Jain P, Balasubramanya RH, Vigneshwaran N (2011) Preparation and characterization of cellulose nanowhiskers from cotton fibres by controlled microbial hydrolysis. CarbohydR Polym 83(1):122–129
Sharma A, Thakur M, Bhattacharya M, Mandal T (2018) Commercial application of cellulose nano-composites—a reeview. Biotechnol Rep. https://doi.org/10.1016/j.btre.2019.e00316
Silva R, Pereira G, Voiry D, Chhowalla M, Asefa T (2015) Co 3 O 4 nanoparticles/cellulose nanowhiskers-derived amorphous carbon nanoneedles: sustainable materials for supercapacitors and oxygen reduction electrocatalysis. RSC Adv 5(61):49385–49391
Siqueira G, Oksman K, Tadokoro SK, Mathew AP (2016) Re-dispersible carrot nanofibers with high mechanical properties and reinforcing capacity for use in composite materials. Compos Sci Technol 123:49–56
Spence KL, Venditti RA, Rojas OJ, Habibi Y, Pawlak JJ (2011) A comparative study of energy consumption and physical properties of microfibrillated cellulose produced by different processing methods. Cellulose 18(4):1097–1111
Sucaldito MR, Camacho DH (2017) Characteristics of unique HBr-hydrolyzed cellulose nanocrystals from freshwater green algae (Cladophora rupestris) and its reinforcement in starch-based film. CarbohydR Polym 169:315–323
Tan XY, Hamid SBA, Lai CW (2015) Preparation of high crystallinity cellulose nanocrystals (CNCs) by ionic liquid solvolysis. Biomass Bioenergy 81:584–591
Tang L-R, Huang B, Ou W, Chen X-R, Chen Y-D (2011) Manufacture of cellulose nanocrystals by cation exchange resin-catalyzed hydrolysis of cellulose. Biores Technol 102(23):10973–10977
Tang J, Shi Z, Berry RM, Tam KC (2015a) Mussel-inspired green metallization of silver nanoparticles on cellulose nanocrystals and their enhanced catalytic reduction of 4-nitrophenol in the presence of β-cyclodextrin. Ind Eng Chem Res 54(13):3299–3308
Tang Y, Shen X, Zhang J, Guo D, Kong F, Zhang N (2015b) Extraction of cellulose nano-crystals from old corrugated container fiber using phosphoric acid and enzymatic hydrolysis followed by sonication. Carbohydr Polym 125:360–366
Tehrani AD, Neysi E (2013) Surface modification of cellulose nanowhisker throughout graft polymerization of 2-ethyl-2-oxazoline. Carbohydr Polym 97(1):98–104
Teixeira RSS, da Silva ASA, Jang J-H, Kim H-W, Ishikawa K, Endo T, Lee S-H, Bon EP (2015) Combining biomass wet disk milling and endoglucanase/β-glucosidase hydrolysis for the production of cellulose nanocrystals. Carbohydr Polym 128:75–81
Torres-Rendon JG, Köpf M, Gehlen D, Blaeser A, Fischer H, Laporte LD, Walther A (2016) Cellulose nanofibril hydrogel tubes as sacrificial templates for freestanding tubular cell constructs. Biomacromolecules 17(3):905–913
Trache D, Hussin MH, Chuin CTH, Sabar S, Fazita MRN, Taiwo OFA, Hassan TM, Haafiz MKM (2016) Microcrystalline cellululose: isolation, characterization and biocomposites – a review. Int J Biol Macromol 93:789–804
Trache D, Hussin MH, Haafiz MM, Thakur VK (2017) Recent progress in cellulose nanocrystals: sources and production. Nanoscale 9(5):1763–1786
Turbak AF, Snyder FW, Sandberg KR (1983) Microfibrillated cellulose, a new cellulose product: properties, uses, and commercial potential. In: J Appl Polym Sci Appl Polym Symp (United States), vol CONF-8205234-Vol. 2. ITT Rayonier Inc., Shelton, WA
Valo H, Arola S, Laaksonen P, Torkkeli M, Peltonen L, Linder MB, Serimaa R, Kuga S, Hirvonen J, Laaksonen T (2013) Drug release from nanoparticles embedded in four different nanofibrillar cellulose aerogels. Eur J Pharm Sci 50(1):69–77
Villanova J, Ayres E, Carvalho S, Patrício P, Pereira F, Oréfice R (2011) Pharmaceutical acrylic beads obtained by suspension polymerization containing cellulose nanowhiskers as excipient for drug delivery. Eur J Pharm Sci 42(4):406–415
Viswanathan G, Murugesan S, Pushparaj V, Nalamasu O, Ajayan PM, Linhardt RJ (2006) Preparation of biopolymer fibers by electrospinning from room temperature ionic liquids. Biomacromolecules 7(2):415–418
Wågberg Decher G, Norgren M, Lindström T, Ankerfors M, Axnäs K (2008) The build-up of polyelectrolyte multilayers of microfibrillated cellulose and cationic polyelectrolytes. Langmuir 24:784–795
Wan C, Jiao Y, Li J (2017) Flexible, highly conductive, and free-standing reduced graphene oxide/polypyrrole/cellulose hybrid papers for supercapacitor electrodes. J Mater Chem A 5(8):3819–3831
Wang T, Drzal LT (2012) Cellulose-nanofiber-reinforced poly (lactic acid) composites prepared by a water-based approach. ACS Appl Mater Interfaces 4(10):5079–5085
Wang B, Sain M, Oksman K (2007a) Study of structural morphology of hemp fiber from the micro to the nanoscale. Appl Compos Mater 14(2):89
Wang N, Ding E, Cheng R (2007b) Thermal degradation behaviors of spherical cellulose nanocrystals with sulfate groups. Polymer 48(12):3486–3493
Wang Q, Zhu J, Gleisner R, Kuster T, Baxa U, McNeil S (2012a) Morphological development of cellulose fibrils of a bleached eucalyptus pulp by mechanical fibrillation. Cellulose 19(5):1631–1643
Wang Q, Zhu JY, Reiner RS, Verrill SP, Baxa U, McNeil SE (2012b) Approaching zero cellulose loss in cellulose nanocrystal (CNC) production: recovery and characterization of cellulosic solid residues (CSR) and CNC. Cellulose 19(6):2033–2047
Wang H, Li D, Zhang R (2013a) Preparation of ultralong cellulose nanofibers and optically transparent nanopapers derived from waste corrugated paper pulp. BioResources 8(1):1374–1384
Wang Q, Zhu JY, Considine JM (2013b) Strong and optically transparent films prepared using cellulosic solid residue recovered from cellulose nanocrystals production waste stream. ACS Appl Mater Interfaces 5(7):2527–2534
Wang R, Guan S, Sato A, Wang X, Wang Z, Yang R, Hsiao BS, Chu B (2013c) Nanofibrous microfiltration membranes capable of removing bacteria, viruses and heavy metal ions. J Membr Sci 446:376–382
Wang Z, Ma H, Hsiao BS, Chu B (2014) Nanofibrous ultrafiltration membranes containing cross-linked poly (ethylene glycol) and cellulose nanofiber composite barrier layer. Polymer 55(1):366–372
Wang R, Chen L, Zhu J, Yang R (2017) Tailored and integrated production of carboxylated cellulose nanocrystals (CNC) with nanofibrils (CNF) through maleic acid hydrolysis. ChemNanoMat 3(5):328–335
Wang Z, Pan R, Sun R, Edström K, Strømme M, Nyholm L (2018) Nanocellulose structured paper-based lithium metal batteries. ACS Appl Energy Mater 1(8):4341–4350
Wang J, Tavakoli J, Tang Y (2019) Bacterial cellulose production, properties and applications with different culture methods- a review. Carbohydr Polym 219:63–76
Wei J, Zhou Y, Lv Y, Wang J, Jia C, Liu J, Zhang X, Sun J, Shao Z (2019) C Carboxymethyl cellulose nanofibrils with a treelike matrix: preparation and behavior of pickering emulsions stabilization. ACS Sustain Chem Eng 7(15):12887–12896
Wu B, Geng B, Chen Y, Liu H, Li G, Wu Q (2017) Preparation and characteriastics of TEMPO-oxidized cellulose nanofibrils from bamboo pulp and their oxygen-barrier application in PLA films. Front Chem Sci Eng 11(4):554–563
Xie H, Du H, Yang X, Si C (2018) Recent strategies in preparation of cellulose nanocrystals and cellulose nanofibrils derived from raw cellulose materials. Int J Polym Sci 2018
Yahya MB, Lee HV, Hamid SBA (2015) Preparation of nanocellulose via transition metal salt-catalyzed hydrolysis pathway. BioResources 10(4):7627–7639
Yang B, Wyman CE (2008) Pretreatment: the key to unlocking low-cost cellulosic ethanol. Biofuels, Bioprod Biorefining Innov Sustain Econ 2(1):26–40
Yang W, Feng Y, He H, Yang Z (2018) Environmentally-friendly extraction of cellulose nanofibers from steam-explosion pretreated sugar beet pulp. Materials 11(7):1160. https://doi.org/10.3390/ma11071160
Yu H, Qin Z, Liang B, Liu N, Zhou Z, Chen L (2013) Facile extraction of thermally stable cellulose nanocrystals with a high yield of 93% through hydrochloric acidhydrolysis under hydrothermal conditions. J Mater Chem A 1(12):3938–3944
Yu H, Yan C, Lei X, Qin Z, Yao J (2014) Novel approach to extract thermally stable cellulose nanospheres with high yield. Mater Lett 131:12–15
Yu H-Y, Zhang H, Song M-L, Zhou Y, Yao J, Ni Q-Q (2017) From cellulose nanospheres, nanorods to nanofibers: various aspect ratio induced nucleation/reinforcing effects on polylactic acid for robust-barrier food packaging. ACS Appl Mater Interfaces 9(50):43920–43938
Zhang J, Elder TJ, Pu Y, Ragauskas AJ (2007) Facile synthesis of spherical cellulose nanoparticles. Carbohydr Polym 69(3):607–611
Zhang L, Tsuzuki T, Wang X (2015) Preparation of cellulose nanofiber from softwood pulp by ball milling. Cellulose 22(3):1729–1741
Zhang K, Sun P, Liu H, Shang S, Song J, Wang D (2016) Extraction and comparison of carboxylated cellulose nanocrystals from bleached sugarcane bagasse pulp using two different oxidation methods. Carbohydr Polym 138:237–243
Zhang T, Cheng Q, Ye D, Chang C (2017a) Tunicate cellulose nanocrystals reinforced nanocomposite hydrogels comprised by hybrid cross-linked networks. Carbohydr Polym 169:139–148
Zhang T, Zuo T, Hu D, Chang C (2017b) Dual physically cross-linked nanocomposite hydrogels reinforced by tunicate cellulose nanocrystals with high toughness and good self-recoverability. ACS Appl Mater Interfaces 9(28):24230–24237
Zhang L, Jia Y, He H, Yin J, Chen R, Zhang C, Shen W, Wang X (2018) Multiple factor analysis on preparation of cellulose nanofiber by ball milling from softwood pulp. BioResources 13(2):2397–2410
Zhao H-P, Feng X-Q, Gao H (2007) Ultrasonic technique for extracting nanofibers from nature materials. Appl Phys Lett 90(7):073112
Zhao Y, Zhang Y, Lindström ME, Li J (2015) Tunicate cellulose nanocrystals: preparation, neat films and nanocomposite films with glucomannans. Carbohydr Polym 117:286–296
Zhou Y, Fuentes-Hernandez C, Khan TM, Liu J-C, Hsu J, Shim JW, Dindar A, Youngblood JP, Moon RJ, Kippelen B (2013) Recyclable organic solar cells on cellulose nanocrystal substrates. Sci Rep 3:1536
Zhou Y, Khan TM, Liu J-C, Fuentes-Hernandez C, Shim JW, Najafabadi E, Youngblood JP, Moon RJ, Kippelen B (2014) Efficient recyclable organic solar cells on cellulose nanocrystal substrates with a conducting polymer top electrode deposited by film-transfer lamination. Org Electron 15(3):661–666
Zhou L, Li N, Shu J, Liu Y, Wang K, Cui X, Yuan Y, Ding B, Geng Y, Wang Z, Duan Y, Zhang J (2018) One-pot preparation of carboxylated cellulose nanocrystals and their liquid crystalline behaviors. ACS Sustain Chem Eng 6(9):12403–12410. https://doi.org/10.1021/acssuschemeng.8b02926
Zhu R, Yadama V (2018) Isolation and characterization of cellulose micro/nanofibrils from douglas fir. J Polym Environ 26(3):1012–1023
Zimmermann T, Pöhler E, Geiger T (2004) Cellulose fibrils for polymer reinforcement. Adv Eng Mater 6(9):754–761
Zu G, Shen J, Zou L, Wang F, Wang X, Zhang Y, Yao X (2016) Nanocellulose-derived highly porous carbon aerogels for supercapacitors. Carbon 99:203–211
Acknowledgments
Authors acknowledge the following funding programs: DST-Biorefinery Program; NRF-CPRR Program; NRF-SA/Australia bilateral and NRF-Incentive Funding.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mokhena, T.C., John, M.J. Cellulose nanomaterials: new generation materials for solving global issues. Cellulose 27, 1149–1194 (2020). https://doi.org/10.1007/s10570-019-02889-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02889-w