Abstract
Nowadays, there is less emphasis on the aesthetic traits of textiles and more on their functionality. Traditionally functional textiles are prepared in two steps including dyeing and then finishing. This adds extra cost to the process through more energy consumption and water effluent. Current research deals with a single bath application of antibacterial finish with reactive dyeing. L-cysteine (L-cys) is a natural defensive thiolated amino acid found in many living organisms and is applied to cotton fabric through simple conventional reactive dyeing method. Fabrics with L-cys were assessed against both Escherichia Coli and Staphylococcus Aureus strains and promising results were found containing antibacterial activity up to 20 washes. The fastness properties of antimicrobial dyed fabrics were unaffected when compared with control samples. However, shade depth (k/s) was decreased with increasing amount of antibacterial agent while control samples showed highest value. This proposed process reduces chemical- and energy consumption, as well as water effluent.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The textile industry is constantly striving for innovative production techniques to improve product quality, incorporate environment friendly approaches and introduce cost effective processes. Therefore, the conventional trend is now shifting towards reducing the number of steps in the application processes e.g. multifunctional finishing in a single bath (Hou and Sun 2013; Ibrahim et al. 2009), combining pre-treatment processes (Eren et al. 2009) and introducing functionality along with dyeing (Alebeid and Zhao 2016). Functional textiles are much in demand as people now expect more out of it, and antibacterial textile is one of the emerging fields. The growth of bacteria on fabric can cause hygienic problems as well as destroy aesthetics of fabrics. An antibacterial finish can provide protection against these germs. It holds great value for industrial textiles which are exposed to outdoor usages such as tents, tarpaulins, ropes as well as areas where chances of disease spreading are high, for example, hospitals, schools, hotels, nursing homes and public areas (Pan and Sun 2011; Schindler and Hauser 2004). Although their importance in human life cannot be overstated, their application as a finishing process can be lengthy and expensive. Therefore, there is a need to establish processes there they can be applied along with other necessary processes, such as textile coloration.
There are some approaches that can be adopted for simultaneous dyeing and antibacterial finishing. Reactive dye can be synthesized in a way that it can also provide protection against germ, but the drawback associated with them is tailing, as these dyes are cationic in nature (Alihosseini and Sun 2011; Farouk and Gaffer 2013; Kim and Son 2005). Natural dyes extracted from different sources, for example turmeric, walnut and henna showed antibacterial activity (Haque et al. 2015; Umbreen et al. 2008) but their improper fixation, lengthy extraction process, poor fastness properties and durability restricted their application.
Many antimicrobial products have been used in past that are now strictly prohibited because of their toxicity and potential for environmental damage. Some of them include copper naphthenate and numerous organo mercury compounds and formalin (Schindler and Hauser 2004). Silver and other metallic salts or oxides have attracted attention as alternate antimicrobial agents for textile but their toxicity to humans and aquatic life is still an issue (Kim et al. 2009). In addition, when these materials are applied to textiles their bioactivity substantially reduces, hence requiring higher concentration in order to maintain their activity (Gouveia et al. 2012). Thus, the trend shifted towards nature-based antibacterial agents. The significant antimicrobial natural compounds employed are chitosan, modified chitosan (Martins et al. 2011, 2013, 2014), natural dyes, aloe vera, essential oils and some plant extracts (Gouveia 2010). However, limited colour range, narrow antibacterial spectrum, unspecified application conditions and durability limit their application in industry. Therefore, there is a need to explore more antibacterial agents that can overcome the stated problems. L-cysteine is a naturally occurring compound which contains uncharged polar amino acid with R groups containing polarity due to their sulfhydryl groups (Caldeira et al. 2013; Gouveia et al. 2012). These sulfhydryl agents can react with sulfhydryl groups located in gram positive and gram negative bacteria (Nelson et al. 2008). L-cysteine was successfully applied on cotton surface, however, an oxidation reaction was required to activate the cotton surface and hence an alternate application approach was necessary.
In this study, reactive dye will act as a medium to bind L-cysteine to cotton fabric. In the past, reactive dye was used as a bridging agent between sulfamethoxazole, trimethoprim (antibacterial agent) and cotton, and hence imparted durable antibacterial activity (Chun and Gamble 2007). The most distinguishing characteristic of reactive dyes is that they form covalent bonds with terminal –OH (hydroxyl) group of cellulosic fibres during the application process. Thus, the dye molecule contains specific functional groups that can undergo addition or substitution reactions with the OH groups present in cotton (Shore 1995). Amine based antibacterial agent can react with functional group (e.g. vinyl sulphone) of reactive dye which forms covalent bond with cotton.
Materials and methods
100% cotton fabric containing plain weave was purchased locally with 122 ends per inch and 120 picks per inch while GSM (grams per square meter) was 134. Avitara SE Reactive dye was supplied by Huntsman and L-cysteine (Molecular Weight 240.38, CAS number 56-89-3) as antibacterial agent was purchased from Daejung Chemicals Korea. Nutrient broth was purchased from Merck and Nutrient agar from Aqua Medic Inc. (Michigan, MI). All chemicals were of reagent grade and were used without any treatment.
Three levels were set for both dye and antibacterial agent as shown in Table 1. Design of experiment was developed according to Full Factorial approach as shown in Table 2.
Fabric pre-treatment and dyeing
Mercerization of 100% cotton fabric with 30% NaOH was done conducted at room temperature with Jigger Machine followed by thorough rinsing to remove any residual NaOH. pH of fabric was maintained using acetic acid and checked by water extraction method until neutral pH was attained. Simultaneous dyeing and antibacterial finishing was conducted at Jigger machine. Time-temperature profile and sequence of addition of chemicals is shown in Fig. 1. Final hot washing was done to remove all the unfixed dye and chemicals (antibacterial agent).
Antimicrobial activity
A single colony of bacterial strains of Escherichia coli and Staphylococcus aureus was relocated onto nutrient broth for 24 h. The cells were collected by centrifugation and re-suspended in normal saline until they gave absorbance of 0.7 at 600 nm. These cell suspensions were utilized as inocula for antibacterial testing. The fabric examples were put onto petri dish containing nutrient agar and incubated at 37 °C for 24 h. The antibacterial activity was determined by utilizing the standard test of AATCC 147 for the zone of inhibition. The same test was replicated on the treated fabric after 20 washes. The washing was performed at Launderometer (Shimadzu Ltd) by using ISO 105-C06 standard method.
FTIR analysis
Fabrics were analysed by FTIR to determine any structural changes. It can also provide information about the effective linkage of L-cysteine onto cotton fibres. Briefly cotton fabrics dyed with antibacterial agent, without antibacterial agent (controlled samples) and washed (up to 20 washes) were tested. Measurements were done by Tensor 27, manufactured by Perkin Elmer.
Washing fastness
The purpose of this test is to determine the amount of color removed after washing of the textiles materials. The specimen was cut according to the required size and stitched with white bleached fabric. The sample was then placed in the steel containers comprising soap solution, anhydrous sodium carbonate and 100 steel balls. The steel containers were then placed in the Laundrometer at a temperature of 60 °C for time duration of 30 min. After the treatment, the specimen was checked for color fastness according to AATCC 105-C03.
Rubbing fastness
Two sample pieces of not less than 14 × 5 cm, one piece having the long direction parallel to the warp yarn and the other parallel to the weft yarns were cut. Two pieces were used for dry rubbing test and two other similar pieces were cut to use them for wet rubbing test. A Crock meter was used as a rubbing device and untreated sample was used as a control sample. Rubbing fastness was assessed by using AATCC-8 test method.
Light fastness
Light fastness of samples was evaluated according to AATCC test method 16-1993. All treated and controlled samples were exposed to xenon arc lamp for 20 h. The change in color of the test samples was evaluated with the help of grey scales.
Results and discussion
Reaction of Avitera SE dyes to cotton and L-cysteine
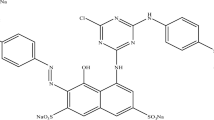
Usually all the reactive dyes offer addition or substitution reaction with the nucleophiles. Initially, the reactive dyes need to be activated from its reactive form to be further covalently bonded with relevant nucleophile. The reactive dyes being sulphatoethyl sulphone needs to be converted to its active vinyl form as shown in Fig. 2.
The activated vinyl sulphone based dye has a potential to react covalently with cellulose as shown in Fig. 3.
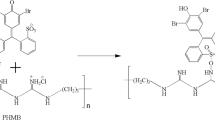
This active vinyl group of the dye has potential to react with L-cysteine as well which has antimicrobial characteristics making the fabric functional dyed with Avitera SE dye shown in Fig. 4. Application of L-cysteine during dyeing eliminates the whole process of finishing which saves time and reduces the cost of chemicals, energy, labour and machinery.
Similarly, the Avitera SE dye might have halogen base reactive groups on its dye molecule which through substitution reaction could covalently bound with the cellulose as shown in Fig. 5.
The dye also has a potential to be covalently bonded with other nucleophiles apart from cellulose such as L-cysteine added to impart the antimicrobial activity of the dyed fabric as shown in Fig. 6.
The reaction mechanisms show that L-cysteine reacts with multi-functional reactive dye which further reacts with cellulose using its second functional group and hence make its reactive bond with cellulosic fabric.
Process schematic
The process schematic is shown in Fig. 7 which illustrates that after mercerization, the cellulosic substrate swells enabling more dyestuff and surfactant to penetrate and react. Later on, after the dyeing treatment, the multifunctional dyestuff reacts with cellulose as well as L-cysteine in either way.
Antibacterial activity
Antibacterial activity of samples can be seen in Figs. 8 and 9 against S. Aureus and E. Coli respectively. Samples containing highest concentration of antibacterial agent showed highest antimicrobial activity. Small zone of inhibition appeared around samples 1, 4 and 7 which were dyed with maximum concentration of antibacterial agent i.e. 6 g/l. However, fabric dyed with low concentration (2 and 4 g/l) showed no clear zone but they inhibit the growth of bacteria’s over their surface. This type of inhibition under the samples always happens whenever the antibacterial agent does not possess the ability to migrate and, therefore, inhibition is restricted to the textile and also to the zones that are in close vicinity of the antimicrobial textiles. Since antibacterial agent was chemically bound to the fabric and dye, therefore it could not leach as much and hence there was controlled growth over samples. Control samples that were prepared for each shade did not show any activity and growth of S. aureus which can be clearly seen over their surface in Fig. 10.
Moreover, the samples sustained their activity after 20 home launderings. Figures 11 and 12 show antibacterial results of washed samples against S. Aureus and E. Coli. The durability of antimicrobial finish on fabric was mainly because of its bond formation with fabric. Reactive dye acted as a bridge between antimicrobial agent and cotton fabric. L-cysteine application did not require binder as it happens with most antibacterial finishing, Furthermore, antibacterial agents that are applied with binders abrade away eventually.
Chemical structure using FTIR
Figure 13 shows FTIR spectrums of one of the samples (7) with all phases, i.e. treated (F7), untreated (F7-C) and treated sample after washing (F7-W). The broad peaks between 3000 and 3600 showed presence of hydroxyl and stretching of primary amine. The major difference between sample 7 and its control was found between 2500 and 3500 cm−1 wavelength where control sample showed less transmittance than the treated sample. This shows that dyed sample also contains amine groups because of the amine group present in Avetira SE. However, difference in transmittance shows that antimicrobial treated sample contain more amine groups. The perfect superimposing of peaks F7 and F7-W showed that there was no appreciable difference found in the chemical composition of both samples. Hence, it can be stated that, the finish was sustained in the sample even after multiple washes.
Wash fastness
Table 3 shows that washing fastness of all samples was approximately same. Washing fastness of control samples and those treated with lower concentration of anti- bacterial agent showed same results. However, samples dyed with 6 g/l L-cysteine showed slightly lower fastness. The reason can be stated that higher amount of antibacterial agent reacted with dye molecule results in larger molecular size which further interrupt the proper penetration and fixation of dye in fabric, and during laundering, traces of dye washed off.
Colour staining
Fastness to colour staining is shown in Table 3 indicating that the amount of antibacterial agent did not influence fastness property of dyed fabric. However, it was observed that control samples showed slightly better rating than the others for all shades. The reason is same as was described for washing fastness. More dye came out during washing and stained the adjacent cotton fabric.
Light fastness
It was observed that light fastness of all samples was same as that of control samples. There was no influence of antibacterial agent and its amount on the light fastness of fabric. A possible reason can be that, the light fastness is an intrinsic property of any dye which mainly depends on the electronic configuration of chromophoric part of the dye, and usually independent of the application methods and sometimes ancillary chemicals as well (Gohl and Vilensky 1983).
Rubbing fastness
In both wet and dry state, the rubbing fastness properties of samples dyed with antibacterial agent are similar to the control samples as shown in Table 3. However wet rubbing fastness is slightly lower than dry rubbing fastness because in wet state reactive dye acquires a medium through which it rubs off.
K/S value
The graph in Fig. 14 shows control samples for each dyed fabric with different shades, and presents the highest absorbance peak among all the samples which were dyed simultaneously with antibacterial finish. On comparing samples treated with different concentration of antibacterial agent, it was found that with increase in L-cysteine concentration, the K/S was decreased. It can be justified, as the amount of antibacterial agent increases, the reactive groups attached to the reactive dye molecules get covalently bonded with the antimicrobial agent rather with cellulose resulting in less fixation as well as low penetration of dye due to bigger molecular size, resulting in decrement of shade depth.
The antibacterial formulations being used in textile products are based on silver and Titanium dioxide which are more expensive than L-cysteine. These formulations are not environmental friendly as well. They contain dispersing agents and binders which have negative impact on environment. Moreover, usage of higher quantities of silver may have negative effect on human cells. On contrary, L-cysteine reacts with dye molecule, therefore, no need for usage of additional binders and it does not have any harmful effect on humans.
Conclusion
This work describes a novel methodology to provide antibacterial functionality during reactive dyeing of cotton using L-cysteine, a natural product emerging these days, as an antimicrobial agent for textiles. The distinctive factor of this antimicrobial finish over others is its non-toxicity both to the potential users and to the environment. It is easily applicable and compatible during dyeing of cotton textiles with reactive dyes. In addition, it does not have deteriorating effect on fastness properties of dyed fabric. It is entirely a novel environment friendly and cost effective approach which may open new applications for cotton, particularly, in health-care sector.
References
Alebeid OK, Zhao T (2016) Simultaneous dyeing and functional finishing of cotton fabric using reactive dyes doped with TiO2 nano-sol. J Text Inst 107:625–635
Alihosseini F, Sun G (2011) 17—antibacterial colorants for textiles. In: Sun G, Pan N (eds) Functional textiles for improved performance, protection and health. Woodhead Publishing, Cambridge. https://doi.org/10.1533/9780857092878.376
Caldeira E, Piskin E, Granadeiro L, Silva F, Gouveia IC (2013) Biofunctionalization of cellulosic fibres with l-cysteine: assessment of antibacterial properties and mechanism of action against Staphylococcus aureus and Klebsiella pneumoniae. J Biotechnol 168:426–435
Chun DT, Gamble GR (2007) Using the reactive dye method to attach antibacterial compounds to cotton. In: 2007 Beltwide cotton conferences, New Orleans, Louisiana, pp 1242–1246
Eren HA, Anis P, Davulcu A (2009) Enzymatic one-bath desizing—bleaching—dyeing process for cotton fabrics. Text Res J 79:1091–1098. https://doi.org/10.1177/0040517508099388
Farouk R, Gaffer H (2013) Simultaneous dyeing and antibacterial finishing for cotton cellulose using a new reactive dye. Carbohydr Polym 97:138–142
Gohl EPG, Vilensky LD (1983) Textile science. CBS Publishers and Distributors, Delhi
Gouveia IC (2010) Nanobiotechnology: a new strategy to develop non-toxic antimicrobial textiles current research, technology and education topics in applied microbiology and microbial biotechnology. Formatex Badajoz 150:407–414
Gouveia IC, Sá D, Henriques M (2012) Functionalization of wool with l-cysteine: process characterization and assessment of antimicrobial activity and cytotoxicity. J Appl Polym Sci 124:1352–1358. https://doi.org/10.1002/app.34587
Haque ANMA, Hannan M, Rana MM (2015) Compatibility analysis of reactive dyes by exhaustion-fixation and adsorption isotherm on knitted cotton fabric. Fash Text 2:1–12
Hou A, Sun G (2013) Multifunctional finishing of cotton fabrics with 3,3′,4,4′-benzophenone tetracarboxylic dianhydride: reaction mechanism. Carbohyd Polym 95:768–772. https://doi.org/10.1016/j.carbpol.2013.02.027
Ibrahim NA, Gouda M, Husseiny SM, El-Gamal AR, Mahrous F (2009) UV-protecting and antibacterial finishing of cotton knits. J Appl Polym Sci 112:3589–3596. https://doi.org/10.1002/app.29669
Kim T-K, Son Y-A (2005) Effect of reactive anionic agent on dyeing of cellulosic fibers with a Berberine colorant—part 2: anionic agent treatment and antimicrobial activity of a Berberine dyeing. Dyes Pigm 64:85–89
Kim S, Choi JE, Choi J, Chung K-H, Park K, Yi J, Ryu D-Y (2009) Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicol Vitro 23:1076–1084
Martins AF, Pereira AG, Fajardo AR, Rubira AF, Muniz EC (2011) Characterization of polyelectrolytes complexes based on N, N, N-trimethyl chitosan/heparin prepared at different pH conditions. Carbohydr Polym 86:1266–1272
Martins AF, Bueno PV, Almeida EA, Rodrigues FH, Rubira AF, Muniz EC (2013) Characterization of N-trimethyl chitosan/alginate complexes and curcumin release. Int J Biol Macromol 57:174–184
Martins AF, Facchi SP, Follmann HD, Pereira AG, Rubira AF, Muniz EC (2014) Antimicrobial activity of chitosan derivatives containing N-quaternized moieties in its backbone: a review. Int J Mol Sci 15:20800–20832
Nelson DL, Lehninger AL, Cox MM (2008) Lehninger principles of biochemistry. W. H. Freeman, New York
Pan N, Sun G (2011) Functional textiles for improved performance, protection and health. Elsevier, Amsterdam
Schindler WD, Hauser PJ (2004) Chemical finishing of textiles. Elsevier, Amsterdam
Shore J (1995) Cellulosics dyeing. Soc Dye Colour, p 189
Umbreen S, Ali S, Hussain T, Nawaz R (2008) Dyeing properties of natural dyes extracted from turmeric and their comparison with reactive dyeing. Res J Text Appar 12:1–11
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Rehman, A., Rehman, A., Khalid, W. et al. Simultaneous dyeing and anti-bacterial finishing on 100% cotton fabric: process establishment and characterization. Cellulose 25, 5405–5414 (2018). https://doi.org/10.1007/s10570-018-1934-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-018-1934-9