Abstract
Common cotton gauze was endowed with both superhydrophobic and antibacterial properties by a dip-coating method that involved sequential deposition of positively charged chitosan (CS), negatively charged gallic acid modified silver nanoparticles (GA@AgNPs) and 1H,1H,2H,2H-perfluorodecanethiol (PFDT) with low surface energy on cotton fabrics. After such surface coating, the wettability of gauze surface was converted from superhydrophilic to superhydrophobic with a water contact angle (CA) of 158 ± 2.2° and sliding angle (SA) of 5.2 ± 1.8°, exhibiting water repellency, antifouling ability as well as bacterially antiadhesive activity. Moreover, such PFDT/GA@AgNPs/CS-coated cotton fabrics showed efficiently antibacterial activities against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus), which was mainly attributed to the synergistic effect of contact-killing of CS and continuous release of Ag+. In addition, it was found that the outer PFDT deposition could act as a barrier to prevent leaching of the AgNPs during laundry, enhancing the antibacterial durability.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Medical gauze made of cotton fabrics is a kind of disposable and sterile device that commonly used for dressing wound because of its low-skin-irritating nature, softness, breathability and low cost (Zhu et al. 2018). Now, cotton gauze used in hospital is inherently superhydrophilic with CA close to 0°, which enables it to absorb blood and other fluids from wounds (Sasaki et al. 2016; Zhu et al. 2018). However, in practical application, especially for cotton gauze as dressing for skin wound, patients have to avoid touching water and suffer great inconvenience in daily life (Liu et al. 2012b). More importantly, a vital problem for cotton gauze is that bacteria in humid atmosphere can easily adhere onto hydrophilic cotton fabric surface and further migrate into fiber interior to finally form a biofilm, which will serve as reservoir for the development of pathogens, leading to health threats such as infection to mankind (Chen et al. 2016). Therefore, water-repellent and antibacterial gauze used in combination with common gauze will be ideal as skin wound dressing (Liang et al. 2016). To satisfy this urgent requirement, fabricating multifunctional cotton fabrics with both superhydrophobic and antibacterial properties via surface coating approach may be a feasible strategy (Xue et al. 2012; Fu et al. 2017; Karthik et al. 2017; Suryaprabha and Sethuraman 2017; Liu et al. 2018b).
Superhydrophobic surface is highly repellent to water with water contact angle larger than 150° and a sliding angle less than 10° (Drelich and Chibowski 2010), which shows many attractive applications in self-cleaning, antifouling, water collection and oil/water separation (Sasmal et al. 2014; Teisala et al. 2014; Kollarigowda et al. 2017; Suryaprabha and Sethuraman 2017; Das et al. 2018). Clinically used cotton gauze has a weave thread structure and each thread is consisted of micro-scaled fabrics with a diameter of 10–20 μm. Hence, the surface of micro-scaled cotton fabric can be further modified by secondary nanostructures and/or hydrophobic molecules to become superhydrophobic. Recently, Zhu’s group (Zhu et al. 2018) developed a superhydrophobic gauze without sacrificing the inherently breathable nature via decorating traditional cotton fabrics by hydrophobic paraffin, which was used for bleeding control with reducing blood loss. However, the antibacterial property of such superhydrophobic gauze was not mentioned, while the paraffin modified on cotton fabric didn’t have any antibacterial activity. Although superhydrophobic surface has been reported that can effectively resist bacterial adhesion (Pernites et al. 2012; Hizal et al. 2017), bacteria are just reduced rather than killed. More importantly, the bacteria will still adhere to the superhydrophobic surface when the surface becomes fully wet after long time exposure to the moist environments (Hwang et al. 2018). Therefore, more attention should be paid on to the combination of superhydrophobic surface and bactericidal materials (Ou et al. 2016; Hwang et al. 2017; Watson et al. 2017). Among numerous bactericidal materials, silver nanoparticles (AgNPs) are acknowledged efficient and safe antibacterial agents with broad-spectrum and long-acting antibacterial activity through the continuous release of silver ions (Goli et al. 2013; Rizzello and Pompa 2014; Ouay and Stellacci 2015; Xie et al. 2017). Besides, nano-sized AgNPs are also suitable to enhance the hydrophobicity of cotton fabric surface via the formed micro-nanoscale structures (Liu et al. 2012a; Ko et al. 2013). Despite, the leaching of AgNPs from cotton fabric is still a major challenge to its practical application. (Wu et al. 2016). Because the loaded AgNPs are usually exposed to the outer environments, which may be easily washed off from the cotton fabric surface, resulting in rapid loss of their antibacterial activity (Zhang et al. 2013; Yu et al. 2016).
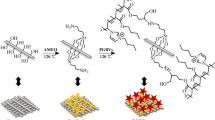
In present work, we report a multifunctional cotton gauze with both superhydrophobic and antibacterial properties, which was fabricated via a solution-dipping method that involves the sequential deposition of positively charged chitosan (CS), negatively charged gallic acid modified silver nanoparticles (GA@AgNPs) and 1H,1H,2H,2H-perfluorodecanethiol (PFDT) with low surface energy (as shown in Scheme 1). This PFDT/GA@AgNPs/CS-coated cotton fabrics with macro- and nano-scaled dual structures covered by hydrophobic PFDT exhibited excellent water repellency as well as efficient bacterially antiadhesive and antibacterial activity. More than that, the fluorinated PFDT could act as a barrier to prevent leaching of the AgNPs during laundry, enhancing the antibacterial durability. Such a superhydrophobic and antibacterial cotton gauze is very promising to be used as outer layer in combination with common gauze as inner layer for dressing skin wound.
Materials and method
Materials
Chitosan (CS, Mw ~ 200 kDa, DAc > 85%) was purchased from Zhejiang Golden-Shell Pharmaceutical Co. Ltd (Zhejiang, China). 1H,1H,2H,2H-perfluorodecanethiol (PFDT, 97%) was purchased from Aladdin Reagent Co. Ltd (Shanghai, China). Ethanol and glutaraldehyde 25% were purchased from Kelong chemistry company (Chengdu, China). Nutrient agar (NA) and nutrient broth (NB) and were purchased from Qingdao Hope Bio-Technology Co. Ltd (Qingdao, China). Escherichia coli (E. coli, ATCC25922) and Staphylococcus aureus (S. aureus, ATCC6538) was purchased from BeNa Culture Collection (Beijing, China). Medical gauze swabs were purchased from Jianghe Medical Material Co. Ltd (Qianjiang, China).
Preparation of gallic acid modified silver nanoparticles (GA@AgNPs)
Silver nanoparticles protected by gallic acid (GA@AgNPs) were synthesized according to our previous work (Liu et al. 2018a). Briefly, 10 mL AgNO3 (5 mM) was first mixed with 10 mL gallic acid solution (5 mM). Then, the above mixture was added dropwise into 30 mL NaBH4 solution with a concentration of 10 mM for reacting 2 h at dark. The average diameter and zeta potential of the obtained GA@AgNPs are 8.5 nm and − 25 mV, respectively (see Fig. S1 in the supporting information). The morphology of GA@AgNPs was measured by transmission electron microscope (JEOL, JEM-2100F, Japan). The zeta potential of the GA@AgNPs was tested by Nano ZS instrument (Malvern, Zetasizer Nano ZS, England).
Preparation of PFDT/GA@AgNPs/CS-coated cotton fabrics
PFDT/GA@AgNPs/CS-coated cotton fabrics were prepared via a convenient solution-dipping method that involves the sequential deposition of CS, gallic acid GA@AgNPs and PFDT. Briefly, pristine cotton fabric was first immersed into an acidic CS solution (5 mg mL−1) with pH of 4.0 for 30 min to obtain a CS coating, which was then rinsed with deionized water to remove the physically adsorbed CS and obtained as CS-coated fabric. Then, this CS coated fabric was immersed into an aqueous solution of GA@AgNPs with a weight ratio of AgNPs solution (0.2 mg mL−1) to cotton fabrics of 100:1 for 30 min under continuous stirring, and the cotton fabric was rinsed by deionized water to remove the physically adsorbed AgNPs and defined as GA@AgNPs/CS coated fabric. Finally, such GA@AgNPs/CS coated cotton fabric was immersed into a PFDT ethanol solution (3 μL mL−1) for 20 min. After rinsing with ethanol, the PFDT/GA@AgNPs/CS coated cotton fabric was dried at room temperature.
The release behavior of silver ions from PFDT/GA@AgNPs/CS-coated cotton fabrics
The release profile of silver ions from PFDT/GA@AgNPs/CS coated cotton fabrics was investigated by using inductively coupled plasma optical emission spectrometry (ICP-OES, Optima 2100DV, PerkinElmer). First, the silver content of PFDT/GA@AgNPs/CS coated gauze was measured by ICP-OES. Briefly, 100 mg dry PFDT/GA@AgNPs/CS coated gauze was cut into pieces and sealed in a high-pressure batch autoclave with 6 mL HNO3 and 1 mL H2O2 for 4 h at 120 °C (Ou et al. 2016). Then, the acidic solution was diluted with water to 250 mL, and the silver concentration was measured by ICP-OES and determined by the silver calibration curve which was plotted based on the standard solution with the silver concentration of 10, 5, 1, 0.5, 0.1 μg mL−1. For determining the release rate of silver ions, 100 mg PFDT/GA@AgNPs/CS coated cotton fabrics was cut into pieces and put into 10 mL PBS solution (pH 7.4) and incubated on an automated shaker at 37 °C. At predetermined time intervals, 10 mL solution was withdrawn from the release media and another 10 mL fresh PBS solution was added. Then the amount of released Ag+ was determined by using ICP-OES.
The antibacterial activity of PFDT/GA@AgNPs/CS-coated cotton fabrics
Bacterial antiadhesion ability
Bacterial antiadhesion ability of cotton fabrics coated by CS, GA@AgNPs/CS or PFDT/GA@AgNPs/CS was evaluated according to a previous work (Lin et al. 2018). Briefly, coated cotton fabrics and control (pristine fabrics) were immersed into 25 mL bacterial suspension (S. aureus or E. coli) with concentration of 107 CFU mL−1, incubating under static conditions for 2 h at 37 °C. Then, cotton fabrics were transferred into a tube with 25 mL of fresh NB, with further incubation for 24 h at 37 °C at a shaking speed of 120 rpm. After the incubation, cotton fabrics were taken out and washed by sterile water to remove the unadhered bacteria. Afterward, the cotton fabrics were put into a test tube containing 5 mL PBS solution, and those bacteria that strongly adhered on the fabric were then ultrasonicated for 2 min. The same operation was performed five times. The above detached bacteria in PBS solution was mixed and homogenized, and then 100 μL of this solution spread onto the NA plates and further incubated at 37 °C for 24 h. After counting the number of colonies, the number of adhered live bacteria was calculated by multiplying the number of colonies by the dilution factor. The bacterial antiadhesion rate was estimated as:
Contact killing efficiency assay
The contact killing efficiency assay of PFDT/GA@AgNPs/CS-Coated cotton fabrics was evaluated against E. coli and S. aureus, respectively, according to a standard procedure of ASTM E 2149-2001(Xiang et al. 2018). Cotton fabric samples with a weight of 100 mg was cut into pieces and put into 10 mL sterilized normal saline with bacterial concentration of 104 CFU mL−1 in an Erlenmeyer flask. Then the Erlenmeyer flask was incubated at 37 °C with shaking at 150 rpm on an automated shaker. At 0 h, and 2 h contact time, 20 µL bacterial solution was withdrawn from flask and spread on NA plate. The culture dish was incubated for another 24 h at 37 °C and counted for colony-forming units. The contact killing efficiency was defined as:
Bacteria morphologies observation on cotton fabrics
Square cotton gauze samples with side length of 10 mm were put into sterile test tubes and rinsed by 40 µL solution with bacterial cell density of 109 CFU mL−1, and then the tubes were incubated at 37 °C for 24 h. After incubation, gauze samples were washed by sterile normal saline 3 times and immersed in sterile glutaraldehyde aqueous solution (3 vol%) 4 h for immobilizing bacteria cells onto gauze surface (Liu et al. 2017). Then, ethanol was used for dehydration of the bacteria cells, and these gauze samples were observed by field emission scanning electron microscope after treatment of drying and spay-gold.
Bacterial growth curves in the presence of PFDT/GA@AgNPs/CS-Coated cotton fabrics
E. coli and S. aureus were first cultured in liquid nutrient broth media for 18 h at 37 °C. For antibacterial test, a portion of pre-cultured E. coli and S. aureus were diluted by culture media to 10 mL with an OD600 value of 0.05 (Wu et al. 2016). Then the 10 mL bacteria in test tubes were incubated with 100 mg gauze samples (cut into pieces) on an automated shaker (150 rpm) at 37 °C. At pre-determined time points, 150 µL of growth media was removed and the OD600 was measured using UV–vis spectrophotometer (722S, Youke, China).
The durable antibacterial activity of PFDT/GA@AgNPs/CS-coated cotton fabrics against washing
The water-fastness of PFDT/GA@AgNPs/CS-Coated gauze sample was tested according to a modified ISO 105-C10: 2006 method (Ou et al. 2016; Wu et al. 2016). The tested gauze sample (50 mm × 50 mm) was washed in a drum at 40 °C with 0.5 wt% detergent for 30 min at a spinning rate of 40 rpm, which was defined as one washing cycle. The mass ratio of water to gauze sample was 50:1. After washing 1, 2, 3, 4, 5, 10, 20 cycles, respectively, gauze sample was taken out, washed by sterilized water for several times and cut into pieces. The killing efficiency was then tested as described in “Contact killing efficiency assay” section.
Characterization
Microstructures of cotton fabrics (CS-coated, GA@AgNPs/CS-coated and PFDT/GA@AgNPs/CS-coated) and bacteria morphologies were observed on an FE-SEM (Quanta 250, FEI, USA) at an accelerating voltage of 5 kV. Water contact angles (CA) was measured by Contact Angle System (OCAH200, Dataphysics, Germany) at ambient temperature. A water droplet of 8 µL was used as the indicator. Average contact angle values were obtained by measuring five different positions on the same sample. The chemical compositions of the coated gauze were investigated by an X-ray photoelectron spectroscopy (XSAM800, Kratos, UK). The instrument was equipped with a monochromatic Al Ka (1486.6 eV) X-ray source operated at 12 kV × 15 mA at a pressure of 2 × 10−7 Pa. The C 1s peak (binding energy 284.8 eV) was used as a reference for charge correction. The concentration of silver was measured by ICP-OES (Optima 2100DV, PerkinElmer, USA).
Results and discussion
Surface morphology and wettability of PFDT/GA@AgNPs/CS-coated cotton fabrics
The surface morphology of PFDT/GA@AgNPs/CS-coated cotton fabrics is observed and investigated by FE-SEM, using pristine, CS-coated and GA@AgNPs/CS-coated cotton fabrics as controls (shown in Fig. 1). Compared to the SEM images of pristine cotton gauze at lower magnifications (Fig. 1a, e), the morphologies of CS-coated, GA@AgNPs/CS-coated and PFDT/GA@AgNPs/CS-coated cotton fabrics don’t show any changes (Fig. 1b–d, f–h). The results clearly demonstrate that the original porous structure of cotton gauze based on interwoven fibers were not affected or destroyed by these coatings. Therefore, according to the recent study reported by Zhu’s group (Zhu et al. 2018), the breathability and moisture-penetrability of CS-coated, GA@AgNPs/CS-coated or PFDT/GA@AgNPs/CS-coated cotton fabrics can maintain because of the micropores between the weave threads. However, the surface of cotton fiber becomes rougher than that of pristine after the CS coating (Fig. 1i, j), which converts the surface wettability from hydrophilic to hydrophobic with a water contact angel (CA) of 125 ± 1.3° (Fig. 2a, b). The hydrophobicity will be further increased to 135 ± 2.1° after the deposition of GA@AgNPs which are witnessed as white dots on the fabric surface (Fig. 1k). Such increased hydrophobicity is ascribed to the micro-scaled texture of cotton fabric decorated by nano-sized silver nanoparticles. Simultaneously, the cotton fabric is dyed a yellow color that inherited from these tiny spherical silver nanoparticles with average dimeter of 8.0 nm (Fig. 2c and Fig. S1). Then, the GA@AgNPs/CS-coated cotton fabric was immersed into a PFDT ethanol dispersion, and the PFDT deposition further increased the surface roughness of the cotton fabric (Fig. 1l). As shown in Fig. 2d, the cotton fabrics retain the yellow color, suggesting that there was no dissolution of the deposited GA@AgNPs during the PFDT coating. Importantly, these PFDT/GA@AgNPs/CS-coated cotton fabrics with macro- and nano-scaled dual structures covered by low-surface-energy PFDT molecules are superhydrophobic with CA of 158 ± 2.2° and SA of 5.2 ± 1.8°. Water droplets can readily roll off this cotton fabrics, exhibiting appreciable waterproofness and excellent antifouling ability (see the dynamic video in the support information).
Characterization of PFDT/GA@AgNPs/CS-coated cotton fabrics by XPS analyses
The depositions of CS, GA@AgNPs and PFDT are also monitored and characterized by X-ray photoelectron spectroscopy (XPS). Compered to XPS spectra of pristine cotton fabric in Fig. 3a, N 1s peak appears at 399.8 eV (N–C) for CS coated sample (Fig. 3b, c), suggesting the formation of CS coating with amino groups(Wu et al. 2011; Wang et al. 2014). Due to the abundant hydroxyl groups on pristine cotton fabric, hydrogen-bond interaction maybe formed between the hydroxyl groups and amino groups of CS, leading to the stable CS coating on cotton fabrics. While for GA@AgNPs/CS-coated cotton fabrics, the sequential deposition of as-prepared GA@AgNPs onto CS layer makes typical Ag 3d signal emerge in the XPS spectra (Fig. 3d). Highly resolved XPS spectra of Ag 3d in Fig. 3e shows two individual peaks located at 368.1 and 374.1 eV that can be assigned to Ag 3d5/2 and Ag 3d3/2 binding energies, respectively (Zhang et al. 2011; Wang et al. 2013). These two peaks are further resolved to demonstrate the successful deposition of metallic AgNPs (Ag0) on CS coating (Schlaich et al. 2018). Furthermore, the N 1s peak of this GA@AgNPs/CS coating is also analyzed. As can be seen from Fig. 3f, a new N 1s peak appears at higher binding energy (400.6 eV) after the deposition of GA@AgNPs. This result suggests a decrease of electronic density around the N atom, which is mainly due to the electrostatic interaction (C–NH3+···−OOC–) between partial amino groups of CS and carboxyl groups of GA@AgNPs (Liang et al. 2014). In fact, the as-prepared GA@AgNPs possess a negatively charged surface with zeta-potential of around − 25 mV (Fig. S1), which tended to form electrostatic interaction with the CS layer during deposition process.
For PFDT/GA@AgNPs/CS coated cotton fabrics, the XPS spectra in Fig. 4a reveals the presence of F1s and S2p signals, indicating the successful deposition of PFDT layer on GA@AgNPs/CS coating. In addition, S2p has been used to evaluate the chemical bonding between alkanethiol and metallic substrates. It is accepted that the peak at around 162.0 eV is corresponding to bound sulfur, and the peak at around 163.0 eV is identified as RS-SR or radiation induced species (Ou et al. 2016). Moreover, it is found a chemical shift (~ 0.3 eV) of Ag 3d signal to higher binding energy after the PFDT deposition (Figs. 3e and 4b). All these results suggest the modification of PFDT on silver nanoparticles via chemical bonding.
The stability and Ag+ releasing of PFDT/GA@AgNPs/CS-coated cotton fabrics
Except for enabling superhydrophobic property to cotton fabrics, the deposition of PFDT also can enhance the stability of the PFDT/GA@AgNPs/CS coating against laundry. The washing durability was evaluated according to ISO 105-C10: 2006 standards. As shown in Fig. 5a, the superhydrophobicity of PFDT/GA@AgNPs/CS coated cotton fabrics is retained after 3 washing cycles (CA = 151 ± 1.2°). Even after 10 cycles, the change of CA is still higher than 140°, indicating that this PFDT/GA@AgNPs/CS coated cotton fabrics is durable to washing. While for GA@AgNPs/CS coated cotton fabric without PFDT layer, the CA dramatically decreases to around 110° after 10 cycles, which implying the coating is destructed during laundry. Therefore, UV–vis spectroscopy was employed to monitor the washing off silver nanoparticles (see Fig. S2 in the supporting information). After 2 h of washing (4 cycles), no obvious absorbance of AgNPs is observed in the washing solution from the PFDT/GA@AgNPs/CS coated cotton fabrics. On the contrary, there is absorbance of AgNPs at 400 nm in the washing solution from the GA@AgNPs/CS coated cotton fabrics. The enhanced stability of this PFDT/GA@AgNPs/CS coating against laundry can be attributed to the deposited PFDT which acts as a barrier layer around AgNPs with high water-repellent ability. As expected, such PFDT barrier can also slow dawn the release of Ag+ from silver nanoparticles, which will give a long-acting antibacterial activity of the PFDT/GA@AgNPs/CS coated cotton fabrics. ICP-OES was performed to monitor the release behavior of Ag+ from the coating, and the silver nanoparticles contents of PFDT/GA@AgNPs/CS and GA@AgNPs/CS coating are both about 0.12 mg per 100 mg dry coated cotton fabrics. As shown in Fig. 5b, both PFDT/GA@AgNPs/CS and GA@AgNPs/CS coating exhibit continuous behavior of Ag+. However, the release rate of Ag+ from PFDT/GA@AgNPs/CS coating is slower than that of GA@AgNPs/CS coating without PFDT.
Antibacterial ability of PFDT/GA@AgNPs/CS coated cotton fabrics
Bacterially antiadhesive ability
Since the superhydrophobic surface can resist the bacteria adhesion, the bacterial antiadhesion ability of the PFDT/GA@AgNPs/CS coated cotton fabric was tested. As shown in Fig. 6a, compared to that of CS coated or GA@AgNPs/CS coated cotton fabrics, nearly no live bacteria adhered to the PFDT/GA@AgNPs/CS coated cotton fabric after incubation with high concentration bacterial suspension. In Fig. 6b, the bacterial antiadhesion rates for CS coated, GA@AgNPs/CS coated and PFDT/GA@AgNPs/CS coated cotton fabrics against E. coli are 89.84, 98.11 and 99.99%, respectively; against S. aureus are 82.15, 97.00 and 99.97%, respectively. This result demonstrates that the more hydrophobic the fabric, the higher is the bacterial antiadhesion rate. Especially for this superhydrophobic surface with low surface energy, its excellent water-repellent ability makes bacteria suspension in the aqueous environment hard to wet and attach the fabric surface, and therefore no bacteria can penetrate into the fiber interior (Fig. 6c). In fact, bacteria adhesion onto solid surface is a key step for biofilm formation which will reservoir for the development of pathogens, leading to infection threats. The efficient bacteria antiadhesive ability is very helpful for this PFDT/GA@AgNPs/CS coated cotton gauze in dressing wound.
Bactericidal activity
Though superhydrophobic surface can resist bacteria adhesion, it is only a passively antibacterial strategy rather than kill them. To achieve the excellent antibacterial effect, the PFDT/GA@AgNPs/CS coating should also rapidly kill the contacted and invasive pathogenic microbes. Therefore, the bactericidal activity of PFDT/GA@AgNPs/CS coated fabrics were studied. As shown in Fig. 7, it is obvious that the killing efficiencies of GA@AgNPs/CS and PFDT/GA@AgNPs/CS coated cotton fabrics against E. coli and S. aureus are similar (higher than 99%), which are much higher than that of single CS coating (lower than 80%). The result indicates the higher bactericidal activity of GA@AgNPs/CS coated or PFDT/GA@AgNPs/CS coated cotton fabrics is ascribed to the synergism of contact killing of CS and Ag+ release killing of GA@AgNPs. To further understand such synergistically bactericidal activity, the morphologies of bacteria on coating surface were observed by SEM. After incubation with bacteria for 24 h, attached bacteria cells could be observed and displayed in Fig. 8. Compared to live bacteria cells on pristine cotton fabric with intact membranes, the membranes of bacteria on CS coating are visibly damaged with lesions. That is mainly because of the contact-active disruption of microbe cell membrane by the cationic chitosan. However, for those bacterial cells attached on GA@AgNPs/CS coated or PFDT/GA@AgNPs/CS coated cotton fabrics, not only lesions but also distorted and wrinkled membranes with holes are observed. Such serious damage is supposed to be caused both by contact-killing activity of CS and Ag+ release-based killing activity of silver nanoparticles, implying the antibacterial advantage of such dual-action bactericidal coating.
Since the Ag ions can be slowly released from GA@AgNPs/CS or PFDT/GA@AgNPs/CS coating (Fig. 5b) into environment with subsequent killing of bacteria, the growth inhibition of bacteria was investigated. Pristine and CS coated cotton fabrics were used as controls. Precultured E. coli or S. aureus with an optical density at 600 nm (OD600) of around 0.05 were cultured in presence of cotton fabric samples. The changes in OD600 values over time are recorded and showed in Fig. 9. Clearly, pristine and CS coated cotton fabrics can’t inhibit the bacterial growth and the OD600 values for both E. coli and S. aureus increased with culture time. It is easy to understand that pristine cotton fabrics don’t have any antibacterial property, while for CS coated cotton fabric, the CS coating can be easily masked by adsorbed bacterial cells (live or dead) due to its poor bacterial antiadhesion ability (As shown in Fig. 6b), causing loss of bactericidal activity. In contrast, in the presence of GA@AgNPs/CS or PFDT/GA@AgNPs/CS coated cotton fabrics, the growth of both types of bacteria is completed inhibited in 24 h of the culture period. That is because the released Ag ions from these two coatings with excellent bacterial antiadhesion ability can steadily bind with bacteria and annihilate them, exhibiting efficient and long-term antibacterial performances.
Durably antibacterial activity
The antibacterial durability of PFDT/GA@AgNPs/CS coated cotton fabrics was assessed by determining the change of killing efficiency against E. coli or S. aureus under different laundering cycles. From Fig. 10, it is found that PFDT/GA@AgNPs/CS coated cotton fabrics maintain excellent antibacterial efficiency higher than 98% even after 20 washing cycles, indicating appreciable antibacterial durability. However, the killing efficiency of GA@AgNPs/CS coated cotton fabrics significantly decrease with increase of accelerated laundering cycles. The result is consistent with the coating stability test in Fig. 5a that the water-repellent PFDT deposition protected the coating not to be dissolved or lost over time, thus providing a durable antibacterial capability.
Conclusions
In summary, the super-hydrophobicity contributed by micro-scaled texture of cotton fabric and nano-sized silver nanoparticles covered by low surface energy PFDT molecules, not only provided complete waterproofness but also greatly enhanced the bacterial antiadhesion, enabling the treated cotton fabrics to have long-lasting antibacterial activity through the sustained release of Ag+ with washable durability. Such multifunctional PFDT/GA@AgNPs/CS coating is very suitable for medical gauze to dress the wound in the moist and insanitary environment.
References
Chen S, Yuan L, Li Q, Li J, Zhu X, Jiang Y, Sha O, Yang X, Xin JH, Wang J, Stadler FJ, Huang P (2016) Durable antibacterial and nonfouling cotton textiles with enhanced comfort via zwitterionic sulfopropylbetaine coating. Small 12:3516–3521. https://doi.org/10.1002/smll.201600587
Das S, Kumar S, Samal SK, Mohanty S, Nayak SK (2018) A review on superhydrophobic polymer nanocoatings: recent development and applications. Ind Eng Chem Res 57:2727–2745. https://doi.org/10.1021/acs.iecr.7b04887
Drelich J, Chibowski E (2010) Superhydrophilic and superwetting surfaces: definition and mechanisms of control. Langmuir 26:18621–18623. https://doi.org/10.1021/la1039893
Fu Y, Jin B, Zhang Q, Zhan X, Chen F (2017) pH-induced switchable superwettability of efficient antibacterial fabrics for durable selective oil/water separation. ACS Appl Mater Interfaces 9:30161–30170. https://doi.org/10.1021/acsami.7b09159
Goli KK, Gera N, Liu X, Rao BM, Rojas OJ, Genzer J (2013) Generation and properties of antibacterial coatings based on electrostatic attachment of silver nanoparticles to protein-coated polypropylene fibers. ACS Appl Mater Interfaces 5:5298–5306. https://doi.org/10.1021/am4011644
Hizal F, Rungraeng N, Lee J, Jun S, Busscher HJ, van der Mei HC, Choi CH (2017) Nanoengineered superhydrophobic surfaces of aluminum with extremely low bacterial adhesivity. ACS Appl Mater Interfaces 9:12118–12129. https://doi.org/10.1021/acsami.7b01322
Hwang GB, Patir A, Allan E, Nair SP, Parkin IP (2017) Superhydrophobic and white light-activated bactericidal surface through a simple coating. ACS Appl Mater Interfaces 9:29002–29009. https://doi.org/10.1021/acsami.7b05977
Hwang GB, Page K, Patir A, Nair SP, Allan E, Parkin IP (2018) The anti-biofouling properties of superhydrophobic surfaces are short-lived. ACS Nano 12:6050–6058. https://doi.org/10.1021/acsnano.8b02293
Karthik S, Suriyaprabha R, Vinoth M, Srither SR, Manivasakan P, Rajendran V, Valiyaveettil S (2017) Larvicidal, super hydrophobic and antibacterial properties of herbal nanoparticles from Acalypha indica for biomedical applications. RSC Adv 7:41763–41770. https://doi.org/10.1039/c7ra05697d
Ko Y, Baek H, Kim Y, Yoon M, Cho J (2013) Hydrophobic nanoparticle-based nanocomposite films using in situ ligand exchange layer-by-layer assembly and their nonvolatile memory applications. ACS Nano 7:143–153. https://doi.org/10.1021/nn3034524
Kollarigowda RH, Abraham S, Montemagno CD (2017) Antifouling cellulose hybrid biomembrane for effective oil/water separation. ACS Appl Mater Interfaces 9:29812–29819. https://doi.org/10.1021/acsami.7b09087
Liang M, Su R, Huang R, Qi W, Yu Y, Wang L, He Z (2014) Facile in situ synthesis of silver nanoparticles on procyanidin-grafted eggshell membrane and their catalytic properties. ACS Appl Mater Interfaces 6:4638–4649. https://doi.org/10.1021/am500665p
Liang D, Lu Z, Yang H, Gao J, Chen R (2016) Novel asymmetric wettable AgNPs/chitosan wound dressing: in vitro and in vivo evaluation. ACS Appl Mater Interfaces 8:3958–3968. https://doi.org/10.1021/acsami.5b11160
Lin J, Chen XY, Chen CY, Hu JT, Zhou CL, Cai XF, Wang W, Zheng C, Zhang PP, Cheng J, Guo ZH, Liu H (2018) Durably antibacterial and bacterially antiadhesive cotton fabrics coated by cationic fluorinated polymers. ACS Appl Mater Interfaces 10:6124–6136. https://doi.org/10.1021/acsami.7b16235
Liu T, Yin B, He T, Guo N, Dong L, Yin Y (2012a) Complementary effects of nanosilver and superhydrophobic coatings on the prevention of marine bacterial adhesion. ACS Appl Mater Interfaces 4:4683–4690. https://doi.org/10.1021/am301049v
Liu Y, Xin JH, Choi CH (2012b) Cotton fabrics with single-faced superhydrophobicity. Langmuir 28:17426–17434. https://doi.org/10.1021/la303714h
Liu G, Li K, Luo Q, Wang H, Zhang Z (2017) PEGylated chitosan protected silver nanoparticles as water-borne coating for leather with antibacterial property. J Colloid Interface Sci 490:642–651. https://doi.org/10.1016/j.jcis.2016.11.103
Liu G, Gao H, Li K, Xiang J, Lan T, Zhang Z (2018a) Fabrication of silver nanoparticle sponge leather with durable antibacterial property. J Colloid Interface Sci 514:338–348. https://doi.org/10.1016/j.jcis.2017.09.049
Liu Q, Huang J, Zhang J, Hong Y, Wan Y, Wang Q, Gong M, Wu Z, Guo CF (2018b) Thermal, waterproof, breathable, and antibacterial cloth with a nanoporous structure. ACS Appl Mater Interfaces 10:2026–2032. https://doi.org/10.1021/acsami.7b16422
Ou J, Wang Z, Wang F, Xue M, Li W, Amirfazli A (2016) Washable and antibacterial superhydrophbic fabric. Appl Surf Sci 364:81–85. https://doi.org/10.1016/j.apsusc.2015.12.113
Ouay BL, Stellacci F (2015) Antibacterial activity of silver nanoparticles: a surface science insight. Nano Today 10:339–354. https://doi.org/10.1016/j.nantod.2015.04.002
Pernites RB, Santos CM, Maldonado M, Ponnapati RR, Rodrigues DF, Advincula RC (2012) Tunable protein and bacterial cell adsorption on colloidally templated superhydrophobic polythiophene films. Chem Mater 24:870–880. https://doi.org/10.1021/cm2007044
Rizzello L, Pompa PP (2014) Nanosilver-based antibacterial drugs and devices: mechanisms, methodological drawbacks, and guidelines. Chem Soc Rev 43:1501–1518. https://doi.org/10.1039/c3cs60218d
Sasaki K, Tenjimbayashi M, Manabe K, Shiratori S (2016) Asymmetric superhydrophobic/superhydrophilic cotton fabrics designed by spraying polymer and nanoparticles. ACS Appl Mater Interfaces 8(1):651–659. https://doi.org/10.1021/acsami.5b09782
Sasmal AK, Mondal C, Sinha AK, Gauri SS, Pal J, Aditya T, Ganguly M, Dey S, Pal T (2014) Fabrication of superhydrophobic copper surface on various substrates for roll-off, self-cleaning, and water/oil separation. ACS Appl Mater Interfaces 6:22034–22043. https://doi.org/10.1021/am5072892
Schlaich C, Li M, Cheng C, Donskyi IS, Yu L, Song G, Osorio E, Wei Q, Haag R (2018) Mussel-inspired polymer-based universal spray coating for surface modification: fast fabrication of antibacterial and superhydrophobic surface coatings. Adv Mater Interfaces 5:1701254. https://doi.org/10.1002/admi.201701254
Suryaprabha T, Sethuraman MG (2017) Fabrication of copper-based superhydrophobic self-cleaning antibacterial coating over cotton fabric. Cellulose 24:395–407. https://doi.org/10.1007/s10570-016-1110-z
Teisala H, Tuominen M, Kuusipalo J (2014) Superhydrophobic coatings on cellulose-based materials: fabrication, properties, and applications. Adv Mater Interfaces 1:1300026. https://doi.org/10.1002/admi.201300026
Wang J, Liu W, Li H, Wang H, Wang Z, Zhou W, Liu H (2013) Preparation of cellulose fiber–TiO2 nanobelt–silver nanoparticle hierarchically structured hybrid paper and its photocatalytic and antibacterial properties. Chem Eng J 228:272–280. https://doi.org/10.1016/j.cej.2013.04.098
Wang M, She Y, Xiao Z, Hu J, Zhou R, Zhang J (2014) The green adsorption of chitosan tripolyphosphate nanoparticles on cotton fiber surfaces. Carbohydr Polym 101:812–818. https://doi.org/10.1016/j.carbpol.2013.10.023
Watson GS, Green DW, Cribb BW, Brown CL, Meritt CR, Tobin MJ, Vongsvivut J, Sun M, Liang AP, Watson JA (2017) Insect analogue to the lotus leaf: a planthopper wing membrane incorporating a low-adhesion, nonwetting, superhydrophobic, bactericidal, and biocompatible surface. ACS Appl Mater Interfaces 9:24381–24392. https://doi.org/10.1021/acsami.7b08368
Wu J, Zhang L, Wang Y, Long Y, Gao H, Zhang X, Zhao N, Cai Y, Xu J (2011) Mussel-inspired chemistry for robust and surface-modifiable multilayer films. Langmuir 27:13684–13691. https://doi.org/10.1021/la2027237
Wu M, Ma B, Pan T, Chen S, Sun J (2016) Silver-nanoparticle-colored cotton fabrics with tunable colors and durable antibacterial and self-healing superhydrophobic properties. Adv Funct Mater 26:569–576. https://doi.org/10.1002/adfm.201504197
Xiang J, Ma L, Su H, Xiong J, Li K, Xia Q, Liu G (2018) Layer-by-layer assembly of antibacterial composite coating for leather with cross-link enhanced durability against laundry and abrasion. Appl Surf Sci 458:978–987. https://doi.org/10.1016/j.apsusc.2018.07.165
Xie X, Mao C, Liu X, Zhang Y, Cui Z, Yang X, Yeung KWK, Pan H, Chu PK, Wu S (2017) Synergistic bacteria killing through photodynamic and physical actions of graphene oxide/Ag/collagen coating. ACS Appl Mater Interfaces 9:26417–26428. https://doi.org/10.1021/acsami.7b06702
Xue CH, Chen J, Yin W, Jia ST, Ma JZ (2012) Superhydrophobic conductive textiles with antibacterial property by coating fibers with silver nanoparticles. Appl Surf Sci 258:2468–2472. https://doi.org/10.1016/j.apsusc.2011.10.074
Yu M, Wang Z, Lv M, Hao R, Zhao R, Qi L, Liu S, Yu C, Zhang B, Fan C, Li J (2016) Antisuperbug cotton fabric with excellent laundering durability. ACS Appl Mater Interfaces 8:19866–19871. https://doi.org/10.1021/acsami.6b07631
Zhang P, Shao C, Zhang Z, Zhang M, Mu J, Guo Z, Liu Y (2011) In situ assembly of well-dispersed Ag nanoparticles (AgNPs) on electrospun carbon nanofibers (CNFs) for catalytic reduction of 4-nitrophenol. Nanoscale 3:3357–3363. https://doi.org/10.1039/c1nr10405e
Zhang D, Chen L, Zang C, Chen Y, Lin H (2013) Antibacterial cotton fabric grafted with silver nanoparticles and its excellent laundering durability. Carbohydr Polym 92:2088–2094. https://doi.org/10.1016/j.carbpol.2012.11.100
Zhu T, Wu J, Zhao N, Cai C, Qian Z, Si F, Luo H, Guo J, Lai X, Shao L, Xu J (2018) Superhydrophobic/superhydrophilic janus fabrics reducing blood loss. Adv Healthcare Mater 7:1701086. https://doi.org/10.1002/adhm.201701086
Acknowledgments
This work was supported by the National Key Point and Invention Program of the Thirteenth (2017YFC1104601), Key Technology Support Program of Sichuan Province (2016SZ0004, 2017SQZX0010 and 2018SZ0174), Support Program of Sichuan University-Luzhou City (2017CDLZ-S02).
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10570_2018_2110_MOESM2_ESM.docx
Schematic illustration of synthetic rout of GA@AgNPs, TEM image and size distribution histograms of GA@AgNPs, UV–vis absorption spectra of the washing solutions of GA@AgNPs/CS-coated (pink line) and PFDT/GA@AgNPs/CS-coated (green line) cotton fabrics after 2 h of washing, and the dynamic video of water droplets rolling off from superhydrophobic cotton gauze can be found in supporting information (DOCX 768 kb)
Rights and permissions
About this article
Cite this article
Liu, G., Xiang, J., Xia, Q. et al. Superhydrophobic cotton gauze with durably antibacterial activity as skin wound dressing. Cellulose 26, 1383–1397 (2019). https://doi.org/10.1007/s10570-018-2110-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-018-2110-y