Abstract
Graphene has been highlighted in a variety of wearable electronics and smart textiles applications due to its unique properties such as high conductivity, transparency, flexibility and other excellent mechanical performance. Although there have been extensive efforts for graphene based conductive fibers/yarns, there are remaining challenges in terms of the seamless integration between 2D flakes, and reduced charge transport in a lower carrier concentration. Unstable resistance probably arises from the creation of gaps in the conductive parts of the smart textile. Also, regional temperatures can get too high, constituting a fire-safety hazard and endangering the wearer's safety. In this work, the synergistic effect of graphene and flame-retardant materials was investigated, and a conductive fabric was developed which is highly conductive and flame retardancy. Graphene has excellent electrical and thermal conductivity and acts synergistically with traditional flame-retardants on common fabrics. The electrical surface resistivity of hybrid material modified fabrics was as low as 0.54 kΩ/sq, so they could serve as safe and highly conductive conductor in a simple circuit and show excellent wash-ability. The limiting oxygen index of the fabric increased from 19 to 32 after modification in conjunction with the residue at 800 °C increased from 17.9 to 31%, which could be used as safe and highly conductive materials for smart textiles and wearable devices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Smart textiles have increased exponentially in recent years, being used in many applications such as health, sport, automotive, aerospace and military (Grancarić et al. 2018; Gu et al. 2010). To function as smart textiles, electrical conductivity is a fundamental essential (Zahid et al. 2017; Qing et al. 2019; Cataldi et al. 2017). Many studies have been carried out to fabricate conductive textiles according to these demands and demonstrated in various applications (Simorangkir et al. 2018; Atalay et al. 2018; Zhang and Shi 2019; Huang et al. 2016). Unfortunately, the risking of combustion of electrically conductive fabrics in case of unstable resistance was neglected. For functional textiles, such as fireman garments, various flame retardants have been used to improve the flame retardancy and thermal stability of textile materials such as cotton, polyester/cotton, silk and wool fabrics (Chen et al. 2019; Pan et al. 2018; Gashti et al. 2012, 2013a; Gashti and Gashti 2013). However, the flame-retardant treatment of the wearable electronics and smart textiles appears rarely in the literature. The aim of the present work was to impart electrical conductivity and flame retardancy to polyester/cotton fabric which is the most widely used fabric material for apparel and technical applications.
Graphene oxide (GO) is a graphene derivative with many polar groups that can easily bind with fiber surfaces (Qu et al. 2019), and it is hydrophilic and can disperse in water readily (Saxena et al. 2011; Cai et al. 2017). Various reduction methods were developed to restore its excellent thermal conductivity, electrical conductivity, and mechanical performance (Gashti and Almasian 2013; Fathy et al. 2019; Nooralian et al. 2016; Voiry et al. 2016). In recent years, many kinds of conductive fabrics have been prepared using reduced graphene oxide (Ding et al. 2019). A cotton fabric with electrical conductivity was fabricated by the deposition of graphene oxide dispersion and hot press reduction, the sheet resistance of modified cotton fabric reduced to 0.9 kΩ/sq (Ren et al. 2017). Nylon-6 yarns, cotton yarns, polyester yarns, and nonwoven fabrics with conductivity were prepared successfully with the addition of graphene oxide and bovine serum albumin (Yun et al. 2013). Additionally, graphene oxide together with other substances has been employed to improve the flame retardancy of materials (Tian et al. 2016; Liu et al. 2019; Feng et al. 2019). The flame retardancy of polypropylene nanocomposites was improved using graphene/multi-walled carbon nanotubes (Huang et al. 2014). The intumescent flame retardant poly(butylene succinate) composites with good flame retardancy were prepared with the application of graphene (Wang et al. 2011). Thermal stability and flame retardancy of epoxy resin were significantly improved with the addition of graphene nanosheets (Liu et al. 2014).
In this study, graphene oxide and phosphorus flame retardant were chosen to modify polyester/cotton fabric using the conventional dip-immersion method. The structure, electrical resistance, flammability and thermal property of the fabric have been investigated, and a universal, safe, highly conductive textile material was provided to be used in smart textiles and wearable devices.
Materials and methods
Materials
Polyester/cotton fabric (PET/CO65/35, 238 g/m2) was purchased from Qingdao Xu Teng Textile Co., Ltd. Phosphate flame retardant (PFR) was provided by Qingdao Lianmei chemical industry. Aqueous solution of GO (5 g/L) was prepared using flake graphite powder according to modified Hummers’ method (Zhao et al. 2018). Large flake graphite powder was kindly provided by Qingdao Huatai Tech Co., Ltd. Hydrochloric acid (37%), sulfuric acid (98%), and other reagents were purchased from Tianjin Chemical Reagent Co. Ltd. The reducing agent powder was provided by Ji'nan Tian Shuo Chemical Co., Ltd. The adhesive Goon was provided by Jiahong organic silicon technology Co., Ltd. The distilled water was used in the experiment.
Preparation of modified polyester/cotton fabric
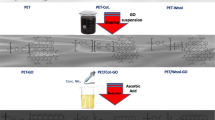
The conventional dip-immersion method was used to modify polyester/cotton fabric. The original fabric (PET/CO), the fabric with reduced graphene oxide (PET/CO-rGO), and the fabric treated with flame retardant (PET/CO-PFR) were chosen as the control samples. The preparation route of the modified fabric (PET/CO-rGO-PFR) was shown in Fig. 1. The fabric was firstly soaked in graphene oxide solution for 2 h to become yellowish-brown and the fabric was squeezed with a small rolling mill to dry at room temperature. Secondly, the fabric was reduced at the temperature of 90 °C for 1.5 h and the color of the fabric changed to black, which was caused by the reduction of graphene oxide. This step could be repeated many times to obtain better electrical conductivity. Finally, the sample was dipped and pressed twice in phosphate flame retardant solution, and dried at 90 °C for 10 min, 150 °C for 3 min. To improve the durability of the modified fabric, the sample was treated in adhesive solution (Goon, 10 wt%) for 30 min and dried at 100 °C for 15 min.
Characterization and measurement
Raman (DXR2, Thermo Scientific, America) spectra were utilized to analyze the G and D peaks of graphene oxide and reduced graphene oxide on the modified fabric. Fourier transform infrared (FTIR) spectra of all samples were obtained by NICOLET iS5 (Thermo Scientific, America) within the wavelength 500–4000 cm−1 (KBr disk). The microstructure of samples was measured by scanning electron microscope (SEM, EVO18, ZEISS, Germany). Four-point probe technique (RTS-8, Probes Tech, China) was utilized to measure the electrical conductivity, the resistances of ten different positions were gauged to obtain the average value.
The limiting oxygen index (LOI) was tested according to ASTMD2863-08 standard, 10 specimens were prepared for each sample with the size of 5 × 15 cm. Burning behaviors of samples were measured by vertical flame test (VFT) based on GB/T5455-1997 with the size of 5 × 12 cm. The thermo gravimetric analysis (TGA, TG209F3, Tarsus, German) was ranged from 50 to 800 °C with the heating rate 10 °C/min.
Results and discussion
Raman spectroscopy and Fourier transform infrared spectroscopy
Raman spectra were utilized to analyze the peaks on PET/CO-GO and PET/CO-rGO. As shown in Fig. 2a, G peak at 1590 cm−1 was higher than D peak at 1310 cm−1 in PET/CO-GO. But the phenomenon is quite different in Fig. 2b, D peak occupied the dominant position and the intensity of D peak was higher than G peak with the ratio of ID/IG was 1.23. It can be attributed to the diminishment of sp2 in average size during reduction process. New graphitic domains established and their increase in number proved the well reduction of GO (Stankovich et al. 2007).
The characteristic peaks of rGO, PFR, PET/CO, PET/CO-rGO, PET/CO-PFR and PET/CO-rGO-PFR were listed in Fig. 2c, d. The peaks of PET/CO appeared at 1709 cm−1, 1238 cm−1, 1104 cm−1, 1025 cm−1 were corresponding to stretching vibration of C=O, asymmetric stretching of aromatic ester, ring asymmetric stretching and stretching vibration of C–O respectively. The peaks between 3600 and 3000 cm−1 represented the hydrogen bonded OH stretching (Carrillo et al. 2004; Alimohammadi et al. 2018; Palaskar et al. 2011). After the final modification, the new peaks appeared at 1667 cm−1, 1545 cm−1, 1420 cm−1, 1238 cm−1, 1025 cm−1, 827 cm−1 corresponding to the bond stretch of C=C, condensed aromatic ring O–H, carboxylic O–H, P–O–C aromatic stretching, C–OH stretching and P–CH2 bond, respectively (Carrillo et al. 2004; Palaskar et al. 2011; Gaan and Sun 2007; Gashti et al. 2013a, b, c; Ren et al. 2007; Shin et al. 1997). It indicated that the flame retardant and rGO were coated on the surface of the fabrics successfully.
Morphology of polyester/cotton fabric
SEM was used to investigate the micromorphology of samples and the images are illustrated in Fig. 3. The surface of PET/CO was smooth without any other substances seen from Fig. 3a1, a2. After modified by rGO, a thin film could be observed vaguely on the surface of the fibers (Fig. 3b1, b2). Being treated by PFR, the surface of fibers was covered with PFR as shown in Fig. 3c1, c2. When reduced graphene oxide and PFR were both introduced to the fabric, the continuous and integrated film was formed on the surface of the fibers showing the effective combination of PFR and rGO (Fig. 3d1, d2).
Electrical surface resistivity
As we all know, polyester/cotton fabric was non-conductive. After once modification, PET/CO-rGO and PET/CO-rGO-PFR showed small electrical surface resistivity 2.104 kΩ/sq and 2.117 kΩ/sq respectively. With the increase of reduction times, the electrical surface resistivity decreased furtherly. After being reduced five times, the electrical surface resistivity of fabric reached 0.551 kΩ/sq and it increased to 0.559 kΩ/sq after adding PFR which indicated that the existence of PFR showed on negative effect on the electrical conductivity. To show the practical application, light bars and button batteries were connected by PET/CO-rGO-PFR on T-shirt (Fig. 4a), the connecting mode of circuit was exhibited in the left bottom of Fig. 4a. When the circuit was switched on, the light bar lightened up.
To improve the washing fastness of PET/CO-rGO-PFR, the adhesive was used after reduction and the electrical surface resistivity changed a little to 0.546 kΩ/sq. The relationship between the electrical surface resistivity and washing cycles was illustrated in Fig. 4b indicating that PET/CO-rGO-PFR had good wash resistance with the existence of adhesive.
Flame retardant property
The images of different samples after Vertical flame test (VFT) were exhibited in Fig. 5a–d. The results showed that PET/CO-PFR and PET/CO-rGO-PFR extinguished themselves, while PET/CO and PET/CO-rGO kept burning after ignition. The introduction of rGO decreased the damage length from 3.7 to 3.3 cm compared with PET/CO-PFR. LOI test was employed to investigate the flame-retardant property of different samples. LOI values of PET/CO, PET/CO-rGO, PET/CO-PFR and PET/CO-rGO-PFR were 19, 20.6, 31, 32, respectively, which indicated that rGO had a synergetic effect on the flame retardancy.
The samples were exerted in the circuit for a long time as shown in Fig. 5e–h. There were smoke and spark of the electrically conductive fabric PET/CO-rGO. After treated by PFR, the circuit operated safely under the same conditions to avoid the combustion.
Thermal property
Thermal decomposing behaviors of four samples were evaluated by thermo gravimetric analysis (TGA). TG and DTG curves were shown in Fig. 6a, b. Figure 6c, d were bar graphs of key data in accordance with TG and DTG curves.
Observing from Fig. 6a, c, the initial decomposition temperature of untreated PET/CO fabric was 274 °C while the temperatures of PET/CO-PFR and PET/CO-rGO-PFR were both 127 °C. The existence of PFR had great effect on the initial decomposition temperature and the addition of rGO did not change the result. The existence of rGO promoted the charring of the fabric and the residues of samples at 800 °C were 17.9%, 30.7%, 31%, respectively.
As shown in Fig. 6b, there were two peaks in the DTG curves. The former corresponded to cotton while the latter to polyester (Rahimi et al. 2011). Figure 6d gave the specific data about DTG curve, the former peaks of the sample treated by PFR and the sample treated by PFR/rGO appeared at 290 °C, which were lower than the original fabric at 355 °C. But the decomposition rate slowed down from 0.37 to 0.23%/°C. As for the second peaks, it slowed down from 1.51 to 1.33%/°C. It indicated that PFR lowered the weight loss rate and the addition of rGO promoted it furtherly. It can be interpreted as that phosphorus had a positive effect on flame retardancy of cellulose fibers including cotton and promoted the formation of carbon (El-Shafei et al. 2015). On the other hand, the reduced graphene oxide affected the flame retardancy of polyester for the char formation (Palaskar et al. 2011; Huang et al. 2012).
Conclusions
In order to develop a non-ignitable electrically conductive fabrics, reduced graphene oxide and phosphate flame retardant were used to modify polyester/cotton fabric. There was cooperative effect between reduced graphene oxide and the flame retardant. Low electrical surface resistivity as 0.54 kΩ/sq was achieved, and the introduction of flame-retardant and adhesive had little effect on the surface resistivity. Also, the modified polyester/cotton fabric had good fastness to washing. The existence of rGO improved the flame retardancy compared with the fabric using PFR only. The value of LOI increased from 19 to 32 and the vertical flame test indicated that the damage length reduced to 3.3 cm during the ignition time, while the original fabric got ruined entirely. TG and DTG curves showed that thermal stability of fabric improved significantly with a decrease in the maximum mass lose rate.
References
Alimohammadi F, Gashti MP, Mozaffari A (2018) Polyvinylpyrrolidone/carbon nanotube/cotton functional nanocomposite: preparation and characterization of properties. Fiber Polym 19:1940–1947
Atalay O, Atalay A, Gafford J, Walsh C (2018) A Highly sensitive capacitive-based soft pressure sensor based on a conductive fabric and a microporous dielectric layer. Adv Mater Technol 3:1700237–1700244
Cai G, Xu Z, Yang M, Tang B, Wang X (2017) Functionalization of cotton fabrics through thermal reduction of graphene oxide. Appl Surf Sci 393:441–448
Carrillo F, Colom X, Sunol JJ, Saurina J (2004) Structural FTIR analysis and thermal characterisation of lyocell and viscose-type fibres. Eur Polym J 40:2229–2234
Cataldi P, Ceseracciu L, Athanassiou A, Bayer IS (2017) Healable cotton–graphene nanocomposite conductor for wearable electronics. ACS Appl Mater Interfaces 9:13825–13830
Chen T, Hong J, Peng C, Chen G, Yuan C, Xu Y, Dai L (2019) Superhydrophobic and flame-retardant cotton modified with DOPO and fluorine-silicon-containing crosslinked polymer. Carbohydr Polym 208:14–21
Ding X, Lei S, Du C, Xie Z, Li J, Huang X (2019) Small-sized CuS nanoparticles/N, S co-doped rGO composites as the anode materials for high-performance lithium-ion batteries. Adv Mater Interfaces 6:1900038
El-Shafei A, El-Shemy M, Abou-Okeil A (2015) Eco-friendly finishing agent for cotton fabrics to improve flame retardant and antibacterial properties. Carbohydr Polym 118:83–90
Fathy M, Moghny TA, Mousa MA (2019) Fast and fully scalable synthesis of graphene oxide from cellulose by catalytic acid spray method. Arab J Sci Eng 44:305–313
Feng Y, Li X, Zhao X, Ye Y, Zhou X, Liu H, Xie X (2019) Synergetic improvement in thermal conductivity and flame retardancy of epoxy/silver nanowires composites by incorporating “Branch-Like” flame-retardant functionalized graphene. ACS Appl Mater Interfaces 10:21628–21641
Gaan S, Sun G (2007) Effect of phosphorus and nitrogen on flame retardant cellulose: a study of phosphorus compounds. J Anal Appl Pyrolysis 78:371–377
Gashti MP, Almasian A (2013) Citric acid/ZrO2 nanocomposite inducing thermal barrier and self-cleaning properties on protein fibers. Composites B 52:340–349
Gashti MP, Gashti MP (2013) Effect of colloidal dispersion of clay on some properties of wool fiber. J Dispers Sci Technol 34:853–858
Gashti MP, Navid MY, Rahimi MH (2012) Coating of macroemulsion and microemulsion silicones on poly (ethylene terephthalate) fibers: Evaluation of the thermal properties and flammability. J Appl Polym Sci 125:1430–1438
Gashti MP, Navid MY, Rahimi MH (2013a) Effects of coating of nano-and microemulsion silicones on thermal properties and flammability of polyethylene terephthalate textile. Pigm Resin Technol 42:34–44
Gashti MP, Pournaserani A, Ehsani H, Gashti MP (2013b) Surface oxidation of cellulose by ozone-gas in a vacuum cylinder to improve the functionality of fluoromonomer. Vacuum 91:7–13
Gashti MP, Elahi A, Gashti MP (2013c) UV radiation inducing succinic acid/silica–kaolinite network on cellulose fiber to improve the functionality. Composites B 48:158–166
Grancarić AM, Jerković I, Koncar V, Cochrane C, Kelly FM, Soulat D, Legrand X (2018) Conductive polymers for smart textile applications. J Ind Text 48:612–642
Gu JF, Gorgutsa S, Skorobogatiy M (2010) Soft capacitor fibers using conductive polymers for electronic textiles. Smart Mater Struct 19:1–13
Huang G, Gao J, Wang X, Liang H, Ge C (2012) How can graphene reduce the flammability of polymer nanocomposites? Mater Lett 66:187–189
Huang G, Wang S, Song P, Wu C, Chen S, Wang X (2014) Combination effect of carbon nanotubes with graphene on intumescent flame-retardant polypropylene nanocomposites. Composites A 59:18–25
Huang G, Liu L, Wang R, Zhang J, Sun X, Peng H (2016) Smart color-changing textile with high contrast based on a single-sided conductive fabric. J Mater Chem C 4:7589–7594
Liu S, Yan H, Fang Z, Wang H (2014) Effect of graphene nanosheets on morphology, thermal stability and flame retardancy of epoxy resin. Compos Sci Technol 90:40–47
Liu L, Chen Y, Liu H, Rehman HU, Chen C, Kang H, Li H (2019) A graphene oxide and functionalized carbon nanotube based semi-open cellular network for sound absorption. Soft Matter 15:2269–2276
Nooralian Z, Gashti MP, Ebrahimi I (2016) Fabrication of a multifunctional graphene/polyvinylphosphonic acid/cotton nanocomposite via facile spray layer-by-layer assembly. RSC Adv 6:23288–23299
Palaskar S, Kale KH, Nadiger GS, Desai AN (2011) Dielectric barrier discharge plasma induced surface modification of polyester/cotton blended fabrics to impart water repellency using HMDSO. J Appl Polym Sci 122:1092–1100
Pan Y, Liu L, Wang X, Song L, Hu Y (2018) Hypophosphorous acid cross-linked layer-by-layer assembly of green polyelectrolytes on polyester-cotton blend fabrics for durable flame-retardant treatment. Carbohydr Polym 201:1–8
Qing X, Wang Y, Zhang Y, Ding X, Zhong W, Wang D, Lu Z (2019) Wearable fiber-based organic electrochemical transistors as a platform for highly sensitive dopamine monitoring. ACS Appl Mater Interfaces 11:13105–13113
Qu J, He N, Patil SV, Wang Y, Banerjee D, Gao W (2019) Screen printing of graphene oxide patterns onto viscose nonwovens with tunable penetration depth and electrical conductivity. ACS Appl Mater Interfaces 11:14944–14951
Rahimi MH, Parvinzadeh M, Navid MY, Ahmadi S (2011) Thermal characterization and flammability of polyester fiber coated with nonionic and cationic softeners. J Surfactants Deterg 14:595–603
Ren H, Sun J, Wu B, Zhou Q (2007) Synthesis and properties of a phosphorus-containing flame retardant epoxy resin based on bis-phenoxy (3-hydroxy) phenyl phosphine oxide. Polym Degrad Stab 92:956–961
Ren J, Wang C, Zhang X, Carey T, Chen K, Yin Y, Torrisi F (2017) Environmentally-friendly conductive cotton fabric as flexible strain sensor based on hot press reduced graphene oxide. Carbon 111:622–630
Saxena S, Tyson TA, Shukla S, Negusse E, Chen H, Bai J (2011) Investigation of structural and electronic properties of graphene oxide. Appl Phys Lett 99:1838–1845
Shin S, Jang J, Yoon SH, Mochida I (1997) A study on the effect of heat treatment on functional groups of pitch based activated carbon fiber using FTIR. Carbon 35:1739–1743
Simorangkir RB, Yang Y, Esselle KP, Zeb BA (2018) A. Method to realize robust flexible electronically tunable antennas using polymer-embedded conductive fabric. IEEE Trans Antennas Propag 66:50–58
Stankovich S, Dikin DA, Piner RD, Kohlhaas KA, Kleinhammes A, Jia Y, Ruoff RS (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45:1558–1565
Tian M, Hu X, Qu L, Du M, Zhu S, Sun Y, Han G (2016) Ultraviolet protection cotton fabric achieved via layer-by-layer self-assembly of graphene oxide and chitosan. Appl Surf Sci 377:141–148
Voiry D, Yang J, Kupferberg J, Fullon R, Lee C, Jeong HY, Chhowalla M (2016) High-quality graphene via microwave reduction of solution-exfoliated graphene oxide. Science 353:1413–1416
Wang X, Song L, Yang H, Lu H, Hu Y (2011) Synergistic effect of graphene on antidripping and fire resistance of intumescent flame-retardant poly (butylene succinate) composites. Ind Eng Chem Res 50:5376–5383
Yun Y, Hong W, Kim WJ, Jun Y, Kim BH (2013) A novel method for applying reduced graphene oxide directly to electronic textiles from yarns to fabrics. Adv Mater 25:5701–5705
Zahid M, Papadopoulou EL, Athanassiou A, Bayer IS (2017) Strain-responsive mercerized conductive cotton fabrics based on PEDOT: PSS/graphene. Mater Des 135:213–222
Zhang X, Shi M (2019) Flame retardant vinylon/poly (m-phenylene isophthalamide) blended fibers with synergistic flame retardancy for advanced fireproof textiles. J Hazard Mater 365:9–15
Zhao H, Tian M, Hao Y, Qu L, Zhu S, Chen S (2018) Fast and facile graphene oxide grafting on hydrophobic polyamide fabric via electrophoretic deposition route. J Mater Sci 53:9504–9520
Acknowledgments
The authors would like to acknowledge the financial assistance provided by National Key R&D Program of China (No. 2017YFB0309000) and National Natural Science Foundation of China (No. 51672141).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, Y., Wang, J., Li, Z. et al. Washable, durable and flame retardant conductive textiles based on reduced graphene oxide modification. Cellulose 27, 1763–1771 (2020). https://doi.org/10.1007/s10570-019-02884-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02884-1