Abstract
In the present study, we pretreated coir fiber with an ionic liquid (IL), n-butylammonium acetate, to expose its cellulose fibers. The best IL pretreatment conditions were determined by central composite design using a desirability function to maximize the enzymatic hydrolysis. To collect data for this optimization, coir fiber was subjected to a pulping process with 9% (w/w) sodium hydroxide for 6 h at 137 °C with a pressure of 2.5 atm. The pulping fiber was then treated with the IL for a range of times and at a range of temperatures. Based on the results, the best treatment conditions were determined to be 90 °C for 40 h. The best conditions for enzymatic hydrolysis were determined to be pH 6 at 44.16 °C for 25.57 h. Under these conditions, 32.33 ± 1.08% of the coir fiber was converted to glucose. The efficiency obtained with pulped coir fiber was 14.34 ± 0.14% and with crude coir fiber was 6.27 ± 0.15%, demonstrating the benefits of the proposed IL pretreatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the consistent increases in the world population and agricultural production, the production of lignocellulosic residues has grown significantly. Thus, lignocellulosic derivatives, such as second-generation bioethanol, have been proposed as a promising alternative to reduce the demand for lignocellulosic residues. In this context, many studies have been conducted on materials such as sugar cane, corn stover, wheat straw, wood chips, rice straws, bamboo, Napier grass, and coir fiber (Andrade Neto et al. 2016; Zhao et al. 2016; Uju et al. 2015).
Additionally, considering the scarcity and high prices of fossil fuels and the pollution that they generate, the interest in lignocellulosic derivatives is further intensified by the possibility of increasing the market demand for bioethanol as an environmentally friendly alternative to meet the energy demand in the world (You et al. 2016).
However, the production of second-generation bioethanol involves the transformation of lignocellulosic biomass (with a fiber composition of 40–45% cellulose, 30–35% hemicellulose, and 25–30% lignin) into simple sugars and the subsequent fermentation of these simple sugars into alcohols. Several processes can be used to produce sugars from cellulose and hemicellulose, the most important being chemical and biological processes (Andrade Neto et al. 2016; Xu et al. 2015).
The accessibility of cellulose to cellulase enzymes for acid or enzymatic saccharification can be improved by applying a pretreatment to the lignocellulosic biomass that increases the surface area of the cellulose, decreases the cellulose crystallinity, and reduces the content of other components such as lignin and hemicellulose. Physical, physico-chemical, chemical, and biological pretreatments are all used as effective methods for this purpose (You et al. 2016; Asakawa et al. 2015; Mood et al. 2012).
A few specific examples are as follows: processes with acids or bases, treatments with organic solvents, vapor explosion, irradiation, alkali-microwave treatment, wet oxidation, autohydrolysis, supercritical carbon dioxide treatment, ammonia fiber/freeze explosion, microbial treatment, and treatments with ionic liquids (ILs). Treatment with alkaline solutions is the most widely used mainly due to the high delignification efficiency; this process increases the exposure of the fiber for hydrolysis and increases the concentration of free hydrolyzable sugars in the product, especially when a subsequent IL treatment step is used (Andrade Neto et al. 2016; Elgharbawy et al. 2016; Zhou and Runge 2015; Liu et al. 2013).
The use of ILs for chemical pretreatments has been investigated as a new alternative to the use of organic solvents due to their unique properties: melting points lower than 100 °C, very low vapor pressure, high solvent power, catalytic activity, low volatility, incombustibility, low viscosity, recyclability, thermal stability, and good interactions with cellulose chains. Some ILs with imidazolium cations exhibit particularly strong interactions with cellulose: 1-ethyl-3-methylimidazolium chloride, 1-ethyl-3-methylimidazolium hexafluorophosphate, 1-ethyl-3-methylimidazolium acetate, 1-methyl-3-octyloxymethylimidazolium tetrafluoroborates, 1-methyl-3-nonyloxymethylimidazolium tetrafluoroborates, 1-butyl-3-methylimidazolium chloride, 1-butyl-3-methylimidazolium acetate, 1-butyl-3-methylimidazolium hydrogen sulfate, 1-allyl-3-methylimidazolium acetate and 1,3-dimethyl imidazolium dimethylphosphate (Merino et al. 2017; Elgharbawy et al. 2016; Lienqueo et al. 2016; Asakawa et al. 2015; He et al. 2015; Miyafuji 2015; Liu et al. 2010, 2011), and ILs containing pyridinium cations, including 1-decyloxymethyl-4-dimethylaminopyridinium chloride and 1-decyloxymethyl-4-dimethylaminopyridinium acesulfamate. These interactions are improved by the combination of anions such as chloride, methanoate, alkyl phosphates, and acetate, which favor the breakdown of the lignin–hemicellulose–cellulose structure via hydrogen bonding, hydrophobic interactions, and π–π interactions with the polysaccharides and lignin (Elgharbawy et al. 2016; Xin et al. 2016; Miyafuji 2015; Liu et al. 2010).
The disadvantage of the IL treatment of vegetable fiber is the need to remove the IL from the fiber after the pretreatment to prevent the IL from interfering with the later hydrolysis step. However, as reported by Elgharbawy et al. (2016), the use of ILs with anions as described above reduces the interference in this step. Andrade Neto et al. (2016) reported that ILs derived from aliphatic amines are highly effective for the acid hydrolysis of coir fiber and are less costly than those obtained from imidazolium or pyridinium cations.
In addition to determining the best pretreatment conditions of the lignocellulosic material, further research is needed to make the hydrolysis step more feasible, such as the use of acidic and enzymatic processes. In this case, several processes involving enzymatic reactions and dilute acid saccharification of the residual biomass have been investigated (Lee et al. 2015). Specifically, acids can be used to generate fermentation inhibitors, such as acetic acid, hydroxymethyl furfural, furfural, glycol aldehyde, and soluble phenolic compounds. Therefore, an increasing number of studies have been done using cellulases, which consist of three major components: endoglucanase, exoglucanase, and β-glucosidase (Victor et al. 2015).
However, for the use of cellulases in industrial applications, some process parameters (pH, temperature, and reaction time) should be characterized and optimized to enhance the enzymatic production. In addition, the influence of various inhibitors in this process should be investigated, such as organic solvents or ILs (Kshirsagar et al. 2015).
Materials and methods
In order to produce the n-butylammonium acetate IL used to treat the coir fiber, the following analytical-grade reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA): n-butylamine (99.5%), acetic acid (99.5%), chloroform-d (≥ 99.96 atomic % D), sodium hydroxide, potassium hydroxide, glucose, sulfuric acid (95.0–98.0%), and a cellulase enzyme complex from Trichoderma reesei (ATCC 26921). Coir fibers with lengths of 12–14 cm were supplied by Projeto Coco Vivo (Arraial d’Ajuda, BA, Brazil). All other reagents were purchased from Dinâmica Química Contemporânea (Brazil).
Experimental
IL synthesis
The n-butylammonium acetate IL was obtained by the acid–base neutralization reaction. The acetic acid was slowly added to an aliphatic amine (n-butylamine) under stirring (Andrade Neto et al. 2016). The stoichiometric ratio was 1:1 and the reaction temperature was maintained between 20 and 40 °C. The product was then characterized by nuclear magnetic resonance (NMR) in Chloroform-d (CDCl3) with a Bruker Avance III 600 HD spectrometer (German) operating at 600 MHz to obtain the 1H spectrum and 150 MHz to obtain the 13C NMR spectrum. The chemical shifts (δ, ppm) were reported relative to tetramethylsilane (TMS).
The COSY technique (homonuclear correlation spectroscopy), HSQC (heteronuclear single quantum coherence), and HMBC (heteronuclear multiple bond coherence) were used to attribute the signals to hydrogens and carbons by analyzing the correlations between neighboring hydrogens and between neighboring or adjacent hydrogens and carbons in bidirectional spectra.
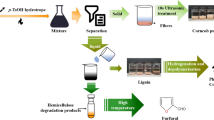
Treatment of coir fiber
The coir fiber was dried at 80 ± 3 °C for 72 h and milled in a knife mill (model MA-680, Marconi, Piracicaba, SP, Brazil). Then, the fiber was submitted to chemical pulping in a 9% (w/w) sodium hydroxide solution using a 5 L-capacity Metalquim stainless steel reactor (Brazil) for 6 h; the pressure was 2.5 atm, the rotation rate was 200 rpm, and the temperature was 137 °C. The fiber was filtered, washed, and dried at 80 °C.
After the pulping process, the coir fiber was exposed to a second pretreatment with the n-butylammonium acetate IL. During this pretreatment, the temperature and reaction time were varied according to a central composite rotatable design (CCRD) with two center points (Table 1). The resulting lignin and holocellulose contents were used to determine the best IL treatment conditions to maximize the concentration of holocellulose and minimize the lignin content.
All reactions were conducted in duplicate with a ratio of 10 g fiber:100 mL IL and performed under constant agitation at 280 rpm. At the end of the treatment, 100 mL of water were added to facilitate the filtration process. Then, the samples were filtered, washed with water, and dried in an oven at 80 °C.
After the coir fiber treatment with IL, all the samples were submitted to enzymatic hydrolysis at a pH of 4.3 for a reaction time of 40 h at 40 °C with an enzyme concentration of 0.25 mg mL−1 (FPU = 53.09 ± 0.94 U g−1). This condition was chosen based on a preliminary analysis and previous studies (Morandim-Giannetti et al. 2017; Hafid et al. 2017). The glucose concentration was measured after treatment with each set of conditions and was used to determine the best IL treatment condition for the pulped coir fiber.
The lignin and holocellulose concentrations in the coir fiber were measured before and after the pulping and the IL treatment with the optimized conditions (TAPPI test method T222 os-76, 1979; Wise et al. 1946).
Enzymatic hydrolysis
After the optimization of the treatment conditions for coir fiber pulped with the IL (40 h at 90 °C), experiments were conducted to determine the best conditions for the enzymatic hydrolysis. The reaction time, pH, and temperature were treated as the input variables and the concentration of fermentable sugar was treated as the output variable. All reactions with each set of pH, temperature, and reaction time conditions were conducted in duplicate according to CCRD with two center points (Table 2) to optimize these conditions. The FPU of the cellulose used was 53.09 ± 0.94 U g−1.
The final concentration of fermentable sugars (the output variable) was determined by high-performance liquid chromatography using a Shimadzu Chromatograph (Japan) consisting of LC-20AD pumps, a RID-10A refractive index detector, an SPD-20A ultraviolet light detector, a CTO-20A column oven, and a CBM-20A controller. The mobile phase was 0.5% phosphoric acid in water and was applied at a flow rate of 0.7 mL min−1 in a Shim-Pack CLC-NH2 (M) column with a Shim-Pack G-NH2 pre-column.
Data analysis
The lignin and holocellulose concentration measurements obtained after the treatment of the coir fiber with the ILs and the glucose concentration measurements obtained after the hydrolysis were processed using the Statistica 12.0 software (StatSoft Inc., 2014, USA). The analyses were performed using the design of experiments approach, which is a well-known technique to conduct optimizations with simultaneous variation of all parameters. The conditions were optimized by using a desirability function considering the geometric mean of the individual desirability of each parameter according to Eq. 1 as described previously (Barros Neto et al. 2007; Calado and Montgomery, 2003). A model with linear and quadratic terms was used and the equations correlating the input and output variables were obtained.
where d describes the individual desirability of each parameter and m is the number of parameters analyzed.
Results and discussion
Synthesis and characterization of ILs
The n-butylammonium acetate IL was synthesized in the laboratory (Fig. 1) and characterized by hydrogen NMR (H1NMR) and carbon NMR (C13NMR). Based on the H1NMR spectra, the amino group of n-butylamine was chemically displaced after the formation of the n-butylammonium ion. This indicates a protonation of the amino group and a change in the multiplicity of the signal related to amine hydrogens. It was possible to observe a change from two to three in the integration of the amine hydrogens regarding the n-butylamine and the n-butylammonium ion, respectively.
n-butylamine:
H1NMR (600 MHz, CDCl3): δ 2.59 (2H, t, H-1), 1.32 (2H, m, H-2), 1.25 (2H, m, H-3), 0.81 (3H, t, H-4), 0.96 (2H, dd, NH2). C13NMR (150 MHz, CDCl3): δ 42.15 (C-1), 36.04 (C-2), 20.30 (C-3), 13.90 (C-4).
n-butylammonium acetate:
H1NMR (600 MHz, CDCl3): δ 1.90 (3H, s, H-2), 2.77 (2H, t, H-3), 1.58 (2H, m, H-4), 1.34 (2H, m, H-5), 0.88 (3H, t, H-6), 9.56 (3H, s, NH3 +). C13NMR (150 MHz, CDCl3): δ 178.07 (C-1), 30.04 (C-2), 38.58 (C-3), 23.73 (C-4), 19.29 (C-5), 13.26 (C-6).
Treatment of the coir fiber
Many studies are currently using statistical planning to reduce the number of experiments required for process optimization when the response of interest is affected by several variables (Andrade Neto et al. 2016; Morandim-Giannetti et al. 2013). In this context, treatments for lignocellulosic materials are being investigated to increase the concentration of fermentable sugars produced by the hydrolysis process and thereby create ideal conditions for the fermentation step. Thus, in this study, the coir fiber was subjected to two treatments to reduce the lignin content and increase the concentration of fermentable sugars.
The alkaline pulping process was performed and the lignin and holocellulose concentrations were analyzed. The results indicated a 61.98% delignification and a 17.78% increase in the holocellulose content. The percent delignification was calculated based on the lignin concentrations before and after the pulping process as determined using the TAPPI test method (method T222 os-76). The concentrations of holocellulose were determined based on the methodology described by Wise et al. (1946), as mentioned above. The holocellulose concentration was 63.26 ± 0.97% in the crude fiber and 74.51 ± 0.35% in the fiber after pulping. The lignin concentration was 30.04 ± 0.85% in the crude coir fiber and 11.42 ± 0.44% after treatment with soda. This change in the fiber composition is consistent with the results of other studies (Andrade Neto et al. 2016).
After the pulping process and the subsequent treatment of the pulped coir fiber with the n-butylammonium acetate IL using different reaction times at various temperatures (Table 1), all the samples were submitted to enzymatic hydrolysis. Then, the glucose content was determined to identify the best treatment conditions. Statistical analyses of the results were performed using the Statistica software. The most appropriate model for this case was chosen (a model with both linear and quadratic terms) and the input variables (time and temperature) that most significantly affected the coir fiber treatment in terms of the resulting concentration of fermentable sugars (mainly glucose) were analyzed (Table 3). Equation 2 correlates the input variables (time and temperature) with the output variable (glucose concentration).
where X1 is the time (h) and X2 is the temperature (°C).
The statistical analysis revealed that the temperature affects the changes in the characteristics of the coir fiber and the resulting glucose concentration more strongly than the process time (Fig. 2). This is due to the great energy of the system that favors the breakdown of the lignin–cellulose–hemicellulose bonds, as well as the breakdown of the cellulose structure itself. Based on an analysis using the necessary restrictions, the optimal temperature to maximize the glucose concentration is 90 °C and the optimal process time is 40 h (Fig. 3).
Optimization of the enzymatic hydrolysis conditions for the treated coir fiber
In addition to the optimization of the IL treatment, the hydrolysis was performed in duplicate under the various conditions shown in Table 2. All samples were analyzed to determine the glucose content (Table 4). The results show significant variations in the glucose concentration under all conditions tested. In addition, all variables significantly influenced the hydrolysis of the coir fiber. However, at very high or very low pH values, cellulose catalysis was not as efficient and the intermediate pH values had a considerable influence on enzymatic processes. Similarly, high temperatures resulted in inefficient catalysis because this condition favors enzyme denaturation and, in this way, reduces catalytic activities (Fig. 4).
Equation 3 correlates the input variables (pH, process time, and temperature) with the output variable (glucose concentration). A statistical analysis with the necessary restrictions indicated the optimal conditions to maximize the conversion of cellulose into fermentable sugars: pH of 6.00, process time of 25.57 h, and temperature of 44.16 °C (Fig. 5).
where X1 is the time (h), X2 is the temperature (°C), and X3 is the pH.
To validate the optimum conditions, the reactions were carried out in triplicate on crude coir fiber, pulped coir fiber and pulped coir fiber treated with the n-butylammonium acetate IL. The concentration of sugars resulting from the treatment of pulped coir fiber with the n-butylammonium acetate IL was approximately tripled compared to that in the pulped fiber. When the coir fiber was treated using the optimized treatment conditions, the resulting lignin content was 6.32 ± 1.37% and the holocellulose content was 83.34 ± 2.14%, and 32.33 ± 1.08% of the holocellulose in this sample was subsequently converted into glucose.
A comparison of the results obtained for the crude fiber, the pulped fiber, and the treated coir fiber, showed that 6.27 ± 0.15% of the holocellulose in the crude fiber was converted into glucose while the conversion percentage for the pulped fiber was 14.34 ± 0.14%. This represents a 124.98% increase in the conversion due to the IL treatment, which justifies the use of the process in the production of second-generation bioethanol.
These values can be considered significant because cellulase from the fungus Trichoderma reesei is composed of 60–80% cellobiohydrolases or exogluconases, 20–36% endogluconases, and 1% β-glucosidases; however, it is deficient in cellobiase, which catalyzes the breakdown of cellulose into glucose, cellobiose, and higher glucose polymers (Smuga-Kogut et al. 2017; Hafid et al. 2017). The use of cellulases from other microorganisms, such as Trichoderma longibrachiatum, would favor the formation of greater amounts of glucose by catalyzing the breakdown of the disaccharide cellobiose for more efficient hydrolysis of cellulosic materials (Ahamed and Vermette 2008; Wen et al. 2005; Gusakov et al. 1985).
Conclusion
In the present study, we have synthesized and characterized the n-butylammonium acetate IL. We then verified the effectiveness of the use of this IL for the treatment of lignocellulosic residues to obtain bioethanol. Analyses of the effects of the treatment of coir fiber revealed that the n-butylammonium acetate IL treatment facilitated the subsequent hydrolysis process and resulted in a 120% increase in the final glucose concentration. The use of experimental planning enabled the determination of the best conditions for the IL treatment and the enzymatic hydrolysis. The implementation of these conditions resulted in a 32.33 ± 1.08% conversion of the coir fiber into glucose, demonstrating the effectiveness of the proposed IL treatment when compared with the conversion results obtained for crude coir fiber and pulped coir fiber (6.27 ± 0.15 and 14.34 ± 0.14%, respectively). Thus, the use of easily obtained ILs has proved to be a sustainable and viable alternative. Yet, it is important to investigate potential new treatments of lignocellulosic materials to further increase the production of fermentable sugars.
References
Ahamed A, Vermette P (2008) Culture-based strategies to enhance cellulase enzyme production from Trichoderma reesei RUT-C30 in bioreactor culture conditions. Biochem Eng J 40(3):399–407
Andrade Neto JC, Cabral AS, Oliveira LRD, Torres RB, Morandim-Giannetti AA (2016) Synthesis and characterization of new low-cost ILs based on butylammonium cation and application to lignocellulose hydrolysis. Carbohydr Polym 143:279–287
Asakawa A, Kohara M, Sasaki C, Asada C, Nakamura Y (2015) Comparison of choline acetate ionic liquid pretreatment with various pretreatments for enhancing the enzymatic saccharification of sugarcane bagasse. Ind Crops Prod 71:147–152
Barros Neto B, Scarminio IS, Bruns RE (2007) Como fazer experimentos: pesquisa e desenvolvimento na ciência e na indústria. Editora da Unicamp, Campinas, pp 305–338
Calado V, Montgomery D (2003) Planejamento de experimentos usando o Statistica, Rio de Janeiro, pp 120–158
Elgharbawy AA, Alam MZ, Kabbashi NA, Moniruzzaman M, Jamal P (2016) Evaluation of several ionic liquids for in situ hydrolysis of empty fruit bunches by locally-produced cellulose. 3. Biotech 6:128
Gusakov AV, Sinitsyn AP, Gerasimas VB, Savitskene RYu, Steponavichus YuYu (1985) A product inhibition study of cellulases from Trichoderma longibrachiatum using dyed cellulose. J Biotechnol 3(3):167–174
Hafid HS, Rahman NAA, Shah UKM, Baharuddin AS, Ariff AB (2017) Feasibility of using kitchen waste as future substrate for bioethanol production: a review. Renew Sustain Energy Rev 74:671–686
He YC, Liu F, Gong L, Zhu ZZ, Ding Y, Wang C, Xue YF, Rui H, Tao ZC, Zhang DP, Ma CL (2015) Significantly improving enzymatic saccharification of high crystallinity index’s corn stover by combining ionic liquid [Bmim]Cl–HCl–water media with dilute NaOH pretreatment. Bioresour Technol 189:421–425
Kshirsagar SD, Saratale GD, Saratale RG, Govindwar SP, Oh MK (2015) An isolated Amycolatopsis sp. GDS for cellulase and xylanase production using agricultural waste biomass. J Appl Microbiol 120:112–125
Lee OK, Oh YK, Lee EY (2015) Bioethanol production from carbohydrate-enriched residual biomass obtained after lipid extraction of Chlorella sp. KR-1. Bioresour Technol 196:22–27
Lienqueo ME, Ravanal MC, Pezoa-Conte R, Cortínez V, Martínez L, Niklitschek T, Salazar O, Carmona R, García A, Hyvärinen S, Mäki-Arvela P, Mikkola JP (2016) Second generation bioethanol from Eucalyptus globulus Labill and Nothofagus pumilio: ionic liquid pretreatment boosts the yields. Ind Crops Prod 80:148–155
Liu H, Sale KL, Holmes BM, Simmons BA, Singh S (2010) Understanding the interactions of cellulose with ionic liquids: a molecular dynamics study. J Phys Chem B 114:4293–4301
Liu CZ, Wang F, Stiles AR, Guo C (2011) Ionic liquids for biofuel production: opportunities and challenges. Appl Energy 92:406–414
Liu Y, Xiao W, Xia S, Ma P (2013) SO3H-functionalized acidic ionic liquids as catalysts for the hydrolysis of celulose. Carbohydr Polym 92:218–222
Merino O, Almazán V, Martínez-Palou R, Aburto J (2017) Screening of ionic liquids for pretreatment of taiwan grass in Q-tube minireactors for improving bioethanol production. Waste Biomass Valoriz 8:733–742
Miyafuji H (2015) Application of ionic liquids for effective use of woody biomass. J Wood Sci 61:343–350
Mood SH, Golfeshan AH, Tabatabaei M, Jouzani GS, Najafi GH, Gholami M, Ardjmand M (2012) Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew Sustain Energy Rev 27:77–93
Morandim-Giannetti AA, Albuquerque TS, de Carvalho RKC, Araújo RMS, Magnabosco R (2013) Study of “napier grass” delignification for production of cellulosic derivatives. Carbohydr Polym 92(1):849–855
Morandim-Giannetti AA, Campos PR, Viana AP, Martinez RPT, Daminato M, Silva RC, Santoro MA (2017) Influência de líquidos iônicos no tratamento da palha de milho para a produção de bioetanol. J Eng Exact Sci 03(02):130–143
Smuga-Kogut M, Zgórska K, Kogut T, Kukiełka K, Wojdalski J, Kupczyk A, Drózdz B, Wielewska I (2017) The use of ionic liquid pretreatment of rye straw for bioethanol production. Fuel 191:266–274
Uju ATW, Goto M, Kamiya N (2015) Great potency of seaweed waste biomass from the carrageenan industry for bioethanol production by peracetic acid–ionic liquid pretreatment. Biomass Bioenergy 81:63–69
Victor A, Pulidindi IN, Gedanken A (2015) Assessment of holocellulose for the production of bioethanol by conserving Pinus radiate cones as renewable feedstock. J Environ Manag 162:215–220
Wen Z, Liao W, Chen S (2005) Production of cellulase/β-glucosidase by the mixed fungi culture Trichoderma reesei and Aspergillus phoenicis on dairy manure. Process Biochem 40(9):3087–3094
Wise LEM, Murphy A, Addieco AD (1946) Chlorite holocellulose, its fractionationand bearing on summative wood analysis and on studies on the hemicellulose. Paper Trade J 122:35-43
Xin D, Yang M, Zhang Y, Hou X, Wu J, Fan X, Wang J, Zhang J (2016) Physicochemical characterization and enzymatic digestibility of Chinese pennisetum pretreated with 1-ethyl-3-methylimidazolium acetate at moderate temperatures. Renew Energy 91:409–416
Xu J, Wang X, Hu L, Xia J, Wu Z, Xu N, Dai B, Wu B (2015) A novel ionic liquid-tolerant Fusarium oxysporum BN secreting ionic liquid-stable cellulase: consolidated bioprocessing of pretreated lignocellulose containing residual ionic liquid. Bioresour Technol 181:18–25
You TT, Zhang LM, Xu F (2016) Progressive deconstruction of Arundo donax Linn. to fermentable sugars by acid catalyzed ionic liquid pretreatment. Bioresour Technol 199:271–274
Zhao ZP, Wang XL, Zhou GY, Cao Y, Lu P, Liu WF (2016) Hydrolysis kinetics of inulin by imidazole-based acidic ionic liquid in aqueous media and bioethanol fermentation. Chem Eng Sci 151:16–24
Zhou S, Runge TM (2015) Mechanism of improved cellulosic bio-ethanol production from alfalfa stems via ambient-temperature acid pretreatment. Bioresour Technol 193(288–296):2015
Acknowledgments
We are grateful to Fundação Educacional Inaciana Padre Sabóia de Medeiros (FEI) for supporting this research. We thank Dr. Nivaldo Boralle from the Chemical Institute of UNESP for the NMR measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oliveira Ribeiro, W.C., Silva Lima, A.C. & Araújo Morandim-Giannetti, A. Optimizing treatment condition of coir fiber with ionic liquid and subsequent enzymatic hydrolysis for future bioethanol production. Cellulose 25, 527–536 (2018). https://doi.org/10.1007/s10570-017-1554-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1554-9