Abstract
The rheological properties of fluids such as paints, coatings, and cosmetics play an important role in determining the effectiveness and desirability of these materials. Typically, these fluids must be relatively viscous and must exhibit shear-thinning characteristics. Previous work has shown that a variety of fibrous cellulosic materials, particularly nanocellulosic products, can impart shear-thinning characteristics to fluids to which they are added. In the work reported here, highly refined micro/nanofibrillated cellulose (MNFC) was assessed for its potential to impart desirable rheological properties to aqueous systems. When endoglucanases were assessed for their ability to influence the rheological properties of MNFC suspensions it was apparent that significant changes in the viscosity and shear-thinning properties of MNFC could be achieved using very low enzyme loadings (< 0.5 mg/g cellulose) within a relatively short timeframe (< 30 min). It is likely that the observed reductions in viscosity arise not just from reductions in the aspect ratio of the cellulosic fibrils, but also through disentanglement of interfibril interactions resulting from enzymatic smoothing of the fibre surfaces.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulosic materials can be used to enhance the rheological characteristics of a number of commercial products, including paints, coatings, and cosmetics. In recent years, a number of new nanocellulosic materials have been developed, including nanocrystalline cellulose, nanofibrillated cellulose, and a wide range of intermediate fibrillated materials containing both nanoscale and microscale particles, referred to as micro/nanofibrillated cellulose (MNFC) (Klemm et al. 2011; Abitbol et al. 2016). These materials have been shown to exhibit desirable properties for use as rheology modifiers, including viscosity enhancement, gel formation, imparting shear-thinning characteristics, and, for some materials, optical transparency (Siró and Plackett 2010).
However, each of the various applications requires a different set of optimal rheological properties, with some applications, such as certain cosmetics, requiring exceptionally high viscosities, while other applications, such as in some paints and coatings, requiring much lower viscosities. Additionally, optimal shear-thinning profiles (i.e. the extent of reduction in viscosity at increasing shear rates) of these materials varies between applications (Eley 2005). While it is possible to reduce the viscosity-enhancing properties of nanocellulosic materials by simply reducing the amount of cellulose added to the system (Tatsumi et al. 2002; Wu et al. 2014), this can result in undesirable changes to the shear-thinning profile. These include reductions in the gel-forming ability of the system, weakening of the network structure of the suspension (Chen et al. 2012), loss of potential crack-bridging effects (especially in paints and coatings) (Low et al. 2007), and undesirable textural changes in the material impacting application such as cosmetics (Zheng and Loh 2016).
One way to maintain adequate nanocellulose loadings without producing solutions that are too viscous is to optimise the intrinsic properties of the nanocellulosic materials so that the rheological properties can be adjusted while maintaining a constant concentration of cellulose within the system. This is typically achieved by the targeted reduction of the aspect ratio of the cellulose fibrils, i.e. the relationship between the fibrils’ width and length, using either chemical or enzymatic processes (Wu et al. 2014). Chemical processes, such as those used to produce small aspect ratio nanocrystalline cellulose products, generally utilize a strong acid treatment. A chemical approach typically requires significant chemical and water inputs, as well as acid-resistant equipment. An alternative enzymatic approach would involve the use of cellulases, particularly endoglucanases. Endoglucanases have been shown to preferentially attack the disordered regions (sometimes termed dislocations) along the fibre (Ander et al. 2008; Thygesen et al. 2011) resulting in a reduction in the aspect ratio of the fibre. Previous work by Yarbrough et al. has also shown that different enzyme systems can be used during the production of nanocellulose to produce material with uniform aspect (Yarbrough et al. 2017). In contrast to acid treatments, enzyme-mediated processes can be carried out under mild conditions, using low enzyme loadings, making it a potentially more attractive way to modify the aspect ratio of fibrillated celluloses.
As well as providing insights into the relationship between the relative aspect ratios of enzyme-treated micro/nanofibrillated cellulose (MNFC) and the rheological properties of the various products, the work reported here describes how an enzymatic approach can be used to specifically modify pulp properties, resulting in enhanced rheological characteristics for a number of different applications.
Materials and methods
Enzymatic hydrolysis
Micro/nanofibrillated cellulose (MNFC) of 70% moisture content was obtained from FPInnovations (Vancouver, Canada). The FPInnovations pilot plant utilizes multiple passes of a northern bleached softwood kraft pulp through a refiner at high consistency (~ 30 wt% solids). The material was removed from the refiner once at least 50 wt% of the material was present as fibrils (defined as having widths of less than 1 µm). The MNFC produced in this way thus consists of ~ 50 wt% larger fibers (i.e. with diameters of 20–30 µm), and 50 wt% micro/nano-fibrillated material (with widths below one micrometer). Enzymatic hydrolysis was carried out using the endoglucanase preparation Novozym 476 (protein content 9.53 mg/ml), generously supplied by Novozymes (Davis, CA). All of the enzymatic reactions were carried out at 2 wt% MNFC concentration using various enzyme loadings at pH ~ 4.8 in 50 mM sodium acetate buffer.
A typical experimental procedure for enzymatic hydrolysis utilised weighed amounts of MNFC, water and buffer, blended in a Waring blender for 30 s to produce a homogeneous 2% suspension. Under these blending conditions we confirmed, using an optical fiber analyzer (Fiber Quality Analyzer; OpTest, Hawkesbury, ON), that fiber degradation was negligible. One hundred grams of this material was then transferred to a 250-ml Erlenmeyer flask and the required amount of enzyme added by pipetting. The flask was then stoppered and incubated at 50 °C, at 180 rpm in an orbital shaker for 0.5–24 h. Hydrolysis was terminated by heating in a water bath at 95 °C for 30 min. Samples were then stored at 4 °C until analysis.
Sedimentation, gel point and aspect ratio
The relative aspect ratios of the MNFC samples were calculated from gel point concentrations, as determined via sedimentation experiments. Due to the exceptionally high aspect ratio and flexibility of the MNFC fibers studied, this technique was used to derive aspect ratio values that were used for relative comparisons between MNFC samples. An additional assumption was that the MNFC material had a similar density to traditional cellulose fibers. From 2 wt% MNFC samples, suspensions of 0.1, 0.08, 0.06, 0.04 and 0.02% (1, 0.8, 0.6, 0.4 and 0.2 kg/m3) were produced and 2 ml of each sample was allowed to settle in glass vials for two days. Photographs of these vials were taken after sedimentation and subsequently analyzed, using ImageJ software, to determine the ratio of the heights of settled fibre sediment to the heights of the total suspension (Fig. 1). The fibre concentration versus sediment ratio was plotted and fitted with linear equations and the intercept set to 0, in the form y = bx. The linear coefficient of this equation (b) is the gel point concentration. The aspect ratio was calculated using the equation below, derived from the work of Martinez et al. (Martinez et al. 2001; Zhang et al. 2012), which describes the gel point and aspect ratio of cellulose pulp fibres:
where A is the aspect ratio; ρ is the fibre density, assumed to be the same as pulp cellulose fibre density 1500 kg/m3 (Martinez et al. 2001); and gc is the gel point concentration.
Sugar release analysis
The quantity of soluble sugar released during hydrolysis was measured using the standard laboratory analytical procedure developed by the National Renewable Energy Laboratory (Sluiter et al. 2012). This procedure has previously been shown to produce reliable sugar content measurements without causing significant sugar dehydration. In brief, the sample (2 g) was diluted tenfold and centrifuged using a table-top centrifuge (2000×g, 10 min) to remove any sediment. The supernatant was sulfuric acid-hydrolyzed to depolymerize soluble oligomers and monomeric sugars were subsequently measured by high performance anion exchange chromatography with pulsed amperometric detection (ICS-3000; Dionex, Sunnyvale, CA) using a CarboPac PA1 column (Dionex) as previously described (Boussaid et al. 1999).
Rheology
An MCR 501 rheometer (Anton Paar GmbH, Graz, Austria) with CC27 cylindrical attachment and gap width of 1.13 mm was used to characterize the rheology of the MNFC samples. The viscosity profiles were measured by increasing shear rate from 0.1 to 30 s−1, collecting fifteen data points. All of the measurements were performed at a controlled temperature of 25 °C. The samples were allowed to rest and stabilize in the sample holder for 15 min prior to measurement.
Scanning electron microscopy
To prepare samples for scanning electron microscopy, the MNFC was diluted to 0.5 wt% in water and frozen into sheets with dimensions of 30 × 30 × 1 cm. Freeze drying was carried out for 24 h. A Hitachi S300 N scanning electron microscope (Hitachi, Tokyo, Japan) was used at 5 kV to obtain images of the freeze-dried MNFC. Samples were gold-coated prior to imaging.
Results and discussion
Does the morphology of the micro/nanofibrillated cellulose (MNFC) influence its use as a rheology modifier?
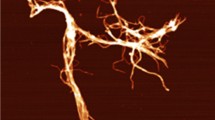
As mentioned earlier, fibrous cellulosic materials can be used as potential modifiers that can impart desirable rheological properties on a variety of commercial products (Dimic-Misic et al. 2013; Li et al. 2015). It has been suggested that the extent and way in which the rheology is modified, through the addition of fibrous cellulose at a given concentration, is influenced by the characteristics of the cellulose particles, particularly their dimensions and surface morphology (Taheri and Samyn 2015). When a variety of different shapes of nanocellulosic materials were prepared in previous work, the starting material and production processes were both shown to significantly impact the properties of the final nanocellulosic product (Dufresne 2012). Although it was likely that these properties could be further modified by both chemical and enzymatic means, for the initial MNFC, it was apparent that a wide range of fibre dimensions were already present at the micro- and nano-fibril levels. This resulted in an entangled, highly hydrated network that would likely influence suspension rheology (Fig. 2).
One inherent challenge when working with highly fibrillated cellulosic materials, such as MNFC, is that the particle size and aspect ratio of the fibrils are difficult to quantify. Thus the effective determination of the aspect ratio of these fibrils is required if we are to develop a good understanding of how these dimensional properties influence the rheological properties of the target fluids. However, a major challenge in accurately determining the aspect ratio is that the relatively small size of the fibrils makes them very difficult to detect using traditional pulp fibre quantification techniques such as optical fibre analysis (Haapala et al. 2013). An additional challenge is that, typically, the fibrils form a complex, entangled network, which makes it difficult to accurately determine fibril length or aspect ratio using traditional microscopy and image-analysis techniques (Pääkkö et al. 2007; Karppinen et al. 2012). Thus one of the first goals was to assess if a gel point determination technique could be used to quantify the relative aspect ratios of MNFC before and after enzyme treatments.
Determination of the aspect ratio of micro/nanofibrillated cellulose (MNFC) and the influence of enzyme treatment
Previous work has shown that, for rod-like particles, aspect ratio significantly influences suspension rheology (Powell 1991; Wu et al. 2014). Higher aspect ratios result in more interparticle interactions, leading to network formation, resulting in restricted suspension flow and increasing viscosity. However, unlike the relatively rod-like kraft pulp fibres, which have well-defined dimensions of length and width, MNFC fibers/fibrils vary dramatically in dimensions from particle to particle, typically forming complex entangled webs. As a result, this prevents accurate determination of MNFC length and width using the traditional analysis tools mentioned earlier. It has also previously been shown that, although particle dimensions can be determined through high-resolution microscopy techniques such as electron microscopy or atomic force microscopy, such quantification is tedious and challenging (Haapala et al. 2013). Thus, the approximate relative aspect ratios of MNFC samples before and after enzyme treatment here were determined using the relatively simple gel-point technique (Varanasi et al. 2013). The gel point is the critical concentration at which fibres in a suspension form a continuous network that enhances mechanical strength of the suspension (Martinez et al. 2001; Varanasi et al. 2013). In general, fibres/filaments with a higher aspect ratio require lower “pulp” concentrations in order to form a continuous load-bearing network within the solution. Thus, this relationship between the aspect ratio and the concentration at which gelation occurs provides a way to determine the approximate aspect ratio.
As earlier work had shown that cellulases, particularly endoglucanases, could specifically aid in fibre length reduction by weakening fibers at disordered regions (Ander et al. 2008; Thygesen et al. 2011; Gourlay et al. 2015), we next tried to selectively modify the aspect ratio of the various MNFC fractions using low enzyme loadings. It was apparent that a range of sedimentation profiles were obtained for the enzyme-treated MNFC pulps after 0.5–24 h of hydrolysis (Fig. 3). To determine the gel point concentration of the samples, the various enzyme-treated MNFC pulps were suspended at a range of different consistencies and dispersed in a given volume of water. The ratio of the height of the fibre sediment to the total height of the water plus suspension was then calculated for each sample. As expected, higher consistency suspensions (containing more MNFC in the same volume) produce higher sediment heights compared to those containing less MNFC.
Although linear and quadratic fits have been used previously to determine gel point from sedimentation heights (Zhang et al. 2012; Mosse et al. 2012; Varanasi et al. 2013), in the work reported here, the linear equation fit all sedimentation plots well (R2 > 0.97). Thus, this model was chosen and the gel point and aspect ratio were calculated using the linear coefficient.
It was apparent that a gradual decrease in the sedimentation height of the MNFC occurred after enzymatic treatment (Fig. 3). After 24 h the sedimentation height had fallen to just 25% of the untreated MNFC control. As a result, the gel point plots (Fig. 4) became correspondingly steeper with increasing hydrolysis time resulting in a higher gel point concentration, indicative of a lower aspect ratio. The change in the relative aspect ratio, calculated from the data in Fig. 4 using Eq. 1, showed a steep decrease within the first 6 h, followed by a slower rate of change over longer times (Fig. 5).
Although there was some enhancement in the rate and extent of aspect ratio reduction when the enzyme loading was increased from 1 to 2.5 mg/g (Fig. 5), no significant increase was observed when the enzyme loading was further increased to 5 mg/g. Previous work (Thygesen et al. 2011; Hidayat et al. 2012) has suggested that the endoglucanases might be acting at fibre dislocations (periodic disordered regions found along the length of the fibres/fibrils), which make up only a small percentage of the total accessibility of the fibre. Thus it is possible that these limited sites become saturated with endoglucanases at relatively low enzyme loadings.
Although, due to the exceptionally high heterogeneity of MNFC, the aspect ratio calculated from the gel point method may not provide a completely accurate value for the average aspect ratio of the MNFC fibrils, it did provide a simple way to characterize and compare MNFC samples after various treatments. It is likely that the aspect ratio measurements provided an averaged population value that includes both the larger fibres and the smaller nanoscale fibrils. It is also likely that the flexibility and entanglement of the nanoscale fibrils further affects the gelation concentration. Thus, the calculated aspect ratios should be considered only as relative values, and should not be taken to indicate the true average aspect ratio of each sample. However, the results reported here closely match other studies that utilized the gel point method for micro- and nano-fibrillated cellulose fibres, strongly supporting the use of gel point determination for this application (Zhang et al. 2012; Varanasi et al. 2013).
The gel point determination and aspect ratio calculations each indicated that endoglucanase treatment could effectively reduce the aspect ratio of the MNFC samples, with the majority of the reduction occurring within the first 6 h of hydrolysis. To try to determine how changes in aspect ratio might influence the rheology of the enzyme treated MNFC suspensions, viscosity measurements were carried out on each of the samples.
Viscosity profiles and modification of MNFC
Previous work has shown that the rheological properties of MNFC suspensions are significantly influenced by factors such as the nature of the biomass source, refining/treatment processes, consistency, temperature and pH (Pääkkö et al. 2007; Karppinen et al. 2012; Charani et al. 2013a, b; Grüneberger et al. 2014). In the presence of very low shear forces, the entangled network of fibrils is resistant to flow and therefore exhibits high viscosity. At higher shear forces the fibrils are more likely to align themselves in the direction of flow, breaking the fibril network, consequently lowering viscosity in a shear-thinning manner. When the viscosity profiles of the untreated MNFC and enzyme-hydrolyzed samples were determined (Fig. 6), it was apparent that a 1 mg/g enzyme treatment greatly modified viscosity within less than 30 min. During this time the viscosity, at a shear rate of 0.1 s−1, decreased by 75% when compared to the untreated MNFC. However, as reported earlier, the calculated average aspect ratio of the MNFC decreased by only 5% over the same time period. This suggested that, while the decrease in the aspect ratio of the fibrils likely does play a role in reducing the viscosity, the reduction in aspect ratio is not the sole factor that influences viscosity.
We next assessed the viscosity profiles using a power law model. The viscosity versus shear rate data were fitted into the power law equation η = KPL γn−1. This model is often used for non-Newtonian materials (i.e. materials whose viscosity is dependent on shear rate) (Knutsen and Liberatore 2009; Wiman et al. 2011; Rao 2013) and typically, two distinct parameters are generated. These are the consistency factor (KPL) and the flow index (nPL). The KPL value is the viscosity at a shear rate of 1 s−1 with a high KPL value indicative of a more viscous fluid. The nPL parameter describes the viscoelastic behaviour of a non-Newtonian fluid under shear forces. Since a completely Newtonian fluid has a value of 1 (i.e. viscosity independent of shear force), the further the value is from 1 the more distinct the fluid is from Newtonian behaviour. When the calculated KPL and nPL values of various enzyme-treated MNFC samples were plotted (Fig. 7), it was apparent that, while the KPL changed drastically during the initial stages of hydrolysis (three to fourfold reduction over the first 30 min), the nPL varied only slightly from ~ 0.24 to ~ 0.35 over the first 30 min. As an order of 0 < nPL < 1 normally indicates that viscosity decreases with increasing shear force (i.e. shear-thinning) while a value of nPL > 1 indicates that viscosity increases with increasing shear force (i.e. shear-thickening), this indicated that, even at a lower overall viscosity, the MNFC still exhibited strong shear-thinning properties after enzyme treatment.
As relatively low enzyme loadings (1 mg/g pulp) over a relatively short time (30 min) had been effective in enhancing the rheology of MNFC, we next assessed the efficacy of even lower enzyme loadings (0.25 and 0.5 mg/g pulp) and shorter reaction times (5 and 30 min). It was apparent (Fig. 8) that, other than the lowest enzyme concentration for the shortest time (i.e. 0.25 mg/g pulp for 5 min), all of the treatments resulted in reduced viscosity.
It appeared that low concentrations of endoglucanase could reduce both the viscosity and aspect ratio of the MNFC suspension and that increasing enzyme loading above this threshold did not result in significant additional reductions in viscosity. One possible explanation is that the exposed cellulose surface chains were readily removed at low enzyme loadings. The ease of hydrolysis of highly exposed cellulose chains on the surface of fibres/fibrils has been reported previously (Pribowo 2014; Gourlay et al. 2015), where it took less than 15 min to remove the bulk of the exposed chains from the cellulose surface. This rapid removal of surface chains and the lack of enhanced viscosity reduction at enzyme loadings above 1 mg/g suggests that a 1 mg/g endoglucanase loading may be all that is required to remove the bulk of these surface chains from the cellulose surface over this time frame.
Despite the rapid reduction in the viscosity of the MNFC dispersions, HPLC analysis of potential oligomeric and monomeric sugars that might be solubilized during this process indicated that the dramatic viscosity changes that were observed occurred in the absence of significant solubilisation of the cellulosic material, with maximum solubilisation of 4–5% of the original material by weight (data not shown).
Conclusions
Earlier work had shown that the surface of cellulosic fibres contained a small amount of highly accessible cellulosic strands and that these fibres/fibrils were readily accessible to enzymatic attack (Gourlay et al. 2015). A rapid and dramatic reduction in the amount of these isolated cellulose chains occurred within the first 15 min of endoglucanase treatment, while only 2% of the cellulose was hydrolyzed. In the work reported here, endoglucanase treatments were assessed to see if they could enhance the rheological properties of MNFC. It was hoped that endoglucanase treatments would reduce the aspect ratio of the fibrillated cellulose, thereby reducing pulp viscosity. However, while a reduction in aspect ratio was observed, this reduction occurred relatively slowly (12.5% reduction in 30 min), while the viscosity dropped rapidly and dramatically (75% reduction in 30 min). This suggested that it was not just the reduction in aspect ratio that was responsible for the observed reduction in the MNFC viscosity following enzymatic treatment. It is likely that the primary determinant for the endoglucanase-promoted reduction in MNFC viscosity is the hydrolysis and removal of exposed, individual cellulose chains at the fibril surface. This results in reduction in fibril–fibril interactions and entanglement, thereby leading to a reduction in the overall viscosity of the enzyme treated pulp. It is likely that other enzymes and treatments can be used to selectively enhance the rheological properties of pulps.
References
Abitbol T, Rivkin A, Cao Y et al (2016) Nanocellulose, a tiny fiber with huge applications. Curr Opin Biotechnol 39:76–88. https://doi.org/10.1016/j.copbio.2016.01.002
Ander P, Hildén L, Daniel G (2008) Cleavage of softwood kraft pulp fibres by HCl and cellulases. BioResources 3:477–490
Boussaid A, Robinson J, Cai Y et al (1999) Fermentability of the hemicellulose-derived sugars from steam-exploded softwood (Douglas fir). Biotechnol Bioeng 64:284–289
Charani PR, Dehghani-Firouzabadi M, Afra E et al (2013a) Production of microfibrillated cellulose from unbleached kraft pulp of Kenaf and Scotch Pine and its effect on the properties of hardwood kraft: microfibrillated cellulose paper. Cellulose 20:2559–2567. https://doi.org/10.1007/s10570-013-9998-z
Charani PR, Dehghani-Firouzabadi M, Afra E, Shakeri A (2013b) Rheological characterization of high concentrated MFC gel from kenaf unbleached pulp. Cellulose 20:727–740. https://doi.org/10.1007/s10570-013-9862-1
Chen P, Yu H, Liu Y et al (2012) Concentration effects on the isolation and dynamic rheological behavior of cellulose nanofibers via ultrasonic processing. Cellulose 20:149–157. https://doi.org/10.1007/s10570-012-9829-7
Dimic-Misic K, Gane PAC, Paltakari J (2013) Micro- and nanofibrillated cellulose as a rheology modifier additive in CMC-containing pigment-coating formulations. Ind Eng Chem Res 52:16066–16083. https://doi.org/10.1021/ie4028878
Dufresne A (2012) Nanocellulose: from nature to high performance tailored materials. De Gruyter, Berlin
Eley RR (2005) Applied rheology in the protective and decorative coatings industry. Rheol Rev 3:173–240
Gourlay K, Hu J, Arantes V et al (2015) The use of carbohydrate binding modules (CBMs) to monitor changes in fragmentation and cellulose fiber surface morphology during cellulase- and swollenin-induced deconstruction of lignocellulosic substrates. J Biol Chem 290:2938–2945. https://doi.org/10.1074/jbc.M114.627604
Grüneberger F, Künniger T, Zimmermann T, Arnold M (2014) Rheology of nanofibrillated cellulose/acrylate systems for coating applications. Cellulose 21:1313–1326. https://doi.org/10.1007/s10570-014-0248-9
Haapala A, Laitinen O, Karinkanta P et al (2013) Optical characterization of micro-scale cellulose particles and wood powder. Appita Annu Conf 4:70–77
Hidayat BJ, Felby C, Johansen KS, Thygesen LG (2012) Cellulose is not just cellulose: a review of dislocations as reactive sites in the enzymatic hydrolysis of cellulose microfibrils. Cellulose 19:1481–1493. https://doi.org/10.1007/s10570-012-9740-2
Karppinen A, Saarinen T, Salmela J et al (2012) Flocculation of microfibrillated cellulose in shear flow. Cellulose 19:1807–1819. https://doi.org/10.1007/s10570-012-9766-5
Klemm D, Kramer F, Moritz S et al (2011) Nanocelluloses: a new family of nature-based materials. Angew Chem Int Ed 50:5438–5466. https://doi.org/10.1002/anie.201001273
Knutsen JS, Liberatore MW (2009) Rheology of high-solids biomass slurries for biorefinery applications. J Rheol 53:877–892. https://doi.org/10.1122/1.3143878
Li MC, Wu Q, Song K et al (2015) Cellulose nanoparticles as modifiers for rheology and fluid loss in bentonite water-based fluids. ACS Appl Mater Interfaces 7:5006–5016. https://doi.org/10.1021/acsami.5b00498
Low IM, McGrath M, Lawrence D et al (2007) Mechanical and fracture properties of cellulose-fibre-reinforced epoxy laminates. Compos Part A Appl Sci Manuf 38:963–974. https://doi.org/10.1016/j.compositesa.2006.06.019
Martinez DM, Buckley K, Lindström A et al (2001) Characterizing the mobility of papermaking fibres during sedimentation. In: Baker CF (ed) The science of papermaking: transactions of the 12th fundamental research symposium. Pulp and Paper Fundamental Research Society, Oxford, pp 225–254
Mosse WKJ, Boger DV, Simon GP, Garnier G (2012) Effect of cationic polyacrylamides on the interactions between cellulose fibers. Langmuir 28:3641–3649. https://doi.org/10.1021/la2049579
Pääkkö M, Ankerfors M, Kosonen H et al (2007) Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromol 8:1934–1941. https://doi.org/10.1021/bm061215p
Powell RL (1991) Rheology of suspensions of rodlike particles. J Stat Phys 62:1073–1094. https://doi.org/10.1007/BF01128178
Pribowo A (2014) Enzyme-substrate interactions and their influence on enzyme recycling strategies as a way of reusing cellulases. Dissertation, The University of British Columbia
Rao A (2013) Rheology of fluid, semisolid, and solid foods: principles and applications, 3rd edn. Springer, Boston
Siró I, Plackett D (2010) Microfibrillated cellulose and new nanocomposite materials: a review. Cellulose 17:459–494. https://doi.org/10.1007/s10570-010-9405-y
Sluiter A, Hames B, Ruiz R et al (2012) Determination of structural carbohydrates and lignin in biomass. NREL/TP-510-42618, US National Renewable Energy Laboratory, Golden, Colorado
Taheri H, Samyn P (2015) Rheological properties and processing of polymer blends with micro- and nanofibrillated cellulose. In: Hakeem KR, Jawaid M, Alothman OY (eds) Agricultural biomass based potential materials. Springer, Berlin, pp 259–291
Tatsumi D, Ishioka S, Matsumoto T (2002) Effect of fiber concentration and axial ratio on the rheological properties of cellulose fiber suspensions. Nihon Reoroji Gakkaishi 30:27–32. https://doi.org/10.1678/rheology.30.27
Thygesen LG, Hidayat BJ, Johansen KS, Felby C (2011) Role of supramolecular cellulose structures in enzymatic hydrolysis of plant cell walls. J Ind Microbiol Biotechnol 38:975–983. https://doi.org/10.1007/s10295-010-0870-y
Varanasi S, He R, Batchelor W (2013) Estimation of cellulose nanofibre aspect ratio from measurements of fibre suspension gel point. Cellulose 20:1885–1896. https://doi.org/10.1007/s10570-013-9972-9
Wiman M, Palmqvist B, Tornberg E, Lidén G (2011) Rheological characterization of dilute acid pretreated softwood. Biotechnol Bioeng 108:1031–1041. https://doi.org/10.1002/bit.23020
Wu Q, Meng Y, Wang S et al (2014) Rheological behavior of cellulose nanocrystal suspension: influence of concentration and aspect ratio. J Appl Polym Sci 131:40525. https://doi.org/10.1002/app.40525
Yarbrough JM, Zhang R, Mittal A et al (2017) Multifunctional cellulolytic enzymes outperform processive fungal cellulases for coproduction of nanocellulose and biofuels. ACS Nano 11:3101–3109. https://doi.org/10.1021/acsnano.7b00086
Zhang L, Batchelor W, Varanasi S et al (2012) Effect of cellulose nanofiber dimensions on sheet forming through filtration. Cellulose 19:561–574. https://doi.org/10.1007/s10570-011-9641-9
Zheng YJ, Loh XJ (2016) Natural rheological modifiers for personal care. Polym Adv Technol 27:1664–1679. https://doi.org/10.1002/pat.3822
Acknowledgments
We thank FPInnovations (Vancouver, BC) for providing the micro/nanofibrillated cellulose and the Natural Sciences and Engineering Research Council for financial support of this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Gourlay, K., van der Zwan, T., Shourav, M. et al. The potential of endoglucanases to rapidly and specifically enhance the rheological properties of micro/nanofibrillated cellulose. Cellulose 25, 977–986 (2018). https://doi.org/10.1007/s10570-017-1637-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1637-7