Abstract
Waterborne bio-based polymer pigments are promising environmentally friendly coloring materials. In this work, novel colored cellulose suspensions applicable for coloring, labelling and anti-counterfeiting were prepared from waste cotton fabrics. It was demonstrated that cellulose regenerated from a phosphoric acid solution of waste cotton fabrics could be conveniently colored by reacting with reactive dyes. The high specific surface area of the regenerated cellulose (RC) allowed for high color build-up of up to 210 mg/g dye fixation and K/S value of up to 31. The resulting colored regenerated cellulose (CRC) suspension had typical characteristics of regenerated cellulose and was used to color polyurethane (PU). The resulting colored PU films exhibited excellent color fastness/resistance to leaching when soaked in water. This study demonstrated a way of alleviating the environmental burden of waste cotton fabrics by converting them to high value-added colored waterborne cellulose suspension.
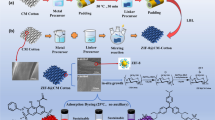
Graphical abstract
Preparation and application of CRC suspensions from waste cotton fabrics

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Millions of tons of waste cotton fabrics (WCF) are produced by the textile and apparel industries every year (Shen et al. 2013), yet only a small fraction of these fabrics was recycled. Some WCF is processed into low-grade products such as mops and plush toys (Wang et al. 2017), and some is processed into high value-added microcrystalline cellulose (Sun et al. 2014), cellulose nanofibrils (CNFs) (Li et al. 2014; Farahbakhsh et al. 2014), cellulose nanocrystals (CNCs) (Moon et al. 2011; Xiong et al. 2012; Yue et al. 2012) and regenerated cellulose (Wang et al. 2016), which find applications as reinforcing materials (Farahbakhsh et al. 2015), supports for catalysts (Wu et al. 2014), flame retardant, and heat-insulting materials (Han et al. 2015). The majority of WCF has gone to landfills or been incinerated, taking over huge amount of land and releasing pollutants into the atmosphere (Miranda et al. 2007; Wang et al. 2017). Developing value-added products from WCF is not only necessary for sustainable development and environmental protection, it could also bring considerable economic benefits (García et al. 2016).
One of the emerging trends in the field of cellulose utilization is to develop colored cellulose-based materials (Huang et al. 2013; Leng et al. 2017; Navarro et al. 2015; Schyrr et al. 2014; Tian et al. 2018). Compared with conventional polymer pigments (Beija et al. 2011; Hu et al. 2016; Jang et al. 2012; Mao et al. 2015; Tang et al. 2006), the colored cellulose-based materials are biodegradable, biocompatible and renewable (Jonoobi et al. 2015; Nechyporchuk et al. 2016; Wang et al. 2016), ideal to be used in color-on-demand areas. Abitbol et al. (2013) labelled CNCs with an aminofluorescein derivative for homogeneity detection of CNCs in composites. Nielsen et al. (2010) developed dual fluorescent labelled CNCs for analyte sensing. Navarro and Bergström (2014) grafted the modified rhodamine on CNFs to be used in imaging. These studies focused on highly crystallized cellulose such as CNCs and CNFs, however, high crystallinity is not conducive to chemical modification of cellulose.
In our previous work, we have successfully prepared RC by phosphoric acid method, which was used to prepare oleogels and reinforce polylactic acid (Jiang et al. 2018; Zhang et al. 2018). RC has enormous amorphous region, allowing more dyes to penetrate and get fixed so that higher color yield is possible. Additionally, RC is easily prepared in very high yield, leading to significantly cut on costs (Jia et al. 2013). In this work, colored waterborne cellulose suspensions were obtained by reacting RC with reactive dyes in deionized water. The obtained CRC suspensions were characterized by Fourier transform infrared spectroscopy, X-ray diffraction, transmission electron microscopy, thermogravimetric analysis, rheological analysis and color analysis. The CRC suspensions were further used to prepare colored polyurethane composite films. This study paves a new way for developing high value-added full-color cellulose materials.
Materials and methods
Materials
The waste plain woven cotton fabrics (107 g/m2) were supplied by Hua Fang Co., Ltd (Binzhou, China). Phosphoric acid (85%) was supplied by Shanghai Titan Scientific Co., Ltd (Shanghai, China). Sodium chloride (NaCl), sodium carbonate (Na2CO3) and sulfuric acid were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). C. I. Reactive Red 194 (RR194), C. I. Reactive Yellow 145 (RY145) and C. I. Reactive Blue 19 (RB19) were purchased from Shaoxing Huafang Chemical Co., Ltd (Shaoxing, China). Soap flake was purchased from Shanghai Soap Factory (Shanghai, China). Isopropyl alcohol was purchased from Shanghai Lingfeng Chemical Reagents Co., Ltd (Shanghai, China). Waterborne polyurethane (WPU, PUD-8051 H, 36.5 wt%) was purchased from Shanghai Sisheng Polymer Materials Co., Ltd (Shanghai, China). All other chemicals were used as received.
Preparation of RC suspension
The waste cotton fabrics (2.00 g) were grinded by MF 10 grinder (IKA, Germany). The grinded waste cotton (2.00 g) and deionized water (18 mL) were added to a three-mouth flask equipped with a mechanical stirrer, and the grinded cotton was wetted for 30 min. Then precooled phosphoric acid (0 °C, 300 mL, 85%) was slowly poured into the three-mouth flask and the mixture was stirred at 350 rpm in an ice bath (4 °C) for 24 h to get the cellulose/phosphoric acid solution. Subsequently, the obtained solution was slowly poured into deionized water (1500 mL) at 500 rpm until a milky suspension was formed. The phosphoric acid was gradually removed from the suspension by repeated centrifugation (12,000 rpm Thermo, USA), separation, and dilution with deionized water until the suspension was neutral. Finally, the neutral suspension was treated with a high pressure homogenizer (APV-2000, Germany) to obtain a final RC suspension.
Preparation of CRC suspension
RC suspension, NaCl and RR194 dye were added into a reactor, and then the reactor was put in Dye Plus Water Bath Shaker (Dae Lim, Korea) at 30 °C for 10 min. The temperature of dye bath was gradually raised to fixed temperature at a rate of 2 °C/min. The reactor was taken out from Dye Plus Water Bath Shaker and Na2CO3 was added into the reactor, then the reactor was put back and kept at fixed temperature for 90 min. The dye bath was subsequently cooled to room temperature and the dyed RC was purified by repeated centrifugation-soaping–centrifugation-rinsing process. Finally, the CRC suspension was obtained after the soaping experiment was conducted at 95 °C for 10 min by using sodium carbonate (2 g/L) and soap flake (2 g/L). The preparation method of colored cotton fabrics (CCF) was similar to CRC. The formulations of CRC and CCF were shown in Tables 1 and 2. The yellow and blue CRC suspensions (CRC-RY145 and CRC-RB19) were prepared using RY145 and RB19, and their formulas were the same as that of CRC-5 in Table 2. These reactive dyes were chosen because they form a set of dyes frequently used in combination by textile manufactures to produce desirable colors and shades.
Preparation of WPU/CRC and WPU/RC/dye films
WPU (20 g, 36.5 wt%) was mixed with CRC-5 (CRC-RR194), CRC-RY145 and CRC-RB19 suspensions (7.5 g, 3 wt%) at room temperature, respectively. The mixtures were stirred at 300 rpm for 1 h to obtain homogeneous dispersions, then poured onto a glass slide and dried at ambient conditions for 48 h.
WPU (20 g, 36.5 wt%), RC suspension (7.5 g, 2.5 wt%) and reactive dyes (RR194, RY145 or RB19, 0.04 g) were used to prepare the control samples.
Characterizations
Fourier transform infrared spectroscopy (FT-IR)
FT-IR spectra of the dry samples were recorded using a PerkinElmer Spectrum-Two (American) equipped with an attenuated total reflectance (ATR) accessory. Spectra were recorded in wavenumber range from 500 to 4000 cm−1. The RC and CRC suspensions were freeze-dried for 48 h to prepare the corresponding dry samples.
X-ray diffraction (XRD)
The crystallinity and crystalline structure of WCF, RC and CRC were analyzed by XRD (Rigaku D/max 2550PC, Japan) equipped with Cu Kα radiation (40 kV and 200 mA) in a 2θ between 5° and 60°. According to the XRD pattern, degree of crystallinity of sample was estimated by the following equation (Eq. 1),
where CI represented the crystallinity index, \({\text{I}}_{\text{Max}}\) represented the maximum intensity of crystalline cellulose, and \({\text{I}}_{\text{Am}}\) represented the intensity of amorphous cellulose.
Transmission electron microscopy (TEM)
The morphological features of RC and CRC were analyzed by JEM-2100 transmission electron microscope (JEOL, Japan). The samples were diluted to a concentration of 0.1 mg/mL, then dropped on 200-mesh carbon-coated copper grid and dried in the atmosphere.
Thermogravimetric analysis (TGA)
Thermal behaviors of the RC and CRC samples were characterized using a TG 209F1 thermogravimetric analyzer (NETZACH, Germany) under nitrogen atmosphere at a flow rate of 40 mL/min. The samples were heated from 30 °C to 600 °C at a heating rate of 10 °C/min. The derivative of thermal analysis curves of the samples were also recorded.
Rheological properties
The rheological behaviors of RC and CRC suspensions were investigated using a Thermo HAAKE-MARS 60 rheometer equipped with a diameter of 35 mm cone-plate. Viscosity, storage modulus (G′) and loss modulus (G″) of the samples were observed from 0.1 to 10 Hz at a constant temperature of 25 °C. The concentrations of the samples were all 1.0 wt%.
Determination of grafted dye
The amount of grafted dye in CRC was determined spectroscopically using a PerkinElmer (USA) UV–visible spectrophotometer at the maximum absorption wavelength of dye. Pre-dried CRC powder (0.1 g) was completely dissolved in sulfuric acid (5 mL, 70 wt%), and diluted to a suitable concentration for absorption measurements.
Color measurement
Measurement of the color properties (K/S, L, a*, b*, C*, h°) of RC, CRC and colored WPU films were conducted on a Datacolor-650 instrument (Datacolor, USA) equipped with D65 illuminant. L represents the lightness, a* represents red (+ a*) or green (− a*), b* represents yellow (+ b*) or blue (− b*), C* represents the chroma and h° represents hue. K/S value that is color strength is calculated according to the Kubelka–Munk equation (Eq. 2),
where K is the absorption coefficient, S is the scattering coefficient and R is the reflectance of the sample.
The test samples were the RC and CRC films which were prepared by filtering their suspensions (50 g, 0.3 wt%), washing with isopropyl alcohol and drying at room temperature.
Color fastness
The colored WPU films (0.25 g) were immersed in deionized water (15 mL) for 15 days. The soak solutions were observed and tested with PerkinElmer (USA) UV–visible spectrophotometer.
Results and discussion
Effects of temperature, NaCl, Na2CO3 and dye dosage on dye fixation
Fixation of reactive dyes on cellulose is significantly affected by temperature, neutral salt and alkali agent. As shown in Fig. 1a, higher fixation could be achieved when temperature of the dye bath was raised from 50 to 65 °C. However, at higher temperatures fixation of RR194 on RC was compromised by the competing dye hydrolysis (Ahmed 2005). Therefore, 65 °C was chosen as the optimum fixed temperature. Figure 1b and c showed the effects of NaCl and Na2CO3 concentrations on dye fixation, respectively. Results showed that 120 g/L (NaCl) and 30 g/L (Na2CO3) should be used to achieve maximum fixation.
The build-up property of RR194 on RC was investigated and compared to that of cotton fabrics (CF). The results are shown in Fig. 1d. It demonstrated that up to 186.6 mg RR194 could be fixed on 1 g of RC as compared to 56.2 mg/g for CF. More dyes could be anchored on RC due to its larger amorphous areas.
Colored regenerated cellulose from waste cotton fabrics
The FT-IR spectra of RR194, RC and CRC are compared in Fig. 2. The FT-IR spectrum of RR194 dye showed characteristic peaks of triazine C=N vibration at 1554 cm−1 and typical aromatic ring vibration at 1483 cm−1. The FT-IR spectrum of CRC mirrored that of RC except that two weak peaks appeared at 1554 and 1483 cm−1, confirming the presence of triazine and aromatic ring systems due to the attachment of RR194.
The change of crystalline structure of cellulose was verified by the XRD analysis. As shown in Fig. 3, the XRD spectrum of WCF exhibited characteristic peaks at 2θ = 14.8°, 16.5°, 22.7° and 34.3°, corresponding to the (\(\bar{1}10\)), (110), (200) and (004) crystallographic planes of cellulose I, respectively (Sebe et al. 2012). The spectrum of RC showed characteristic crystalline peaks at 2θ = 12.5° and 20.7°, which correspond to (\(\bar{1}10\)) and (110) of cellulose II, indicating a shift from cellulose I to cellulose II during regeneration (Jia et al. 2013). The CIs of cellulose calculated from the spectra were 63% and 41% for the WCF and RC, respectively. The higher amorphous content of the RC indicates that it could be more ready to be modified chemically, and it could be verified by above-mentioned RC and CF dyeing.
The XRD spectra of RC prior to and after dyeing are compared in Fig. 3. The characteristic diffraction peaks related to (\(\bar{1}10\)) and (110) of cellulose II at 2θ = 12.5° and 20.7° were also observed for all the CRC, indicating that modification did not change crystal form of RC. The calculated CIs for CRC-1, CRC-3, CRC-5 and CRC-8 were 32, 26, 25 and 24%, respectively. The noticeable decrease in CI of the CRC could be due to the disruption of intramolecular hydrogen bonds between cellulose chains by the bulky and negatively charged dye moieties (Ashori et al. 2014).
The morphology of RC before and after dyeing was investigated using TEM, and TEM images of RC and CRC are shown in Fig. 4a and b, respectively. As shown in the figures, both RC and CRC featured networked structure are well-dispersed in the suspensions, which consist of nano-fibrils of several tens of nanometers wide and hundreds of nanometers long, respectively. The results indicated that the dyeing treatment didn’t obviously change the morphology of the RC suspension.
The TGA and DTG were conducted to study the thermal stability of the CRC. The TGA and DTG curves of the CRC dyed with different dosages (20, 100, 300 and 1000 mg) of RR194 are shown in Fig. 5 along with those of the RC. The small weight loss of the samples observed in the temperature range of 30–100 °C, could be attributed to evaporation of adsorbed water. As the initial dye dosages increased from 0 to 1000 mg, thermal stability of cellulose was gradually lowered with a change of maximum decomposition temperature (Tmax) from 341 to 308 °C. This could again be attributed to the disruption of intramolecular hydrogen bonds between cellulose chains by the grafted RR194 molecules (Nallathambi and Venkateshwarapuram Rengaswami 2016; Zhang et al. 2015). As the dye content increased, the material became less order and more prone to thermal decomposition.
The effect of dye content on the rheological properties of the CRC suspension was investigated, and the results are summarized in Fig. 6a and b. As shown in Fig. 6a, with the increase of shear frequency, the viscosity of all the cellulose suspensions were gradually decreased. This was because the entanglements of cellulose were continuously and simultaneously destroyed and reformed with the variation of shear frequency (Bekkour et al. 2014). When the disentanglement rate of the cellulose suspensions surpassed their entanglement rate, they exhibited a shear thinning behavior. The trends shown in Fig. 6a suggested that CRC suspension still had typical rheological characteristics of regenerated cellulose.
Compared with the RC, the CRC tended to have lower viscosity, storage modulus (G′) and loss modulus (G″). The reason might be that the interaction and entanglement of cellulose molecules became weaker due to the presence of bulky and negatively charged dye moieties. Within the series of CRC suspensions, there was a trend of decreasing viscosity, G′ and G″ with increasing dye contents according to the Fig. 6. The CRC-8 which contained more RR194, had the lowest viscosity, G′ and G″ of 1.22 Pa·s, 0.95 Pa, and 0.30 Pa at low frequency, respectively, because there were weakest interaction and entanglement between cellulose chains in CRC-8.
The color parameters including K/S, L, a*, b*, C* and h° of the CRC films were measured and listed in Table 3. The K/S values of CRC films ranged from 10.94 to 31.35, and agreed well with the observed dye content of the corresponding samples. The L and C* values decreased from 53.81 and 69.51 for CRC-1 to 35.21 and 63.32 for CRC-8, showing that the color became duller as more RR194 was grafted onto RC.
Application of CRC suspensions
Yellow and blue CRC suspensions were also prepared using commercial RY145 and RB19 (Fig. 7). The CRCs were used for coloring of polyurethane as novel waterborne bio-based pigments. Soak property of WPU/CRC films in deionized water was compared to that of WPU/RC/dye films in Fig. 8a. For the WPU/RC/dye films, leaching of the dyes occurred within 3 h of immersing, while no leaching of color was detected for the WPU/CRC films after 15 days. The UV–vis spectra of the soak solutions of WPU/RC/dye and WPU/CRC films are shown in Fig. 8b. The amount of leached dyes were up to 58.3 mg/L. These results indicated that color fastness of WPU/CRC films was significantly better than the WPU/RC/dye films.
The color parameters of colored WPU films are summarized in Table 4. As shown in Table 4, K/S values of WPU/CRC films were slightly less than those of the WPU/RC/dye films. On the whole, there were very small differences for WPU/CRC and WPU/RC/dye films of the same color, which indicated that CRC as waterborne bio-based pigment was comparable to reactive dye in coloring WPU films.
Conclusions
Colored cellulose suspensions were prepared from waste cotton fabrics (WCF) via a facile exhaust dyeing process. High color build-up of up to 210 mg/g dye fixation and K/S of up to 31 were achieved. Morphology, crystallinity, thermal properties, rheological behaviors and color properties of CRC were investigated. Results indicated that the dyeing treatment didn’t change the morphology of RC suspension but lowered the CI of RC by 17%. Maximum decomposition temperature of CRC gradually decreased to 308 °C with increasing dye content. Within the series of CRC suspensions, there was a trend of decreasing viscosity, storage modulus and loss modulus with increasing dye contents. It was demonstrated that the CRC suspensions could be used to prepare high color fastness WPU/CRC composite films that are resistant to leaching when soaked in deionized water. This study described a simple and feasible method for preparing full-color cellulose materials, and the obtained colored cellulose suspension has great potential applications as waterborne bio-based polymer pigment in waterborne coating, ink, colored filler, textile dyeing and finishing.
References
Abitbol T, Palermo A, Moran-Mirabal JM, Cranston ED (2013) Fluorescent labeling and characterization of cellulose nanocrystals with varying charge contents. Biomacromolecules 14:3278–3284
Ahmed NSE (2005) The use of sodium edate in the dyeing of cotton with reactive dyes. Dyes Pigm 65:221–225
Ashori A, Babaee M, Jonoobi M, Hamzeh Y (2014) Solvent-free acetylation of cellulose nanofibers for improving compatibility and dispersion. Carbohydr Polym 102:369–375
Beija M, Charreyre M-T, Martinho JMG (2011) Dye-labelled polymer chains at specific sites: synthesis by living/controlled polymerization. Prog Polym Sci 36:568–602
Bekkour K, Sun-Waterhouse D, Wadhwa SS (2014) Rheological properties and cloud point of aqueous carboxymethyl cellulose dispersions as modified by high or low methoxyl pectin. Food Res Int 66:247–256
Farahbakhsh N, Venditti RA, Jur JS (2014) Mechanical and thermal investigation of thermoplastic nanocomposite films fabricated using micro- and nano-sized fillers from recycled cotton T-shirts. Cellulose 21:2743–2755
Farahbakhsh N, Roodposhti PS, Ayoub A, Venditti RA, Jur JS (2015) Melt extrusion of polyethylene nanocomposites reinforced withnanofibrillated cellulose from cotton and wood sources. J Appl Polym Sci 132:41857
García A, Gandini A, Labidi J, Belgacem N, Bras J (2016) Industrial and crop wastes: a new source for nanocellulose biorefinery. Ind Crops Prod 93:26–38
Han YY, Zhang XX, Wu XD, Lu CH (2015) Flame retardant, heat insulating cellulose aerogels from waste cotton fabrics by in situ formation of magnesium hydroxide nanoparticles in cellulose gel nanostructures. ACS Sustain Chem Eng 3:1853–1859
Hu Y, Shen BY, Li BT, Xu M, Jiang GQ, Kan CY (2016) Preparation and properties of a novel polymerizable amphiphilic anthraquinone derivative and its cationic colored copolymer latexes. RSC Adv 6:37765–37772
Huang J-L, Li C-J, Gray DG (2013) Cellulose nanocrystals incorporating fluorescent methylcoumarin groups. ACS Sustain Chem Eng 1:1160–1164
Jang B, Kim SY, Do JY (2012) Dye-incorporated water-soluble polymer via click triazole formation. Dyes Pigm 94:217–223
Jia X, Chen YW, Shi C, Ye YF, Wang P, Zeng XX, Wu T (2013) Preparation and characterization of cellulose regenerated from phosphoric acid. J Agric Food Chem 61:12405–12414
Jiang Y, Liu LL, Wang BJ, Sui XF, Zhong Y, Zhang LP, Mao ZP, Xu H (2018) Cellulose-rich oleogels prepared with an emulsion-templated approach. Food Hydrocolloids 77:460–464
Jonoobi M, Oladi R, Davoudpour Y, Oksman K, Dufresne A, Hamzeh Y, Davoodi R (2015) Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: a review. Cellulose 22:935–969
Leng TY, Jakubek ZJ, Mazloumi M, Leung ACW, Johnston LJ (2017) Ensemble and single particle fluorescence characterization of dye-labeled cellulose nanocrystals. Langmuir 33:8002–8011
Li JJ, Song ZQ, Li DG, Shang SB, Guo Y (2014) Cotton cellulose nanofiber-reinforced high density polyethylenecomposites prepared with two different pretreatment methods. Ind Crops Prod 59:318–328
Mao HY, Wang CX, Wang YJ (2015) Synthesis of polymeric dyes based on waterborne polyurethane for improved color stability. New J Chem 39:3543–3550
Miranda R, Sosa_Blanco C, Bustos-Martínez D, Vasile C (2007) Pyrolysis of textile wastes. J Anal Appl Pyrolysis 80:489–495
Moon RJ, Martini A, Nairn J, Simonsen J, Youngblood J (2011) Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev 40:3941–3994
Nallathambi A, Venkateshwarapuram Rengaswami GD (2016) Salt-free reactive dyeing of cotton hosiery fabrics by exhaust application of cationic agent. Carbohydr Polym 152:1–11
Navarro JRG, Bergström L (2014) Labelling of N-hydroxysuccinimide-modified rhodamine B on cellulose nanofibrils by the amidation reaction. RSC Adv 4:60757–60761
Navarro JRG, Conzatti G, Yu Y, Fall AB, Mathew R, Eden M, Bergstrom L (2015) Multicolor fluorescent labeling of cellulose nanofibrils by click chemistry. Biomacromolecules 16:1293–1300
Nechyporchuk O, Belgacem MN, Bras J (2016) Production of cellulose nanofibrils: a review of recent advances. Ind Crops Prod 93:2–25
Nielsen LJ, Eyley S, Thielemans W, Aylott JW (2010) Dual fluorescent labelling of cellulose nanocrystals for pH sensing. Chem Commun 46:8929–8931
Schyrr B, Pasche S, Voirin G, Weder C, Simon YC, Foster EJ (2014) Biosensors based on porous cellulose nanocrystal-poly(vinyl alcohol) scaffolds. ACS Appl Mater Interfaces 6:12674–12683
Sebe G, Ham-Pichavant F, Ibarboure E, Koffi ALC, Tingaut P (2012) Supramolecular structure characterization of cellulose II nanowhiskers produced by acid hydrolysis of cellulose I substrates. Biomacromolecules 13:570–578
Shen F, Xiao WX, Lin LL, Yang G, Zhang YZ, Deng SH (2013) Enzymatic saccharification coupling with polyester recovery from cotton-based waste textiles by phosphoric acid pretreatment. Bioresour Technol 130:248–255
Sun XW, Lu CH, Liu Y, Zhang W, Zhang XX (2014) Melt-processed poly(vinyl alcohol) composites filled with microcrystalline cellulose from waste cotton fabrics. Carbohydr Polym 101:642–649
Tang BT, Zhang SF, Yang JZ, Liu F (2006) Synthesis of a novel water-soluble crosslinking polymeric dye with good dyeing properties. Dyes Pigm 68:69–73
Tian WG, Zhang JM, Yu J, Wu J, Zhang J, He JS, Wang FS (2018) Phototunable full-color emission of cellulose-based dynamic fluorescent materials. Adv Funct Mater 28:1703548
Wang S, Lu A, Zhang LN (2016) Recent advances in regenerated cellulose materials. Prog Polym Sci 53:169–206
Wang ZH, Yao ZJ, Zhou JT, Zhang Y (2017) Reuse of waste cotton cloth for the extraction of cellulose nanocrystals. Carbohydr Polym 157:945–952
Wu XD, Lu CH, Zhou ZH, Yuan GP, Xiong R, Zhang XX (2014) Green synthesis and formation mechanism of cellulose nanocrystal-supported gold nanoparticles with enhanced catalytic performance. Environ Sci Nano 1:71–79
Xiong R, Zhang XX, Tian D, Zhou ZH, Lu CH (2012) Comparing microcrystalline with spherical nanocrystalline cellulose from waste cotton fabrics. Cellulose 19:1189–1198
Yue YY, Zhou CJ, French AD, Xia G, Han GP, Wang QW, Wu QL (2012) Comparative properties of cellulose nano-crystals from native and mercerized cotton fibers. Cellulose 19:1173–1187
Zhang FL, Pang ZQ, Dong CH, Liu Z (2015) Preparing cationic cotton linter cellulose with high substitution degree by ultrasonic treatment. Carbohydr Polym 132:214–220
Zhang YC, Jiang Y, Han L, Wang BJ, Xu H, Zhong Y, Zhang LP, Mao ZP, Sui XF (2018) Biodegradable regenerated cellulose-dispersed composites with improved properties via a pickering emulsion process. Carbohydr Polym 179:86–92
Acknowledgments
This work was financially supported by the Fundamental Research Funds for the Central Universities (Nos. 2232018A3-04 and 2232018-02), the National Key Research and Development Program of China (No. 2016YFC0802802), and the Programmer of Introducing Talents of Discipline to Universities (No. 105-07-005735).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ding, L., Jiang, Y., Wang, B. et al. A waterborne bio-based polymer pigment: colored regenerated cellulose suspension from waste cotton fabrics. Cellulose 25, 7369–7379 (2018). https://doi.org/10.1007/s10570-018-2068-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-018-2068-9