Abstract
A new Pd catalyst chemically bonded to cellulose nanocrystals surfaces (CNC-BIA-Pd) was developed. 2-(1H-Benzo[d]imidazol-2-yl) aniline (BIA), tosylated cellulose nanocrystals (CNC-TOS) and PdCl2 were the reactants for the synthesis of the new catalyst designed to be used for the Ullmann reaction. The modified cellulose nano catalyst was characterized with various analytical tools. The catalyst had excellent selectivity and activity in a short reaction time (45 min) and outstanding TON (490) and TOF (1960) values. The nano catalyst was determined to be reusable for at least eight cycles. The CNC-BIA-Pd catalyst indicated excellent catalytic efficiency for the Ullmann C–O reaction when it was compared to commercial palladium catalysts.
Graphical abstract
A new Pd catalyst chemically bonded to cellulose nanocrystal surfaces (CNC-BIA-Pd) was developed and used for the Ullmann reactions. The catalyst had excellent selectivity and activity in a short reaction time and outstanding TON (490) and TOF (1960) values. The nano catalyst was determined to be reusable for at least eight cycles.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Ullmann reaction, after being introduced in 1901, has been used widely for C–C bonding between two aromatic nuclei using copper as a catalyst (Handa et al. 2015; Altenhoff et al. 2003). Ni and Pd were used as catalysts in later modifications of this reaction for aryl bromides or chlorides (Snelders et al. 2009; Pratap et al. 2009; Martin and Buchwald 2008). Different catalysts for the Ulmann reaction have been reported using palladium complexes (Hennings et al. 1999) which usually use several reducing agents such as zinc (Qafisheh et al. 2002), sodium formate (Arcadi et al. 1990) and trimethylamine (Han et al. 2000). Despite good efficiency of these catalysts in the Ulmann reaction, purification of products is reported to be challenging (Jiang and Cai 2007) due to the homogeneous reaction conditions. To solve this problem, heterogeneous catalysts have been developed to simplify their recovery and reusability (Li et al. 2017). Some polymer-supported transition metal catalysts are silica-supported metallocene/MAO (Grasa et al. 2002), silica‐supported zirconocene (Bourissou et al. 2000), silica-supported metals (Baran et al. 2017), layered double hydroxide supported nano palladium and clay-supported catalyst (Tehrani and Basiryan 2015). The main heterogeneous catalysts are expensive, not biodegradable, and unstable thermodynamically, and tend to aggregate in bulk metal form.
Cellulose nanocrystals (CNC) are an abundant, accessible, highly crystalline biopolymer that can be extracted from cellulosic fibers like wood, cotton, non-wood plants, agricultural residues, bacteria and algae (Tehrani and Basiryan 2015; Fraschini et al. 2014; Brito et al. 2012; Lopez et al. 2010; Favier et al. 1995; Le Normand et al. 2014; Silvério et al. 2013). Production, modification and application of cellulose nanocrystals have been considered by researchers and companies in the last decade.
Cellulose nanocrystals have been reported as a substrate for metal containing nano catalysts due to their high surface area, active surface functional groups, water suspension ability, high crystallinity, chirality, high mechanical strength, renewability, biodegradability, and non-toxicity (Yan et al. 2012; Roman 2015; Yanamala et al. 2014; Kümmerer et al. 2011; Kaushik and Moores 2016). Cellulose nanocrystals indicate un-conventional colloidal behavior in liquid form which can be useful for different applications (Kovacs et al. 2010; Moon et al. 2011; Habibi et al. 2010; Lin and Dufresne 2014). Using nano-cellulose as a heterogeneous support has been reported for different catalysts like palladium, platinum, copper, nickel and silver nano-particles (Keshipour and Adak 2016; Keshipour and Khalteh 2016; Kaushik and Moores 2016; Alesi et al. 2008; Cirtiu et al. 2011; Huang et al. 2014; Reddy et al. 2006). Cellulose fibers were reported as a substrate for nano palladium (0) for the Suzuki reaction (Fu et al. 2015; Jamwal et al. 2011; Hu et al. 2016), Heck reaction (Keshipour et al. 2013; Li et al. 2017; Cirtiu et al. 2011), the Ullmann cross-coupling reaction (Zhou et al. 2012), and Au-coupling reaction (Huang et al. 2013).
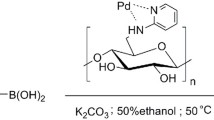
We recently developed a nano catalyst for the formation of C–O bonds between activated aryl halides and phenol derivatives (Khalilzadeh et al. 2011, 2014; Keipour et al. 2016; Salmanpour et al. 2013). In our continued efforts towards the development of heterogeneous catalysts for selective organic transformations, our hypothesis is that cellulose nanocrystals not only can be used as a substrate for our catalyst but that it can enhance catalytic reactions due to its special colloidal behavior. Therefore, in this research we developed nano-cellulose 2-(1H-Benzo[d]imidazol-2-yl) aniline (CNC-BIA-Pd) as a strong and novel nano-catalyst for organic chemistry. As a part of this research, activated and inactivated phenols and aryl halides were reacted using the developed catalyst in DMSO at 80 °C to synthesize diaryl ether with short reaction times (15–60 min). The design and procedure of the catalyst preparation is illustrated in Scheme 1.
Experimental
Chemicals and instruments
All chemicals were purchased from Merck and Fluka and used as received. A Vector 22-Bruker FT-IR was used for Fourier transform infrared (FT-IR) in the range of 400–4000 cm−1 at room temperature. The X-ray diffraction (XRD) of the Nano-cellulose and its modified catalyst prepared as powder and were measured with a Philips PW 1830 X-ray diffractometer with Cu Kα source (λ = 1.5418 Å). The data sets were collected in reflection geometry in the range of 10° ≤ 2θ ≤ 80° at room temperature. Thermo gravimetric analysis (TGA) was carried out on a Stanton Red Craft STA-780 (London, UK) using N2 and O2 as carrier gas with a temperature ramp of 10 °C/min from room temperature to 650 °C. A CHNS/O analyzer (Vario Micro cube, Elemental Analysis system (GmbH, Hanau, Germany) was employed for the determination of elemental analysis with helium as the carrier gas. The morphology of the prepared nano catalysts was investigated using scanning electron microscopy (SEM; EM-3200, KYKY) followed by determination of the elemental composition by energy dispersive X‐ray spectrometry (EDX). Morphology of nano catalysts was carried out with transmission electron microscopy (TEM; EM-10C, ZEISS). The deposited amount of palladium nanoparticles was determined by inductively coupled plasma-atomic emission spectroscopy (ICP-AES) (Perkin Elmer Optima 2000 DV ICP-AES). X-ray photoelectron spectroscopy (XPS) measurements were recorded on an electron spectrometer XPS/UVS—SPECS System with PHOIBOS 150 analyzer equipped with Al/Mg Kα radiation. NMR spectra of products were recorded with a Bruker DRX-400 AVANCE instrument (400.1 MHz for 1H, 100.6 MHz for 13C) in DMSO-d6 as solvent.
Preparations of cellulose nanocrystals (CNC) (1)
Cellulose nanocrystals were prepared by acidic hydrolysis of Whatman filter paper (#1) as reported in the literature (Sadeghifar et al. 2011). An amount of 2 g of cellulose fiber was heated in 100 mL of 2.5 M HBr for 3 h at 100 °C under ultra-sonication. The hydrolyzed fibers were diluted with deionized water followed by centrifugation. The washing/centrifugation cycles were repeated five times to remove excess acid and water-soluble fragments. The fine cellulose nanoparticles started to disperse in the aqueous supernatant after reaching a pH around 5 and then were collected using centrifugation at 12,000 RPM for 60 min. The product was kept in a refrigerator without drying.
Tosylation of cellulose nanocrystals yielding CNC-Tos (2)
CNC-Tos was synthesized using a reported method (Sadeghifar et al. 2011; Feese et al. 2011). An amount of 0.5 g of non-dried cellulose nanocrystals was washed with pyridine five times to replace water with pyridine. The mixture in pyridine was stirred for 2 days at room temperature after the addition of tosyl chloride (0.9 g, 5 mmol). An amount of 100 mL of ethanol was added to the reaction mixture and precipitated material was collected by filtration. The product was washed with ethanol (50 mL) five times and kept in a refrigerator without drying (Scheme 1, CNC-Tos).
Preparation of CNC-BIA (4)
An amount of 400 mg of CNC-Tos in methanol was washed and centrifuged three times with DMF to exchange methanol with DMF. An amount of 400 mg of 2-(1H-Benzo[d]imidazol-2-yl) aniline (BIA) was added to the mixture and stirred for 24 h at 100 °C. An amount of 50 mL of deionized water was added to the reaction mixture to recover precipitated product using centrifugation. The product was washed with ethanol and water several times to purify the final product. Purified product was then washed with DMF three times to replace the water and the washed product was kept in a refrigerator (Scheme 1, CNC-BIA).
Preparation of CNC-BIA-Pd catalyst
An amount of 0.50 g of CNC-BIA in DMF was added to a solution of PdCl2 (0.10 g, 0.45 mmol) in 10 mL of DMF under N2 atmosphere and the mixture was stirred for 24 h at 60 °C. After completion of the reaction, the mixture was cooled to room temperature and the resulting product was collected by filtration. The obtained solid black colored product was washed carefully with ethanol (2 × 25 mL), then with diethyl ether (2 × 25 mL) and then three times with distilled water (3 × 25 mL), and finally dried in a vacuum oven at room temperature (Scheme 1, CNC-BIA-Pd).
General procedure of Ullman reaction of aryl halides with phenols by nano catalyst
In a mixture of aryl halide (1 mmol) and phenol derivatives (1.2 mmol) in DMSO (2.5 mL) specific amounts of base and 3.8 mg of CNC-BIA-Pd (0.2 mol%) as nano catalyst were added. The mixture was stirred at 80 °C for different times. Finally, the solid catalyst was filtered and washed carefully with distilled water and absolute ether. To recover the Ullman reaction product, the solution was vaporized and the residue solid was purified by plate chromatography on silica gel and characterized with FT-IR, 13C NMR and 1H NMR techniques (Scheme 2).
Results and discussion
Evaluation of catalyst preparation
The focus of the present report is the preparation of cellulose nanocrystals and its surface modification with a catalyst to be used in the Ullmann coupling reaction. Evidence of cellulose nanocrystals preparation was indicated by TEM images (Fig. 1).
Due to the use of hydrobromic acid for nano-cellulose preparation, there are no sulfonated groups on the cellulose surface which are usually created when using sulfuric acid. The absence of sulfonate groups on the surface of the cellulose nanocrystals increases the thermal and chemical stability of the material.
The new catalyst was prepared using surface modification of cellulose nanocrystals through chemical bonding with 2-(1H-Benzo[d]imidazol-2-yl) aniline (BIA). Chemical bonding of catalyst on the cellulose nanocrystal surface should provide longer lasting catalyst with less leaching. It also should prevent catalyst aggregation. The covalent attachment of the ligand on the cellulose nanocrystals surface was achieved via surface tosylation of CNC (1) with tosyl chloride in DMF to prepare CNC-Tos (2) followed by reaction with 2-(1H-Benzo[d]imidazol-2-yl) aniline (BIA) yielding CNC-BIA (3). In the final step, PdCl2 solution was reacted with CNC-BIA in DMF solution to prepare the final CNC-BIA-Pd (4) catalyst.
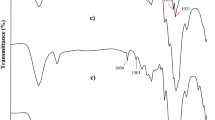
Figure 2 indicates FT-IR spectra of CNC, CNC-Tos and CNC-BIA-Pd. The absorption peaks at 1060 cm−1 and 1373 cm−1 reveal vibration of C–O–C in pyranose ring of glucose in cellulose nanocrystals structure and C–H vibrations, respectively. The absorption bands at 3446 cm−1 and 2998 cm−1 were assigned to the O–H and C–H stretching vibrations, respectively (Baran et al. 2017). Sulfonyl symmetrical and unsymmetrical stretching vibration in tosylated cellulose nanocrystals (CNC-Tos) are visible at 1163 cm−1 and 1350 cm−1 respectively. Absorption bands at 1440 cm−1 and 1543 cm−1 confirm the aromatic structure of the tosyl group (Tehrani and Basiryan 2015) in the sample. After the amination process by 2-(1H-Benzo[d]imidazol-2-yl) aniline, hydroxyl groups on the CNC surface were converted to amino groups. Therefore, the absorption bands of sulfonyl groups at 1163 cm−1 and 1350 cm−1 were not apparent in the FT-IR spectrum of the CNC-BIA-Pd (Yan et al. 2012). New absorption peaks at 1653 and 1260 cm−1 are related to the N (sp2) and N (sp3)–C bonds of the stretching vibration and the vibration in CNC-BIA-Pd. In addition, the intensity of the absorption band at 650 cm−1 that is assigned to the bending vibration of C–H in the pyridine heterocyclic ring is reduced after formation of the CNC-BIA complex with Pd (Hu et al. 2016). The FT-IR spectra confirmed the grafting of 2-(1H-Benzo[d]imidazol-2-yl) aniline (BIA) as ligand connected to Pd metal on cellulose nanocrystals.
Figure 3 indicates a comparison of crystal structures of pure cellulose nanocrystals and its surface modified form with catalyst (CNC-BIA-Pd). The pattern for prepared CNC is very much for a mixture of cellulose I and II, with strong peaks at 12° and 20° [the (020) cellulose II peak at 22° is merged with the (200) cellulose I peak at 22.5°] (French 2014). Relative crystallinity was calculated from the intensity measurements using the Segal method (Segal et al. 1959) (Eq. 1).
where I200 represents the maximum intensity of (200) lattice diffraction peak at a diffraction angle around 2θ = 22.5°, IAM represents the intensity scattered by the amorphous component in the sample, evaluated as the lowest intensity at 18°. The crystallinity of the unmodified cellulose nano particles was around 76% which is in agreement with a previously reported result (Sadeghifar et al. 2011). However, the calculated crystallinity number is only an estimate due to the strong cellulose II (110) peak at around 20°. The CNC maintained its morphology and crystal structure after modification, which is important to maintain the surface area, thermal and physical properties, and colloidal properties of the material. The crystalline structure of palladium is also clearly visible in the modified cellulose nanocrystals as evidenced by the presence of catalyst on the surface. The index peaks at 2θ = 40.0°, 46° and 68° are ascribed to diffractions from various lattice planes of (111), (200) and (220) present in the cubic Palladium (Hu et al. 2016).
To have an estimation of catalyst loading on the surface of cellulose nanocrystals, nitrogen content determination, EDX and thermal analysis were carried out. Nitrogen content in the product was 2.1% or 0.021 g of nitrogen per gram of final product. Considering the presence of three nitrogen atoms in the prepared catalyst structure, the amount of catalyst should be around 4.4 × 10−4 mol ((0.021/3)/15)) per each gram of the product.
Thermo-gravimetric analysis (TGA) on the cellulose nanocrystals and the final product in nitrogen and oxygen atmospheres are indicated in Fig. 4a, b, respectively. Under both gasses, modified cellulose nanocrystals indicated stability up to 220 °C, which makes the material possible to be used for high temperature reactions. After the degradation of samples at temperatures up to 550 °C in nitrogen atmosphere, the modified cellulose nanocrystals indicated more char than the pure cellulose nanocrystals. The remaining char in cellulose nanocrystals was 24% whereas in the modified sample it was around 31% (Fig. 4a). The modified samples contain catalyst with high levels of carbon and Pd, which leads to higher char mass after thermal degradation.
Figure 4b illustrates the sample behavior after heating using oxygen gas, which combusts all organic materials and leaves only minerals such as Pd for this study. The remaining sample weight after burning in oxygen for pure cellulose nanocrystals and the modified sample were zero and 4.7% respectively. An amount of 4.7% Pd in the product indicates the presence of 0.047 g Pd/g product. Due to presence of one atom of Pd in the catalyst, the mole of Pd in each gram of the prepared catalyst was calculated to be around 4.4 × 10−4 (0.047/106.4) which is very close to the number calculated with nitrogen content.
SEM images (Fig. 5) indicate precipitation of nano catalyst on the cellulose nanocrystals surface. After functionalization of CNC with 2-(1H-Benzo[d]imidazol-2-yl) aniline (BIA) connected to Pd, particles with nano size were observed on the CNC-BIA-Pd (final product).
To obtain additional insight into the shapes and particle sizes of the prepared nano catalyst, transmission electron microscopy (TEM) was used. As clearly indicated in Fig. 6, dark areas in the images revealed Pd grafted to 2-(1H-Benzo[d]imidazol-2-yl) aniline (BIA) on the surface of the cellulose nanocrystals.
EDX was used to determine the elemental compositions of the CNC-BIA-Pd (Fig. 7). The obtained results indicate the presence of C, O and Pd in the prepared catalyst. It can be concluded that Pd nano particles were embedded into 2-(1H-Benzo[d]imidazol-2-yl) aniline (BIA) inserted on the surface of cellulose nanocrystals. These observations confirm that chemical modification of the CNC was achieved.
The ICP-AES analysis was performed to determine the amount of Pd (56,140 mg/Kg) in CNC-BIA-Pd as catalyst. The inductively coupled plasma–atomic emission spectroscopy (ICP-AES) analysis showed the weight percentage of the Pd to be 5.2%, in agreement with results obtained from thermal analysis and CHN.
The XPS spectrum of the Pd nanoparticles, dispersed on CNC-BIA-Pd for the Pd 3d region is presented in Fig. 8. The results show that for the binding energies of Pd 3d5/2, two peaks are observed at about 334.5 and 337.8 eV, and for the Pd 3d3/2 there are also two peaks at about 341.4 and 343.1 eV, respectively. This indicates that the Pd are in both forms, metallic state Pd(0) and Pd(II), simultaneously on the CNC-BIA-Pd catalyst (Gniewek et al. 2005; Narayana et al. 1985).
A possible mechanism of reaction between cellulose and catalyst is proposed in Scheme 3 (Wu et al. 2013). It is believed that the electron-rich feature of the hydroxyl groups in the cellulose structure reduce PdCl2 to Pd NPs. In the next step the functional groups on the CNC surface containing oxygen by electrostatic interaction act as anchor points to immobilize Pd NPs. The BIA in this catalyst acts as homogeneous distributer of Pd(II) on the CNC surface after reduction.
Ullmann coupling reaction using CNC-BIA-Pd catalyst
To evaluate the performance of the developed catalyst in the Ullmann reaction, the effects of catalyst loading, solvents, temperature, base and reaction times were investigated on a Carbon–Oxygen reaction. A reaction optimization was carried out using phenol and 4-nitro iodo benzene as reactants and CNC-BIA-Pd as catalysts. Table 1 indicates the effects of catalyst loading, temperature and solvents on the reaction efficiency. The best reaction efficiency (96%) occurred when using 0.2 mol% of catalyst, using 3.75 mmol of K2CO3 as the base at 80 °C for 45 min reaction time in the presence of DMSO as a solvent (Table 1).
Generally, the Ullmann reaction indicates weak performance when using active phenols with a withdrawing group (EWG) and inactive aryl halides (with electron donor group). However, CNC-BIA-Pd catalyst indicated better results compared with the classic and improved Ullmann catalysts when using inactive aryl halides and inactive phenols (Banwell et al. 2011).
Moreover, the effect of the substitute groups of the substrate on the yields of the Ullmann coupling reactions using the developed catalyst was examined, and the yields were in the order of para > ortho > meta. Additionally, the order of the catalytic performance of the substrates (containing aryl bromide, aryl chloride and aryl iodide) was determined as follows: I > Br > Cl. All results are in agreement with the previous reported results for Ullmann reaction (Baran et al. 2017).
Turn over number (TON) and turn over frequency (TOF) values were calculated for all Carbon–Oxygen Ullmann reactions, and the results are listed in Table 2. The results indicated that CNC-BIA-Pd catalyst gave remarkable TON and TOF values with small loading of the catalyst in a short time. These values indicated that the catalyst can be used efficiently for different Ullmann coupling reactions.
In addition, the catalytic efficiency of CNC-BIA-Pd catalyst was evaluated against different commercial palladium salts with the model reaction under optimum conditions (Table 2). These tests indicated that the CNC-BIA-Pd catalyst had higher catalytic activity as well as TON and TOF values than the commercial palladium catalysts. All of the synthesized compounds were further identified using the GC/MS and 1H NMR techniques and their spectra are presented in supplementary data.
Another important issue concerning the application of a heterogeneous catalyst is its reusability and stability under reaction conditions. To gain insight into this issue, catalyst recycling experiments were carried out using the Ullmann reaction of 4-nitro iodobenzene and phenol over CNC-BIA-Pd. The results are tabulated in Table 3.
After each cycle, the catalyst was filtered off, washed with water, diethyl ether and acetone. Then it was dried in an oven at 60 °C and reused in the Ullmann reaction. The results indicated that CNC-BIA-Pd could be reused without losing its effectiveness eight times. It should be mentioned that there was low Pd leaching (about 5%) during the reaction and the catalyst exhibited high stability even after eight cycles (Table 3).
Conclusion
A new Pd catalyst bonded to cellulose nanocrystals was developed (CNC-BIA-Pd) and characterized by different spectroscopic methods. The amount of bonded catalyst was calculated as 0.031 mol per each mole of glucose unit in the cellulose nanocrystals. The modified cellulose nanocrystal based catalyst showed thermal stability up to 220 °C which makes it possible to be used for high temperature reactions. The catalytic performance of the catalyst was tested for the Ullmann reaction for aryl fluoride, chloride, bromide and iodides at 80 °C. The highest conversion yield (96%) was obtained at 80 °C with DMSO as solvent using 3.75 mmol K2CO3 as base. The best conversion yield was obtained with 0.2 mol% catalyst for each mole of substrate. The effect of the substitute groups on the substrate on the Ullmann coupling reactions yield was determined and displayed efficiency in the order of para > ortho > meta. The order of the catalytic performance on the substrates (containing aryl bromide, aryl chloride and aryl iodide) was shown to be in the order of I > Br > Cl. The developed catalyst indicated high TON (490) and TOF (1960) values. The reusability of the catalyst activity was tested and the catalytic performance remained high after eight cycles of use. In conclusion, the developed CNC-BIA-Pd catalyst indicated high thermal stability, reusability, and high product yields in Ullmann reactions using small catalyst loadings.
References
Alesi S, Di Maria F, Melucci M, Macquarrie DJ, Luque R, Barbarella G (2008) Microwave-assisted synthesis of oligothiophene semiconductors in aqueous media using silica and chitosan supported Pd catalysts. Green Chem 10:517–523
Altenhoff G, Goddard R, Lehmann CW, Glorius F (2003) An N-heterocyclic carbene ligand with flexible steric bulk allows Suzuki cross-coupling of sterically hindered aryl chlorides at room temperature. Angew Chem Int Ed 42:3690–3693
Arcadi A, Burini A, Cacchi S, Delmastro M, Marinelli F, Pietroni B (1990) The palladium-catalyzed cross coupling of vinyl and aryl triflates with 2-furylzinc chloride: an efficient route to 2-vinyl-and 2-arylfurans. Synlett 1990:47–48
Banwell MG, Jones MT, Reekie TA (2011) The palladium-catalysed Ullmann cross-coupling reaction. Chem NZ 75:122–127
Baran NY, Baran T, Menteş A (2017) Fabrication and application of cellulose Schiff base supported Pd(II) catalyst for fast and simple synthesis of biaryls via Suzuki coupling reaction. Appl Catal A Gen 531:36–44
Bourissou D, Guerret O, Gabbai FP, Bertrand G (2000) Stable carbenes. Chem Rev 100:39–92
Brito BS, Pereira FV, Putaux JL, Jean B (2012) Preparation, morphology and structure of cellulose nanocrystals from bamboo fibers. Cellulose 19:1527–1536
Cirtiu CM, Dunlop-Briere AF, Moores A (2011) Cellulose nanocrystallites as an efficient support for nanoparticles of palladium: application for catalytic hydrogenation and Heck coupling under mild conditions. Green Chem 13:288–291
Favier V, Chanzy H, Cavaille JY (1995) Polymer nanocomposites reinforced by cellulose whiskers. Macromolecules 28:6365–6367
Feese E, Sadeghifar H, Gracz HS, Argyropoulos DS, Ghiladi RA (2011) Photobactericidalporphyrin-cellulose nanocrystals: synthesis, characterization, and antimicrobial properties. Biomacromol 12:3528–3539
Fraschini C, Chauve G, Le Berre JF, Ellis S, Méthot M, O’Connor B, Bouchard J (2014) Critical discussion of light scattering and microscopy techniques for CNC particle sizing. Nord Pulp Pap Res J 29:31–40
French AD (2014) Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 21:885–896
Fu J, Li D, Li G, Huang F, Wei Q (2015) Carboxymethyl cellulose assisted immobilization of silver nanoparticles onto cellulose nanofibers for the detection of catechol. J Electroanal Chem 738:92–99
Gniewek A, Trzeciak AM, Ziolkowski JJ, Kepinski L, Wrzyszcz J, Tylus W (2005) Pd-PVP colloid as catalyst for Heck and carbonylation reactions: TEM and XPS studies. J Catal 229:332–343
Grasa GA, Viciu MS, Huang J, Zhang C, Trudell ML, Nolan SP (2002) Suzuki–Miyaura cross-coupling reactions mediated by palladium/imidazolium salt systems. Organometallics 21:2866–2873
Habibi Y, Lucia LA, Rojas OJ (2010) Cellulose nanocrystals: chemistry, self-assembly, and applications. Chem Rev 110:3479–3500
Han Y, Roy A, Giroux AA (2000) Divergent protocol for solid-phase synthesis of highly substituted Bi-aryl furan derivatives. Tetrahedron Lett 41:5447–5451
Handa S, Wang Y, Gallou F, Lipshutz BH (2015) Sustainable Fe–ppm Pd nanoparticle catalysis of Suzuki–Miyaura cross-couplings in water. Science 349:1087–1091
Hennings DD, Iwama T, Rawal VH (1999) Palladium-catalyzed (Ullmann-type) homocoupling of aryl halides: a convenient and general synthesis of symmetrical biaryls via inter-and intramolecular coupling reactions. Org Lett 1:1205–1208
Hu P, Dong Y, Wu X, Wei Y (2016) 2-Aminopyridine functionalized cellulose based Pd nanoparticles: an efficient and ecofriendly catalyst for the Suzuki cross-coupling reaction. Front Chem Sci Eng 10:389–395
Huang JL, Gray DG, Li CJ (2013) A3-coupling catalyzed by robust Au nanoparticles covalently bonded to HS-functionalized cellulose nanocrystalline films. Beilstein J Org Chem 9:1388–1391
Huang Y, Wang T, Ji M, Yang J, Zhu C, Sun D (2014) Simple preparation of carbonized bacterial cellulose–Pt composite as a high performance electrocatalyst for direct methanol fuel cells (DMFC). Mater Lett 128:93–96
Jamwal N, Sodhi RK, Gupta P, Paul S (2011) Nano Pd (0) supported on cellulose: a highly efficient and recyclable heterogeneous catalyst for the Suzuki coupling and aerobic oxidation of benzyl alcohols under liquid phase catalysis. Int J Biol Macromol 49:930–935
Jiang JZ, Cai C (2007) New role of microemulsion as reducing agent in palladium catalyzed reductive Ullmann reaction. Colloids Surf A Physicochem Eng Asp 305:145–148
Kaushik M, Moores A (2016) Review: nanocelluloses as versatile supports for metal nanoparticles and their applications in catalysis. Green Chem 18(3):622–637
Keipour H, Hosseini A, Afsari A, Oladee R, Khalilzadeh MA, Ollevier T (2016) CsF/clinoptilolite: an efficient solid base in SNAr and copper-catalyzed Ullmann reactions. Can J Chem 94:95–104
Keshipour S, Adak K (2016) Pd (0) supported on N-doped graphene quantum dot modified cellulose as an efficient catalyst for the green reduction of nitro aromatics. RSC Adv 6:89407–89412
Keshipour S, Khalteh NK (2016) Oxidation of ethylbenzene to styrene oxide in the presence of cellulose-supported Pd magnetic nanoparticles. Appl Organomet Chem 30:653–656
Keshipour S, Shojaei S, Shaabani A (2013) Palladium nano-particles supported on ethylene diamine functionalized cellulose as a novel and efficient catalyst for the Heck and Sonogashira couplings in water. Cellulose 20:973–980
Khalilzadeh MA, Hosseini A, Pilevar A (2011) Potassium fluoride supported on natural nanoporous zeolite: a new solid base for the synthesis of diaryl ethers. Eur J Org Chem 2011:1587–1592
Khalilzadeh MA, Keipour H, Hosseini A, Zareyee D (2014) KF/clinoptilolite, an effective solid base in Ullmann ether synthesis catalyzed by CuO nanoparticles. New J Chem 38:42–45
Kovacs T, Naish V, O’Connor B, Blaise C, Gagné F, Hall L, Trudeau V, Martel P (2010) An ecotoxicological characterization of nanocrystalline cellulose (NCC). Nanotoxicology 4:255–270
Kümmerer K, Menz J, Schubert T, Thielemans W (2011) Biodegradability of organic nanoparticles in the aqueous environment. Chemosphere 82:1387–1392
Le Normand M, Moriana R, Ek M (2014) Isolation and characterization of cellulose nanocrystals from spruce bark in a biorefinery perspective. Carbohydr Polym 111:979–987
Li Y, Xu L, Xu B, Mao Z, Xu H, Zhong Y, Zhang L, Wang B, Sui X (2017) Cellulose sponge supported palladium nanoparticles as recyclable cross-coupling catalysts. ACS Appl Mater Interfaces 9:17155–17162
Lin N, Dufresne A (2014) Nanocellulose in biomedicine: current status and future prospect. Eur Polym J 59:302–325
Lopez M, Bizot H, Chambat G, Marais MF, Zykwinska A, Ralet MC, Driguez H, Buléon A (2010) Enthalpic studies of xyloglucan cellulose interactions. Biomacromol 11:1417–1428
Martin R, Buchwald SL (2008) Palladium-catalyzed Suzuki–Miyaura cross-coupling reactions employing dialkylbiaryl phosphine ligands. Acc Chem Res 41:1461–1473
Moon RJ, Martini A, Nairn J, Simonsen J, Youngblood J (2011) Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev 40:3941–3994
Narayana M, Michalik J, Contarini S, Kevan L (1985) Determination of the chemical state of palladium in PdNa–X zeolite by electron spin resonance and X-ray photoelectron spectroscopy. J Phys Chem 89:3895–3899
Pratap R, Parrish D, Gunda P, Venkataraman D, Lakshman MK (2009) Influence of biaryl phosphine structure on C–N and C–C bond formation. J Am Chem Soc 131:12240–12249
Qafisheh N, Mukhopadhyay S, Sasson Y (2002) Highly selective Pd-catalyzed reductive coupling of substituted haloarenes with supported phase-transfer catalyst using Zn as the reducing agent. Adv Synth Catal 344:1079–1083
Reddy KR, Kumar NS, Sreedhar B, Kantam ML (2006) N-arylation of nitrogen heterocycles with aryl halides and arylboronic acids catalyzed by cellulose supported copper (0). J Mol Catal A Chem 252:136–141
Roman M (2015) Toxicity of cellulose nanocrystals: a review. Ind Biotechnol 11:25–33
Sadeghifar H, Filpponen I, Clarke SP, Brougham DF, Argyropoulos DS (2011) Production of cellulose nanocrystals using hydrobromic acid and click reactions on their surface. J Mater Sci 46:7344–7355
Salmanpour S, Khalilzadeh MA, Hosseini A (2013) KF/clinoptilolite: an efficient promoter for the synthesis of thioethers. Comb Chem High Throughput Screen 16:339–344
Segal LGJMA, Creely JJ, Martin AE Jr, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 29:786–794
Silvério HA, Neto WPF, Dantas NO, Pasquini D (2013) Extraction and characterization of cellulose nanocrystals from corncob for application as reinforcing agent in nanocomposites. Ind Crops Prod 44:427–436
Snelders DJ, Van Koten G, Klein Gebbink RJ (2009) Hexacationic Dendriphos ligands in the Pd-catalyzed Suzuki–Miyaura cross-coupling reaction: scope and mechanistic studies. J Am Chem Soc 131:11407–11416
Tehrani AD, Basiryan A (2015) Dendronization of cellulose nanowhisker with cationic hyperbranched dendritic polyamidoamine. Carbohydr Polym 120:46–52
Wu X, Lu C, Zhang W, Yuan G, Xiong R, Zhang X (2013) A novel reagentless approach for synthesizing cellulose nanocrystal-supported palladium nanoparticles with enhanced catalytic performance. J Mater Chem A 1:8645–8652
Yan L, Zhao Y, Gu Q, Li W (2012) Isolation of highly purity cellulose from wheat straw using a modified aqueous biphasic system. Front Chem Sci Eng 6:282–291
Yanamala N, Farcas MT, Hatfield MK, Kisin ER, Kagan VE, Geraci CL, Shvedova AA (2014) In vivo evaluation of the pulmonary toxicity of cellulose nanocrystals: a renewable and sustainable nanomaterial of the future. ACS Sustain Chem Eng 2:1691–1698
Zhou P, Wang H, Yang J, Tang J, Sun D, Tang W (2012) Bacteria cellulose nanofibers supported palladium (0) nanocomposite and its catalysis evaluation in Heck reaction. Ind Eng Chem Res 51:5743–5748
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Seyednejhad, S., Khalilzadeh, M.A., Zareyee, D. et al. Cellulose nanocrystal supported palladium as a novel recyclable catalyst for Ullmann coupling reactions. Cellulose 26, 5015–5031 (2019). https://doi.org/10.1007/s10570-019-02436-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02436-7