Abstract
In this work, a novel mixed matrix membrane (MMM) was developed based on cellulose acetate (CA), graphene oxide (GO) nanosheets, and Fe3O4 magnetic nanoparticles. In the first step, a nanocomposite was prepared by grafting 2-acrylamide-2-methyl propanesulfonic acid-maleic anhydride copolymer [poly(MA-co-AMPS)] on the functionalized GO surface (GO-APTS) and decorating with Fe3O4 nanoparticles. Then, the prepared nanocomposite [Fe3O4@GO-APTS-poly(AMPS-co-MA)] was blended with CA and membranes with different compositions were prepared via phase inversion procedure. Hydrophilicity, thermal properties, morphology, and surface roughness of the MMMs were studied by water contact angle, TGA, FE-SEM, and AFM techniques, respectively. Filtration properties and dye removal performance of the prepared membranes were evaluated. The highest rejection achieved by the membrane containing 0.2 wt% nanocomposite for all of the tested anionic dyes (Acid Blue 7, Reactive Red 120, and Direct Red 23), reaching up to 100% at pH = 9. This observation was interesting, that only 0.2 wt% of the additive had a huge impact on the rejection efficiency compared to the pure CA membrane for about 20%.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The most important properties that a polymer should have for membrane matrix are good film-forming, low cost, and chemical and mechanical stability for specific operating conditions (Lee et al. 2011). Cellulose acetate, polyethersulfone, and polyamide are polymeric materials, which have high volume applications for the preparation of membrane systems. However, these materials require some physicochemical modifications before using them for membrane production of a particular application (Childress and Elimelech 2000). Researchers have used different organic and inorganic compounds and various techniques for fabrication of the cellulose acetate membranes to improve the stability, selectivity, and filtration performance (Baker 2000; Han et al. 2013; Mahdavi and Shahalizade 2015; Battirola et al. 2017; de Faria et al. 2017). Introducing –SO3H and –NH2 functional groups in the chemical structure of the polymer can change the surface charge of the membrane, which can greatly increase the removal of charged solute molecules via Donnan exclusion (Yaroshchuk 2001; Taha et al. 2012).
Graphene oxide (GO) is an inexpensive solid material with a large surface area containing various hydrophilic oxygenated polar groups such as hydroxyl, epoxy, and carboxyl groups, which improve adsorption properties of adsorbents. Functionalization of GO nanosheets surface by grafting on with particular organic materials is an easy method to enhance their compatibility and reactivity with the functional groups of the polymers (Lin et al. 2011). Small amounts of these modified GO nanosheets could apply desired characteristics to the membrane matrix with a minimum decrease in the mechanical stability (Mahdavi et al. 2017). Recently, a mixed matrix membrane was prepared by incorporating organosilane-functionalized GO on polyvinylidene difluoride (PVDF) structure. It was reported that the tensile strength and elongation-at-break were improved by 69.01 and 48.38%, respectively, compared to those of PVDF/GO. This improvement was attributed to the better interfacial compatibility between the polymer matrix and organosilane-functionalized GO (Xu et al. 2014). Other researchers prepared polyethersulfone/GO nanocomposite membranes and found that adding GO to the polymer matrix increased the mechanical strength (Lee et al. 2011; Ionita et al. 2014). While increasing the amount of GO to 2 wt% led to the weaker mechanical strength due to the aggregation of GO in the polyethersulfone matrix. In other studies, the prepared membranes by using 0.05 wt% rGO-TiO2 in PVDF (Safarpour et al. 2014) and 0.3 wt% GO in poly(m-phenylene isophthalamide) (Yang et al. 2017) showed an increase in the quantities of water flux. These studies lead to the conclusion that generally GO has poor dispersion at high loading in most polymer matrices.
Fe3O4 nanoparticles as an inorganic additive are able to improve antifouling properties, mechanical strength, hydrophilicity, and filtration performance of a membrane. This is due to their high surface area, hydrophilicity, and thermal and chemical stability. The membrane prepared from polysulfone/poly(vinylpyrrolidone)/Fe3O4 showed improved filtration and anti-fouling properties (Huang et al. 2008). It has also been reported that low concentrations of the O-carboxymethyl chitosan/Fe3O4 additive in a polyethersulfone/O-carboxymethyl chitosan/Fe3O4-based mixed matrix membrane resulted in high retention of Direct Red 16 dye and enhanced water flux (Zinadini et al. 2014a). In another study, polyvinyl chloride/cellulose acetate/Fe3O4 membrane was prepared and used for the removal of lead from aqueous solution (Gholami et al. 2014). Magnetic nanoparticles embedding in the polymer matrix enhance adsorption properties due to the improvement in electrostatic interactions. Most of these studies involved in direct incorporation of metal or metal oxide to the membrane or blending polymer matrix with metal oxide decorated GO. There are few studies focused on polymer grafted GO embedded in membrane matrix in literature. In this study, a magnetic nanocomposite was synthesized by grafting poly(AMPS-co-MA) on the APTS-functionalized GO (GO-APTS) and decorating with Fe3O4 nanoparticles. The functionalized GO nanosheets are chemically attached to the copolymer chains via ring opening reaction of maleic anhydride of the copolymer with the amine groups of GO-APTS. The prepared hybrid nanocomposite [Fe3O4@GO-APTS-poly(AMPS-co-MA)] possesses magnetic particles and charged functional groups embedded in the GO framework, enabling electrostatic repulsion for efficient charged solute retention. Furthermore, the copolymer chains enable high compatibility between the nanocomposite particles and the membrane matrix. The homogeneous mixtures of different amounts of nanocomposite and CA were prepared in NMP solvent and cast on the glass plates. After casting, the film was immediately immersed in a water bath. This method of preparation of membrane from a solvent/nonsolvent fast exchange process is known as phase inversion. The filtration properties including bulk and surface hydrophilicity, dye removal performance, morphological, and thermal characteristics of the prepared mixed matrix membrane were investigated.
Experimental
Materials
Natural graphite (99.5%), 2-acrylamide-2-methyl-1-propanesulfonic acid (AMPS 99.0%), maleic anhydride (MA ≥ 99.0%) and benzoyl peroxide (BPO) were supplied from Sigma-Aldrich. Ferric chloride (FeCl3·H2O, 98%) and ferrous chloride (FeCl2·4H2O ≥ 99.0%) were purchased from Fluka. Cellulose acetate (average Mw of 100,000 g mol−1 and 38.9% acetyl content), (3-aminopropyl) triethoxysilane (APTS, 99%) and N-methylpyrrolidone (NMP 97%) were purchased from Merck. Acid Blue 7 (Mw = 690.81 g mol−1), Reactive Red 120 (Mw = 1469.98 g mol−1) and Direct Red 23 (Mw = 813.72 g mol−1) as anionic dyes were purchased from Sigma-Aldrich. All chemicals were analytical grade and used without further purification.
Synthesis of poly(AMPS-co-MA)
Poly(AMPS-co-MA) was synthesized via radical copolymerization of MA and AMPS monomers in the presence of benzoyl peroxide (BPO) as the initiator. 1 g MA and 2 g AMPS were dissolved in THF and after degassing and the addition of 0.3 g BPO, the reaction mixture was stirred magnetically for 12 h at 80 °C under N2 atmosphere. Poly(AMPS-co-MA) was isolated from the reaction mixture by precipitation in diethyl ether and then dried under vacuum at 50 °C (Sahraei et al. 2016).
Synthesis of Fe3O4@GO-APTS-poly(AMPS-co-MA)
GO was synthesized according to the improved Hummers’ method and characterized before use (Marcano et al. 2010). GO nanosheets were functionalized with the silane coupling agent (APTS) to generate amine groups on their surfaces. For this purpose 0.1 g GO was sonicated in 20 mL anhydrous toluene. Then a solution of APTS (0.2 g in 5.0 mL dry chloroform) was added to the GO dispersion and the mixture was stirred for 72 h at 100 °C. Fe3O4 nanoparticles were prepared using FeCl3·6H2O (10 mmol, 2.730 g) and FeCl2·4H2O through conventional co-precipitation method (Liu et al. 2004; Hemmati et al. 2016). In the last step, Fe3O4@GO-APTS-poly(AMPS-co-MA) nanocomposite was synthesized according to the previously reported method (Sahraei et al. 2016), by mixing poly(AMPS-co-MA), GO-APTS, and Fe3O4 nanoparticles with a weight ratio of 2:0.2:0.12 in 30 mL THF and deploying triethylamine as the catalyst. The reaction was carried out by sonicating the mixture for 30 min and stirring for additional 12 h. Final product was separated using a neodymium magnet, washed, and vacuum-dried at 50 °C for 24 h °C.
Preparation of mixed matrix membranes
Different amounts of the prepared nanocomposite (1–5 mg) were dispersed in 4 mL NMP via ultra-sonication for 1 h. After preparation of the uniform dispersion, 1 g CA powder was added to the mixture and stirred for 4 h. The solution was sonicated for another 1 h and then degassed using a desiccator to remove bubbles before casting. Dope solution was cast on a smooth glass using a doctor blade with a gap of 400 µm, and the cast film, after a 10 min delay was immersed in coagulation bath filled with 2 L of 0 °C deionized water. The prepared mixed matrix membrane samples were kept in deionized water. The dope solution compositions of the mixed matrix membrane were designated as M-FGO0, M-FGO0.1, M-FGO0.2, M-FGO0.3, M-FGO0.5 and listed in Table 1.
Characterizations
Fourier transform infrared spectroscopy (FTIR) technique was deployed to confirm each synthesis step and chemical composition of the membrane samples using a vector 22 spectrometer, Bruker. Raman spectra of GO and pristine graphite were obtained to investigate the lattice defects (Senterra, Bruker). Atomic force microscopy (AFM) was used to investigate GO and membrane surfaces in tapping mode at a resonance frequency of 250 kHz by a Veeco (AutoProbe CP–Research). Cross-sectional and surface morphologies of MMMs were imaged via a field emission scanning electron microscopy (FE-SEM, S-4160, Hitachi). Thermogravimetric analysis (TGA) was performed on a TGA STA 504 instrument to study the thermal behavior of the nanostructured materials and membrane samples in the temperature range of 25–600 °C under air atmosphere at a heating rate of 10 °C min−1. The water contact angle was performed by OCA 15 Plus, Data-physics instrument to characterize the surface hydrophilicity of pure and mixed matrix membranes. The membrane mean pore radius (rm) was calculated based on gravimetric method. To this end, water content and porosity (ε) values calculated using weights of the wet and dry membrane samples, according to the following equations (Yu et al. 2013):
where W w and W d is weight (kg) of wet and dry membrane samples, respectively. A is the membrane surface area (m2), h represents membrane thickness (m), d w is the water density (1000 kg m−3), Q is the volume of the permeate per unit time (m3 s−1), η is the water viscosity (8.9 × 10−4 Pa s), and ΔP is the operating pressure (Pa).
Evaluation of filtration properties
The flux of pure water and dye solutions were measured by using a dead-end cell and a trance-membrane pressure of 500 kPa. Flux data were calculated according to Eq. (4):
where Q is the quantity of permeate (L), J is flux (L m−2 h−1), Δt is the experiment time (h), and A is the effective surface area (m2).
Dye rejection experiments
Dye rejection performances of the membrane samples were measured using 60 ppm solutions of anionic dyes including Acid Blue 7, Reactive Red 120 and Direct Red 23 at different pH values. A PHS-3C pH-meter, Tianyou was used for pH adjustments. Dye concentrations in the feed (cf) and permeate (cp) solutions were measured by a UV–Vis spectrophotometer (Braic 2100), and rejection (%R) was calculated according to Eq. (5):
Results and discussion
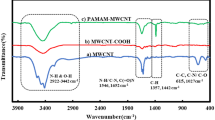
The magnetic nanocomposite GO-APTS-poly(AMPS-co-MA)/Fe3O4 was synthesized by the following steps: (1) Synthesis of GO from graphite via the improved method (Marcano et al. 2010). (2) Amine-functionalization of GO by APTS grafting. (3) Synthesis of poly(AMPS-co-MA) via radical polymerization of AMPS and MA using BPO as an initiator. (4) Chemically attachment of poly(AMPS-co-MA) to GO-APTS nanosheets via ring opening reaction. (5) Finally, the magnetic nanocomposite GO-APTS-poly(AMPS-co-MA)/Fe3O4 was prepared by incorporating Fe3O4 nanoparticles into the GO-APTSpoly(AMPS-co-MA) matrix via physical adsorption. The mixed matrix membranes were fabricated from homogeneous mixtures of the prepared nanocomposite and CA in NMP and by using the solution-casting technique on a glass plate. After casting, the film was immediately immersed in a water bath. This method, known as phase inversion, leads to membranes having an asymmetric structure derived from a solvent/nonsolvent fast exchange process. Surface modification of GO with APTS provides terminal amine groups enabling GO nanosheets to be connected chemically with the copolymer [poly(AMPS-co-MA)] that may be useful for membrane casting. The main idea here is that the interaction between nanocomposite particles and cellulose acetate will mainly depend on the hydrogen bonds. Schematic presentation of the nanocomposite synthesis steps is depicted in Scheme 1. FTIR spectra of GO, GO-APTS, poly(AMPS-co-MA), Fe3O4@GO-APTS-poly(AMPS-co-MA), pure CA membrane (M-FGO0), and M-FGO0.5 are shown in Fig. 1. GO spectrum (Fig. 1a) shows oxygen-containing groups absorption bands at 3500 cm−1 (O–H), 1725 cm−1 (C=O stretching), 1400 cm−1 (C=O vibration), and 1066 cm−1 (C–O vibration). New absorption bands in the spectrum of GO-APTS (Fig. 1b) at ~ 2925 and ~ 2860 cm−1 are related to methyl and methene groups of APTS. Other strong absorption bands also at 1119 and 1567 cm−1 correspond to the vibrations of Si–O–C/Si–O–Si bonds and NH2 scissoring. N–H stretching at 3400–3500 cm−1 overlapped with hydroxyl absorption band. Characteristic absorption bands of poly(AMPS-co-MA) were detected at about 628 cm−1 (C–S stretching) and 1040 cm−1 (S=O stretching). The C=O and cyclic C–O–C absorption bands of MA observed at 1710 and 1220 cm−1, respectively (Fig. 1c). Fe3O4@GO-APTS-poly(AMPS-co-MA) spectrum in Fig. 1d shows absorption bands at 2925 cm−1 (C–H stretching), 1636 cm−1 (C=O stretching), 1039 cm−1 (S=O stretching), 1212 cm−1 (C–O stretching), and 675 cm−1 (Fe–O stretching). Furthermore, the cyclic C–O–C absorption band of MA disappeared and a small shoulder at ~ 3420 cm−1 and a medium absorption band at 1565 cm−1 related to the amine groups of GO-APTS and AMPS monomer were observed. Membrane samples showed characteristic absorption bands of the CA (C=O and C–O stretching as shown in Fig. 1e, f). M-FGO0.5 showed the same absorption bands with a slight red shift, also a medium absorption band at 3400 cm−1 and a weak band at ~ 1560 cm−1 related to the amine group of the nanocomposite (Devrim et al. 2006; Sahraei et al. 2016).
Lattice structure and morphology of GO was analyzed by means of atomic force microscopy (AFM) and Raman spectroscopy, the results are depicted in Fig. 2. As expected, main D and G peaks were observed at 1345 and 1596 cm−1, respectively (Fig. 2a). G peak is very close to 1600 cm−1, indicating that few layers of GO were synthesized (King et al. 2016). The G band is a result of in-plane vibrations of SP2 bonded carbon atoms whereas the D band is due to out of plane vibrations attributed to the presence of structural defects (Kudin et al. 2008; Lalwani et al. 2013). Therefore, higher ID/IG intensity ratio of GO in comparison with graphite is a representative of higher lattice disorders created due to efficient oxidation. In order to perform AFM analysis, a droplet of GO trace dispersion was spin-coated on a clean silicon wafer and the images were recorded (Fig. 2b, c). The Results showed that the prepared GO nanosheets were 4 layers with the average thickness of about 4.1 nm (Stankovich et al. 2007).
Water flux experiments were performed and the results in Fig. 3 show that the permeability properties of water flux of M-FGO0.1 and M-FGO0.2 membranes decreased. This observation could be attributed to the high compatibility and miscibility of the nanocomposite particles and membrane matrix, resulting in efficient dispersion and pore blocking (Vatanpour et al. 2012a; Zinadini et al. 2014b). M-FGO0.3 and M-FGO0.5 displayed higher flux with an irregular trend. M-FGO0.3 showed the highest flux among the modified samples, almost reaching the pure CA membrane (M-FGO0). This behavior could be explained by the poor miscibility and formation of larger surface pores because of the aggregation of the nanocomposite particles. At 0.5 wt% of additive, there is some decrease in water flux showing that the formation of a dense top layer with smaller pores due to higher viscosity and also blocking of pores by the additive is dominating phenomenon again. The pore size of the membrane reduces at high loading of additive due to the slow solidification of the polymer during phase inversion between solvent and nonsolvent. Additionally, it could be because of the viscosity increase due to the adding large amounts of additive (Guillen et al. 2011; Thuyavan et al. 2016). The decrease of water flux at high additive loading has also been reported by another researcher for mixed matrix membranes containing GO (Ammar et al. 2016; Yang et al. 2017). However, all studies anticipate that the controlled dispersion of the nanocomposite particles in the membrane is the main factor to the proposed research.
Water flux trends of pure CA and mixed matrix membranes as a function of time are displayed in Fig. 4. All samples showed a similar trend with a very small decrease under the operation pressure, suggesting that the mixed matrix membrane samples have acceptable resistivity against compaction and flux decline. Dye solution fluxes were also determined under same trans-membrane pressure and the results are shown in Fig. 5. Overall data was accordant with the pure water flux values, but molecular weight of dyes was not necessarily determinative, indicating that molecule rigidity and 3D structure of the dye molecules are also playing roles.
Anionic dye rejection performances were assessed using the filtration cell at pH 7 and 9 and the results are shown in Fig. 6. Chemical structures and molecular weights of dyes are shown in Fig. 7.
According to the results, mostly an inverse relation was observed between the permeability of membranes and dye rejection performances at pH 7, which is well matched with the calculated pore size data. Maximum rejection values obtained by M-FGO0.2 sample (%RAB 7 = 82.27, %RRR 120 = 84.40, %RDR 23 = 87.87 at pH 7). The rejection values of all tested dyes increased by all samples with increasing pH from 7 to 9 and reached up to 100% for M-FGO0.2 sample (%RAB 7 = 100, %RRR 120 = 98.52, and %RDR 23 = 100). Dye removal performance of the samples (M-FGO0.3 and M-FGO0.5) loaded with the higher amounts of nanocomposite showed improvement at pH 9 and the gap between the rejection curves at pH 7 and 9 became broader. This clearly indicates the repulsion between incorporated polar groups such as –OH, –COOH, –SO3H and –NHCO and anionic groups of dye molecules which are charged at pH 9 (Yaroshchuk 2001; Yu et al. 2010). Therefore, Donnan exclusion between negatively charged functional groups of the membrane and anionic groups of dyes is involved (He et al. 2009; Abid et al. 2012). Dye molecules can aggregate in aqueous solution due to the presence of numerous aromatic rings and strong Van der Waals forces. A larger number of sulfonate groups in the molecule structure causes lower aggregation constants of the dyes (Park et al. 2012). This could be the reason that the rejection value of Reactive Red 120 containing six sulfonate groups is slightly lower than the rejection value of Direct Red 23. In other words, the supramolecular self-organization hypothesis is less probable in case of Reactive Red 120. In general, the size and distribution of surface pores are effective factors on water flux and solute rejection. For a given coagulation condition (constant nonsolvent volume and temperature, humidity, etc.), dope solution properties such as viscosity, chemical structure, and concentrations of the polymer and additive are foundational to the surface pore size. Higher viscosity results in delayed demixing and denser membrane structure. In addition, additive agglomeration and pore blocking take place at high additive loading. On the other hand, polar additive tending to migrate to the interface with a nonsolvent increases pore size (Guillen et al. 2011; Ganesh et al. 2013). The main properties of the prepared membranes including water content, mean pore diameter, and porosity are listed in Table 2. The highest porosity and the smallest rm values were obtained for M-FGO0.2. Rejection tests and data in Table 2 showed the rejection of the dyes increased for M-FGO0.1 and M-FGO0.2 due to the decrease in the pore size. The exception is the M-FGO0.3 membrane, where the dye rejection decreased which can be explained by the increase of the pore size due to the additive migration during phase inversion process (Ganesh et al. 2013). Water content values of the M-FGO0.1 and M-FGO0.2 membranes were almost the same as the water content of pure CA membrane (M-FGO0), which can be attributed to the good miscibility of the hydrophilic nanocomposite inside of the membrane matrix. The water content of the membranes of M-FGO0.3 and M-FGO0.5 was higher than that of M-FGO0, showing higher bulk hydrophilicity due to the higher porosity, better connectivity and accessibility to the bulk pores. Water contact angle is an imperative parameter demonstrating surface overall hydrophilicity determined by physicochemical properties. However, surface chemical nature and roughness both affect contact angle and this analysis is much more beneficial when carried out alongside with AFM (Yu et al. 2010). The contact angle values remained almost the same as the pure CA membrane initially when the content of nanocomposite was 0.1 and 0.2 wt% but increased when the content of nanocomposite increased to 0.3 and 0.5 wt%. This increase in the contact angle can be due to the surface roughness because of the aggregation of nanoparticles.
Three-dimensional AFM images of M-FGO0, M-FGO0.2, and M-FGO0.5 samples are shown in Fig. 8. Moreover, some of the roughness parameters obtained from the depicted sections are reported in Table 3. According to the AFM results, surface roughness increased with increasing nanocomposite loading, reaching up to a height of 130 nm in case of M-FGO0.5. The reason behind that could be the migration of large additive molecules to the surface during coagulation process that affects also the porosity of the membrane. AFM results alongside with contact angle analysis suggest that the surface roughness is also affected the surface hydrophilicity due to the trapped air at higher additive loading (Khulbe 2003; Zhong et al. 2012; Mahdavi et al. 2016).
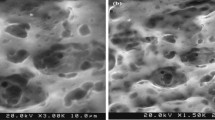
Surface and cross-sectional FE-SEM images of pure and mixed matrix membrane samples are presented in Figs. 9 and 10, respectively. The top surface and sub-layer morphologies of the membranes could be elucidated via FE-SEM imaging. As expected from AFM analysis, surface roughness increased at higher nanocomposite loading and streaks were observed for M-FGO0.5 sample. This morphology could be due to the agglomeration of Fe3O4@GO-APTS-poly(AMPS-co-MA) on the top surface of the membrane.
A cross-sectional view of M-FGO0, M-FGO0.2, and M-FGO0.5 samples were also depicted in Fig. 10. Pure CA sample showed a top thin layer with large channels in sub-layer section (Fig. 10a). Higher viscosity and nucleation rate are the probable reason of denser structure and smaller finger-like structures observed for M-FGO0.2 and M-FGO0.5 samples. However, at higher nanocomposite loading, agglomeration and macro-void formation cause increase of the flux and poor dye rejection values (Vatanpour et al. 2012b).
TGA curves of GO, Fe3O4@GO-APTS-poly(AMPS-co-MA), M-FGO0, M-FGO0.2, and M-FGO0.5 are shown in Fig. 11. GO showed a weight loss under 100 °C with a relatively steep slope, due to loosing of the adsorbed water molecules. Another significant weight loss ascribed to the decomposition of oxygen-containing functional groups started around 150 °C. The final weight loss for GO observed after 400 °C with a low slope, which could be related to pyrolysis of the carbon skeleton (Wu et al. 2012; Hsiao et al. 2013). Fe3O4@GO-APTS-poly(AMPS-co-MA) nanocomposite showed a highly improved thermal behavior with almost no distinguishable weight loss above 100 °C and about 60% weight residue at 600 °C. This is good evidence confirming the presence of Fe3O4 in the nanocomposite structure and modification of GO nanosheets. TGA curves of the pure CA sample (M-FGO0) and mixed matrix membranes (M-FGO0.2, M-FGO0.5) are also depicted in Fig. 11. The main degradation mechanism of CA chains is acetic acid elimination and formation of unsaturated products (Maria da Conceição et al. 2003). The membrane samples demonstrated acceptable thermal stability with a weight residue around 80–90% at 350 °C. The M-FGO0.5 membrane with 0.5 wt% loading of nanofiller showed higher thermal stability (weight residue = ~ 4.5% at 600 °C) than the M-FGO0.2 and the neat CA membrane. The thermal stability of M-FGO0.2 is almost the same as the thermal stability of the neat CA membrane. As M-FGO0.2 contains a very small amount of additive, its effect on thermal properties should be negligible.
Conclusions
In this study, mixed matrix membranes were prepared by incorporation of a highly functionalized GO-based magnetic nanocomposite in the cellulose acetate, and their filtration performances, surface properties, and other key parameters were evaluated. The effect of the loading percent of the nanocomposite in the mixed matrix membranes and pH on the flux and anionic dye removal were also assessed. The magnetic nanocomposite Fe3O4@GO-APTS-poly(AMPS-co-MA) with charged functional groups embedded in the copolymer poly(AMPS-co-MA) grafted on with the functionalized GO (GO-APTS) nanosheets enables the membrane with electrostatic repulsion and enhanced solute retention. The polymeric segments provide compatibility and effective dispersion of hybrid nanostructure in the membrane matrix. The results showed that the Donnan exclusion is an effective phenomenon enabling high dye rejection rates through electrostatic repulsion between charged functional groups of membrane and dye molecules with only a small amount of the nanofiller. However, nanocomposite loading should be optimized to avoid undesired effects such as rough and non-uniform surface morphology. M-FGO0.2 membrane containing 0.2 wt% loading of nanocomposite showed many superior properties compared to the neat CA membrane in terms of permeability, surface properties, and rejection rate which was higher than 98.5% for all three tested dyes at pH 9.
References
Abid MF, Zablouk MA, Abid-Alameer AM (2012) Experimental study of dye removal from industrial wastewater by membrane technologies of reverse osmosis and nanofiltration. Iran J Environ Heal Sci Eng 9:17. https://doi.org/10.1186/1735-2746-9-17
Ammar A, Al-Enizi AM, AlMaadeed MA, Karim A (2016) Influence of graphene oxide on mechanical, morphological, barrier, and electrical properties of polymer membranes. Arab J Chem 9:274–286. https://doi.org/10.1016/j.arabjc.2015.07.006
Baker RW (2000) Membrane technology. Wiley Online Library, New York
Battirola LC, Andrade PF, Marson GV et al (2017) Cellulose acetate/cellulose nanofiber membranes for whey and fruit juice microfiltration. Cellulose 24:5593–5604
Childress AE, Elimelech M (2000) Relating nanofiltration membrane performance to membrane charge (electrokinetic) characteristics. Environ Sci Technol 34:3710–3716. https://doi.org/10.1021/es0008620
de Faria AF, de Moraes ACM, Andrade PF et al (2017) Cellulose acetate membrane embedded with graphene oxide-silver nanocomposites and its ability to suppress microbial proliferation. Cellulose 24:781–796. https://doi.org/10.1007/s10570-016-1140-6
Devrim YG, Rzaev ZMO, Picskin E (2006) Synthesis and characterization of poly[((maleic anhydride)-alt-styrene)-co-(2-acrylamido-2-methyl-1-propanesulfonic acid)]. Macromol Chem Phys 207:111–121
Ganesh BM, Isloor AM, Ismail AF (2013) Enhanced hydrophilicity and salt rejection study of graphene oxide-polysulfone mixed matrix membrane. Desalination 313:199–207
Gholami A, Moghadassi AR, Hosseini SM et al (2014) Preparation and characterization of polyvinyl chloride based nanocomposite nanofiltration-membrane modified by iron oxide nanoparticles for lead removal from water. J Ind Eng Chem 20:1517–1522. https://doi.org/10.1016/j.jiec.2013.07.041
Guillen GR, Pan Y, Li M, Hoek EMV (2011) Preparation and characterization of membranes formed by nonsolvent induced phase separation: a review. Ind Eng Chem Res 50:3798–3817. https://doi.org/10.1021/ie101928r
Han J, Cho YH, Kong H et al (2013) Preparation and characterization of novel acetylated cellulose ether (ACE) membranes for desalination applications. J Memb Sci 428:533–545. https://doi.org/10.1016/j.memsci.2012.10.043
He Y, Li GM, Wang H et al (2009) Experimental study on the rejection of salt and dye with cellulose acetate nanofiltration membrane. J Taiwan Inst Chem Eng 40:289–295. https://doi.org/10.1016/j.jtice.2008.08.008
Hemmati K, Sahraei R, Ghaemy M (2016) Synthesis and characterization of a novel magnetic molecularly imprinted polymer with incorporated graphene oxide for drug delivery. Polym (United Kingdom) 101:257–268. https://doi.org/10.1016/j.polymer.2016.08.074
Hsiao M-C, Ma C-CM, Chiang J-C et al (2013) Thermally conductive and electrically insulating epoxy nanocomposites with thermally reduced graphene oxide–silica hybrid nanosheets. Nanoscale 5:5863. https://doi.org/10.1039/c3nr01471a
Huang ZQ, Chen K, Li SN et al (2008) Effect of ferrosoferric oxide content on the performances of polysulfone-ferrosoferric oxide ultrafiltration membranes. J Memb Sci 315:164–171. https://doi.org/10.1016/j.memsci.2008.02.028
Ionita M, Pandele AM, Crica L, Pilan L (2014) Improving the thermal and mechanical properties of polysulfone by incorporation of graphene oxide. Compos Part B Eng 59:133–139
Khulbe K (2003) Characterization of polyethersulfone-polyimide hollow fiber membranes by atomic force microscopy and contact angle goniometery. J Memb Sci 226:63–73. https://doi.org/10.1016/j.memsci.2003.08.011
King AAK, Davies BR, Noorbehesht N et al (2016) A new raman metric for the characterisation of graphene oxide and its derivatives. Sci Rep. https://doi.org/10.1038/srep19491
Kudin KN, Ozbas B, Schniepp HC et al (2008) Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett 8:36–41. https://doi.org/10.1021/nl071822y
Lalwani G, Henslee AM, Farshid B et al (2013) Two-dimensional nanostructure-reinforced biodegradable polymeric nanocomposites for bone tissue engineering. Biomacromol 14:900–909. https://doi.org/10.1021/bm301995s
Lee KP, Arnot TC, Mattia D (2011) A review of reverse osmosis membrane materials for desalination—development to date and future potential. J Memb Sci 370:1–22. https://doi.org/10.1016/j.memsci.2010.12.036
Lin Y, Jin J, Song M (2011) Preparation and characterisation of covalent polymer functionalized graphene oxide. J Mater Chem 21:3455–3461. https://doi.org/10.1039/C0JM01859G
Liu X, Ma Z, Xing J, Liu H (2004) Preparation and characterization of amino-silane modified superparamagnetic silica nanospheres. J Magn Magn Mater 270:1–6. https://doi.org/10.1016/j.jmmm.2003.07.006
Mahdavi H, Shahalizade T (2015) Preparation, characterization and performance study of cellulose acetate membranes modified by aliphatic hyperbranched polyester. J Memb Sci 473:256–266. https://doi.org/10.1016/j.memsci.2014.09.013
Mahdavi H, Razmi F, Shahalizade T (2016) Polyurethane TFC nanofiltration membranes based on interfacial polymerization of poly(bis-MPA) and MDI on the polyethersulfone support. Sep Purif Technol 162:37–44. https://doi.org/10.1016/j.seppur.2016.02.018
Mahdavi H, Kamyabi A, Shahalizade T, Asadollahi Taheri H (2017) Preparation of highly flexible cellulose acetate membranes modified by hyperbranched poly(amine ester)-epoxidized soybean oil and evaluation of its filtration properties. Cellulose 24:5389–5402. https://doi.org/10.1007/s10570-017-1526-0
Marcano DC, Kosynkin DV, Berlin JM et al (2010) Improved synthesis of graphene oxide. ACS Nano 4:4806–4814. https://doi.org/10.1021/nn1006368
Maria da Conceição CL, de Alencar AEV, Mazzeto SE, de A Soares S (2003) The effect of additives on the thermal degradation of cellulose acetate. Polym Degrad Stab 80(1):149–155
Park JY, Hirata Y, Hamada K (2012) Dye aggregation and interaction of dyes with a water-soluble polymer in ink-jet ink for textiles. Color Technol 128:184–191. https://doi.org/10.1111/j.1478-4408.2012.00370.x
Safarpour M, Khataee A, Vatanpour V (2014) Preparation of a novel polyvinylidene fluoride (PVDF) ultrafiltration membrane modified with reduced graphene oxide/titanium dioxide (TiO2) nanocomposite with enhanced hydrophilicity and antifouling properties. Ind Eng Chem Res 53:13370–13382. https://doi.org/10.1021/ie502407g
Sahraei R, Hemmati K, Ghaemy M (2016) Adsorptive removal of toxic metals and cationic dyes by magnetic adsorbent based on functionalized graphene oxide from water. RSC Adv 6:72487–72499. https://doi.org/10.1039/C6RA12934J
Stankovich S, Dikin DA, Piner RD et al (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon N Y 45:1558–1565. https://doi.org/10.1016/j.carbon.2007.02.034
Taha AA, Wu Y, Wang H, Li F (2012) Preparation and application of functionalized cellulose acetate/silica composite nanofibrous membrane via electrospinning for Cr(VI) ion removal from aqueous solution. J Environ Manage 112:10–16. https://doi.org/10.1016/j.jenvman.2012.05.031
Thuyavan YL, Anantharaman N, Arthanareeswaran G, Ismail AF (2016) Impact of solvents and process conditions on the formation of polyethersulfone membranes and its fouling behavior in lake water filtration. J Chem Technol Biotechnol 91:2568–2581. https://doi.org/10.1002/jctb.4846
Vatanpour V, Madaeni SS, Khataee AR et al (2012a) TiO2 embedded mixed matrix PES nanocomposite membranes: influence of different sizes and types of nanoparticles on antifouling and performance. Desalination 292:19–29. https://doi.org/10.1016/j.desal.2012.02.006
Vatanpour V, Madaeni SS, Rajabi L et al (2012b) Boehmite nanoparticles as a new nanofiller for preparation of antifouling mixed matrix membranes. J Memb Sci 401–402:132–143. https://doi.org/10.1016/j.memsci.2012.01.040
Wu T, Wang X, Qiu H et al (2012) Graphene oxide reduced and modified by soft nanoparticles and its catalysis of the Knoevenagel condensation. J Mater Chem 22:4772. https://doi.org/10.1039/c2jm15311d
Xu Z, Zhang J, Shan M et al (2014) Organosilane-functionalized graphene oxide for enhanced antifouling and mechanical properties of polyvinylidene fluoride ultrafiltration membranes. J Memb Sci 458:1–13. https://doi.org/10.1016/j.memsci.2014.01.050
Yang M, Zhao C, Zhang S et al (2017) Preparation of graphene oxide modified poly(m-phenylene isophthalamide) nanofiltration membrane with improved water flux and antifouling property. Appl Surf Sci 394:149–159. https://doi.org/10.1016/j.apsusc.2016.10.069
Yaroshchuk AE (2001) Non-steric mechanism of nanofiltration: superposition of donnan and dielectric exclusion. Sep Purif Technol 22–23:143–158. https://doi.org/10.1016/S1383-5866(00)00159-3
Yu S, Liu M, Ma M et al (2010) Impacts of membrane properties on reactive dye removal from dye/salt mixtures by asymmetric cellulose acetate and composite polyamide nanofiltration membranes. J Memb Sci 350:83–91. https://doi.org/10.1016/j.memsci.2009.12.014
Yu H, Zhang X, Zhang Y et al (2013) Development of a hydrophilic PES ultrafiltration membrane containing SiO2@N-Halamine nanoparticles with both organic antifouling and antibacterial properties. Desalination 326:69–76. https://doi.org/10.1016/j.desal.2013.07.018
Zhong Z, Li D, Zhang B, Xing W (2012) Membrane surface roughness characterization and its influence on ultrafine particle adhesion. Sep Purif Technol 90:140–146. https://doi.org/10.1016/j.seppur.2011.09.016
Zinadini S, Zinatizadeh AA, Rahimi M et al (2014a) Novel high flux antifouling nanofiltration membranes for dye removal containing carboxymethyl chitosan coated Fe3O4 nanoparticles. Desalination 349:145–154. https://doi.org/10.1016/j.desal.2014.07.007
Zinadini S, Zinatizadeh AA, Rahimi M et al (2014b) Preparation of a novel antifouling mixed matrix PES membrane by embedding graphene oxide nanoplates. J Memb Sci 453:292–301
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sahraei, R., Shahalizade, T., Ghaemy, M. et al. Fabrication of cellulose acetate/Fe3O4@GO-APTS-poly(AMPS-co-MA) mixed matrix membrane and its evaluation on anionic dyes removal. Cellulose 25, 3519–3532 (2018). https://doi.org/10.1007/s10570-018-1800-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-018-1800-9