Abstract
A novel porous adsorbent with high surface area and rigid structure was prepared by crosslinking oxidized microcrystalline cellulose particles with tetrafluoroterephthalonitrile. N2 adsorption–desorption measurements revealed the surface area to be 88.32 m2 g−1 for the porous adsorbent, while it was only 25.74 m2 g−1 for the sample crosslinked using epichlorohydrin. This porous adsorbent showed excellent performance for fast and efficient removal of low-concentration heavy metals from aqueous solution. Adsorption kinetic study demonstrated that, within 5 min, the removal efficiency for Pb2+, Cu2+, and Cd2+ with initial concentration of 10 mg L−1 by this adsorbent was 93.2, 87.5, and 72.3 %, respectively. Moreover, the pseudo-second-order model correlated better to the adsorption kinetic data than the pseudo-first-order model. The effects of other factors, e.g., initial solution pH, adsorption temperature, initial metal concentration, and background electrolytes, on the removal efficiency were also analyzed. Additionally, desorption experiments indicated that this adsorbent could be effectively regenerated using dilute HCl solution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The global problem of micropollutants in water resources has attracted worldwide attention due to the potential harm to human health and the environment. Unlike organic pollutants, heavy-metal ions cannot be degraded by organisms. Consequently, they accumulate in ecosystems and cause gradual toxicity to living organisms (Duan et al. 2013; Fu and Wang 2011). Thus, it is believed that even trace amounts of heavy metals can pose a risk to human beings (Singh et al. 2010; Ma et al. 2015). Various treatment technologies have been established and used for removal of heavy metals from aqueous solution and effluents, including chemical precipitation, ion exchange, oxidation/reduction, membrane processes, and electrochemical technologies (Ge et al. 2016; Gollavelli et al. 2013; Xu et al. 2011). However, inherent drawbacks to these methods including incomplete treatment, requirement for large amounts of reagents, high energy consumption, and generation of toxic sludge have hindered their application for heavy-metal removal (Anwar et al. 2009). The adsorption method has been proved to be a suitable process for removal of several kinds of pollutant from aqueous solution owing to its high removal efficiency, low cost, and simple regeneration. Different types of adsorbent materials have been investigated for heavy-metal removal. However, adsorbents have limited application for wastewater treatment because of various drawbacks of such materials, for instance, long time needed to reach equilibrium, unsatisfactory removal efficiency, unsuitability for low-concentration wastewater treatment, etc. (Ji et al. 2012; Ozturk et al. 2004; Wang et al. 2014). Generally, an effective adsorbent should have cellular structure, abundant functional groups, and high mechanical and chemical stability which could endow it with large contact area, adsorption selectivity, and structural stability (Slater and Cooper 2015; Soler-Illia and Azzaroni 2011; Chen et al. 2016). Against this background, microsized porous adsorbents have attracted increasing attention for potential applications in water treatment because their high surface area and highly interconnected porous structure give rise to high transport rates (Tseng et al. 2007).
In recent years, natural and modified biobased materials have drawn public attention due to their environmental friendliness, cost-effectiveness, biodegradability, regenerability, high uptake capacity, and ready availability (Hokkanen et al. 2016; Yi and Zhang 2008; Cao et al. 2017). Various types of cellulose have been tested and shown to exhibit excellent performance for water pollution control (Xu et al. 2013; Kikuchi and Tanaka 2012). Due to the strong hydrogen bond between glucans, microcrystalline cellulose (MCC) has highly crystalline structure. Thus, MCC has unique mechanical and chemical properties, and can be used as a grafted framework to synthesize adsorbents (Liimatainen et al. 2012). However, the adsorption capacity of MCC might not be as excellent as expected without modification due to the lack of strong binding sites for heavy metals (Zhang et al. 2016b). Having three functional hydroxyl groups in its repeating unit, it can be modified by typical alcohol groups, whose oxidation enables introduction of carboxyls. Tetrafluoroterephthalonitrile (TFTPN) is a very important organic intermediate because of its unique properties resulting from fluorine atoms and cyano groups, as well as its rigid structure. Many kinds of high-surface-area and microporous polymers with intrinsic micropores have been prepared using nucleophilic aromatic substitution reaction between hydroxyl groups and TFTPN. Such materials synthesized using TFTPN have been applied in adsorption processes, having potential for application in wastewater treatment (Carta et al. 2009; Budd et al. 2004; Zhang et al. 2016a).
The aim of this work is to develop a cellulose-based material with greater ability for fast and effective removal of low-concentration heavy metals from aqueous solution. As shown in Fig. 1, sequential oxidation of hydroxyl groups on MCC by sodium periodate and sodium chlorite can be applied as a selective reaction route to prepare anionic dicarboxylic cellulose (DCC) (Liimatainen et al. 2012). NaIO4 can oxidize vicinal hydroxyl groups on cellulose molecules to aldehyde groups while simultaneously breaking the carbon–carbon bond of glucopyranose to form 2,3-dialdehyde cellulose (DAC) (Sirviö et al. 2011). Subsequently, NaClO2 selectively oxidizes the aldehyde groups to form chemically stable DCC (Kim and Kuga 2001). We prepared oxidized cellulose crosslinked by TFTPN and epichlorohydrin (EPI), respectively, and investigated the performance of these materials for removal of Cu2+, Pb2+, and Cd2+ as model heavy metals. Fourier-transform infrared (FTIR) spectroscopy, N2 adsorption–desorption isotherm measurements, scanning electron microscopy (SEM), and thermogravimetric analysis (TGA) were conducted to characterize the structure of the adsorbents. The effects of initial solution pH, contact time, initial metal ion concentration, and temperature on their adsorption performance were also investigated. Additionally, we analyzed adsorption kinetics, adsorption isotherms, and adsorption thermodynamics to investigate the metal ion removal mechanism of this novel adsorbent and explored its regeneration experimentally.
Experimental
Materials
Microcrystalline cellulose (MCC) was purchased from Linghu Xinwang Chemical Co. (Huzhou, China). NaIO4 (99 %), NaClO2 (80 %), acetic acid (99 %), epichlorohydrin (EPI, 99 %), tetrafluoroterephthalonitrile (TFTPN, 99 %), and anhydrous tetrahydrofuran (THF, 99.9 %) were supplied by Shanghai Aladdin Biochemical Technology Co. Ltd. and used without further purification. Other materials were of analytical reagent grade and used as received.
Preparation of adsorbents
Preparation of oxidized cellulose
MCC (1.62 g) was added into 150 mL NaIO4 solution (0.1 mol L−1) and oxidation reaction performed at 50 °C for 4 h in absence of light. The resulting DAC was subsequently filtered, washed, and further reacted with NaClO2 (1.088 g) in 30 mL deionized water, then CH3COOH (0.26 mL) dissolved in 2 mL H2O was added dropwise. The reaction was performed for 7 h at room temperature. The oxidized product DCC was filtered and washed with deionized water to remove impurities (Visanko et al. 2015; Varma and Chavan 1994). The particle size distribution of DCC was measured using a Zetasizer (Nano-zs90, Malvern, UK) and is shown in Fig. S1 (Supplementary Information).
Preparation of adsorbents
Porous adsorbents with different ratios of DCC to TFTPN, named S-1 (DCC/TFTPN, 20/1, w/w) and S-2 (DCC/TFTPN, 4/1, w/w), were prepared. A contrast sample, named S-3, was prepared by crosslinking DCC using EPI. The composition of the three samples is presented in Table 1.
The porous adsorbents were prepared using the following steps (Alsbaiee et al. 2016): A dried pressure bottle was charged with DCC, TFTPN, and K2CO3 and flooded with N2 for 5–10 min, then anhydrous THF was added and N2 was bubbled for 3–5 min. Next, after the N2 inlet was removed, the bottle was placed in an oil bath (85 °C) and the reactants stirred for 48 h. The resulting brown suspension was cooled and filtered, and remaining K2CO3 was removed using HCl (1 mol L−1) until no CO2 was generated. The recovered brown solid was soaked in H2O (10 mL) twice for 15 min, THF (10 mL) twice for 30 min, and CH2Cl2 (15 mL) once for 15 min. Then, the resulting product was dried in vacuo at 50 °C for 48 h.
The contrast sample was prepared using the following steps: DCC was dissolved in a volume of NaOH solution and vigorously stirred for 15 min at room temperature, then EPI was added dropwise for 150 min at 60 °C. The reactant turned into a yellow gel, which was washed with deionized water then soaked in H2O (10 mL) twice for 15 min, THF (10 mL) twice for 30 min, and CH2Cl2 (15 mL) once for 15 min. The product was obtained after drying in vacuo at 50 °C for 48 h (Udoetok et al. 2016; Anirudhan et al. 2012).
Characterization
Fourier-transform infrared (FTIR) spectroscopic analysis was carried out on KBr pellets of the adsorbents using a Tensor 27 infrared spectrometer (Bruker, Switzerland) over the wavenumber range of 400–4000 cm−1. Thermogravimetric analysis (TGA) of adsorbents was carried out using a thermogravimetric analyzer (Mettler TGA/SDTA851) at heating rate of 10 °C min−1 under N2 flow. After being coated with a thin layer of gold powder, the surface morphology of the adsorbents was analyzed by scanning electron microscopy (SEM) measurements (JSM-7600F, JEOL, Tokyo, Japan). To determine the pore surface area of each sample, N2 adsorption–desorption measurements were carried out using a specific surface area and pore analyzer (NOVA 3200e, Quantachrome). Before N2 adsorption measurements, each sample was degassed at 150 °C for 24 h. The specific surface area of each sample was calculated using the Brunauer–Emmett–Teller (BET) equation. Under relative pressure of 0.99, the volume of N2 adsorbed was measured to calculate the total pore volume of each sample. Desorption isotherms were used to investigate the pore size distribution based on the Barrett–Joyner–Halenda (BJH) method. The average pore size D (nm) for each sample was calculated using Eq. (1) (Lowell et al. 2004):
where V total (cm3 g−1) is the total pore volume and A BET (m2 g−1) is the specific surface area.
Adsorption studies
Batch experiments were performed to determine the parameters for adsorption of heavy metals on the adsorbents. In each experimental run, the adsorption behavior was measured by adding 0.050 g adsorbent into 50 mL heavy-metal ion solution. The solution was stirred using a magnetic stirrer throughout the adsorption process. After adsorption, the mixture was separated by centrifugation, and atomic adsorption spectrophotometry (Varian Spectra HP 3510) was used to determine the residual metal concentrations in solution.
To study the effect of pH, the initial pH value was adjusted using 0.1 mol L−1 HCL and 0.1 mol L−1 NaOH solutions. The adsorption time was varied from 0 to 40 min for kinetic analysis. In each experimental run, 0.05 g of adsorbent was added into 50 mL solution of heavy metals with initial concentration varying from 10 to 85 mg L−1 and stirred to obtain the adsorption isotherm. Experiments were conducted at temperatures from 20 to 50 °C for thermodynamic study.
The adsorption capacity q e and removal efficiency R e were calculated using Eqs. (2) and (3), respectively:
where C 0 (mg L−1) is the initial concentration of heavy metals, C e (mg L−1) is the concentration of heavy metals at equilibrium, V (L) is the volume of solution, and m (g) is the mass of adsorbent used.
Adsorption kinetic models
Three adsorption kinetic models were used to evaluate the time required to remove heavy metals and determine the rate-controlling mechanism.
The pseudo-first-order kinetic model can be expressed as (Cao et al. 2017)
where q t and q e are the adsorption capacity (mg g−1) at time t (min) and at equilibrium, respectively, and k 1 (min−1) is the kinetic rate constant.
The pseudo-second-order kinetic model can be expressed as (Cao et al. 2017)
where k 2 (g mg−1 min−1) is the pseudo-second-order rate constant.
The intraparticle diffusion rate model, which determines the rate-controlling step, can be expressed as (Bardajee et al. 2015)
where k 3 (mg g−1 min0.5) is the intraparticle diffusion rate constant and C (mg g−1) is a constant that describes the boundary layer effect.
Adsorption isotherm models
Two common isotherm models were employed to fit the experimental data. The first is the Langmuir isotherm model expressed by Eq. (7) (Cao et al. 2017):
where q e (mg g−1) is the equilibrium adsorption capacity of the adsorbent, C e is the equilibrium ion concentration in solution (mg L−1), q m (mg g−1) is the maximum adsorption capacity of the adsorbent, and K L (L mg−1) is the Langmuir adsorption constant. A dimensionless constant R L for analysis of the Langmuir isotherm was defined by Eq. (8) (Zhang et al. 2003):
The second adsorption isotherm model is the Freundlich isotherm model, which is expressed as (Cao et al. 2017)
where q e and C e are defined as above, K F (L mg−1) is the Freundlich constant, and n is the heterogeneity factor.
Adsorption thermodynamics
Based on the Langmuir isotherm, the thermodynamic properties, such as the free energy change (∆G 0, kJ mol−1), entropy change (∆S 0, J mol−1 K−1), and enthalpy change (∆H 0, kJ mol−1), are defined as follows (Bardajee et al. 2015):
where K L (L g−1) and q e (mg g−1) are defined as above, R (8.314 J K−1 mol−1) is the universal gas constant, and T (K) is temperature.
Desorption studies
After the 30-min adsorption experiment, the adsorbent was filtered out from the heavy-metal solution. The adsorbed heavy-metal ions were removed from the adsorbent by washing with HCl solution (10 mL, 0.1 mol L−1) for 15 min, then the adsorbent was used again in the same heavy-metal solution. Five consecutive such adsorbent reutilization experiments were performed.
Results and discussion
Characterization of adsorbents
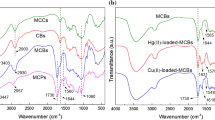
As shown in Fig. 2, the FTIR spectrum of S-1 showed absorbance at 2207 cm−1 corresponding to C≡N vibration and absorbance at 1425 cm−1 corresponding to C–C aromatic stretching. The band at 1265 cm−1 is attributed to C–F stretching vibration (Alsbaiee et al. 2016). Compared with the smaller peak at 1617 cm−1 for MCC, the peak at 1630 cm−1 for DCC and S-1 can mainly be attributed to adsorption by acetyl group, because the peak strength sharply increased and the wavenumber of this peak also increased after oxidation (Zhou et al. 2013). The FTIR spectra exhibit O–H stretching near 3438 cm−1, C–H stretching vibration at around 2930 cm−1, and intense C–O–C stretching at 1033 cm−1. The FTIR results demonstrate successful introduction of functional groups for adsorption and successful crosslinking of DCC using TFTPN. Due to the weak interaction between hydroxyl group and heavy-metal ions, MCC is not suitable for use as an adsorbent for heavy-metal ion removal. However, there are large amounts of carboxyl groups on the surfaces of S-1, S-2, and S-3, providing adsorption sites for heavy-metal ions. As a result, each sample can be used as an adsorbent.
The thermogravimetric analysis (TGA) and differential thermogravimetric (DTG) curves obtained when the adsorbents were heated from 100 to 700 °C in flowing nitrogen are presented in Fig. 3 and Fig. S2. MCC exhibited obvious weight loss at 340 °C corresponding to the maximum rate of decomposition, with almost complete weight loss at the end (Miranda et al. 2013). The TGA curve for DCC showed significant mass loss at two different temperatures. The first mass loss at temperature lower than 200 °C can be attributed to loss of water, while the second loss at 289 °C can be attributed to decomposition of cellulose component. Adsorbent S-1 demonstrated slight mass loss at 175 and 233 °C and significant mass loss at 312 °C. The peak temperature of its main mass loss is shifted to higher temperature compared with that of DCC, which could be due to the introduction of TFTPN. Thermal degradation of DCC resulted in 26.01 wt% residual mass at 700 °C, compared with 31.60 wt% for S-1, both being much greater than the value of 1.58 wt% for MCC.
As shown in Fig. 4 and Table 1, the differences in the microstructure of the three samples were investigated by BET analysis. For S-1, the BET surface area and pore volume were measured to be 88.32 m2 g−1 and 0.2860 cm3 g−1, respectively. However, the BET surface area and pore volume of S-3 were only 25.74 m2 g−1 and 0.1452 cm3 g−1, much lower than for S-1. This difference reflects that crosslinking DCC with TFTPN could enhance the surface area and total pore volume more. For similar molecular structures, higher surface area is beneficial for adsorbing heavy metals from wastewater. The porosity of the three samples was further characterized by BJH analysis to determine their pore size distribution. Comparing S-1 and S-3, it was found that S-3 had smaller total pore volume and lower porosity than S-1, and obviously narrower pore size distribution (mainly 3–10 nm). Regarding S-1 and S-2, even using the same crosslinking agent, the curve for S-1 indicated a pore size distribution extending to above pore width of 10 nm. S-1 exhibited a higher cumulative pore size distribution above 3 nm compared with S-2. Thus, this rigid adsorbent crosslinked using TFTPN showed better pore structure, and this favorable effect became more pronounced with greater TFTPN addition.
The surface morphology of the three adsorbents was investigated by SEM as shown in Fig. 5. According to the images, S-1 showed many nanopores and small aggregates on its surface (Fig. 5a), which could favor diffusion of metal cations into the adsorbent structure and thus enhance its adsorption capacity. S-3 exhibited an entirely different regular structure, being relatively nonporous. It showed irregular granular structures with dominant size larger than 1 μm (Fig. 5d).
Adsorption studies
Adsorption kinetics
The adsorption kinetic curves (R e versus t) are illustrated in Fig. 6. It can be seen that the adsorbents exhibited rapid adsorption and that using TFTPN as crosslinking agent obviously improved the removal efficiency. From Fig. 6a, it can be seen that Cu2+ was most rapidly removed by S-1, eventually reaching equilibrium in about 5 min, while more than 25 min was required for S-3 to reach equilibrium. The removal efficiency of Cu2+ lay in the order S-1 (87.5 %) > S-2 (67.3 %) > S-3 (56.3 %). Figure 6b shows that S-1 exhibited higher adsorption capacity for Pb2+ (93.2 %) and Cu2+ (87.5 %) than Cd2+ (72.3 %). DCC was crosslinked using TFTPN to provide S-1 with high surface area and rigidity. Generally, pores in common adsorbents are twisted and tortuous, resulting in some disadvantages for use as adsorbents, including longer time needed to reach adsorption equilibrium and a more difficult regeneration process caused by the resistance of pore diffusion (Tseng et al. 2007). However, these problems could be mitigated in adsorbent S-1 owing to its special structure and the characteristics of the raw material and crosslinking agent. The produced material had high surface area and rigid structure, promising rapid and efficient removal of low-concentration heavy metals from aqueous solution.
Curve fits for the kinetic models are shown in Figs. S3 and S4. The rate constants, regression coefficients, and other essential parameters for the kinetic rate models are presented in Table 2, revealing that the equilibrium adsorption capacity calculated by fitting the pseudo-second-order kinetic model to the curve was closer to the measured value. The plot of q t versus t 0.5 is shown in Fig. S4. Obviously, the plots have nonzero intercept, indicating that intraparticle diffusion occurs throughout the adsorption process (Seifi et al. 2011). Thus, the rate-controlling steps of the adsorption process include both intraparticle diffusion and liquid film diffusion. There are essentially three stages during adsorption by porous materials: liquid film diffusion (i.e., diffusion to the exterior surface of the adsorbent), intraparticle diffusion, and the equilibrium stage (Mohammed et al. 2015).
Adsorption isotherms
The isotherms for adsorption of heavy metals are shown in Fig. 7, while the calculated parameters of the Freundlich and Langmuir models are summarized in Table 3. The q e value for each adsorbent increased with increasing concentration until equilibrium was reached. From the results in Table 3, one can see that the adsorption isotherm data matched better with the Langmuir than Freundlich model. The Langmuir model is normally used to describe monolayer adsorption on a homogeneous distribution of sites with a sorption energy that does not consider interaction between adsorbed heavy-metal ions (Guo et al. 2009). Meanwhile, the Freundlich model is suitable for describing multilayer adsorption on a heterogeneous adsorbent surface (Wei et al. 2016; Lin and Juang 2009). According to the results, adsorption occurred on a homogeneous adsorbent surface. It has been reported that a value of n in the range of 2–10 represents good, 1–2 moderate, and < 1 poor adsorption characteristics (Yao et al. 2010). The n values for adsorption of heavy metals onto S-1 indicate that this adsorbent is extraordinarily suitable for heavy-metal adsorption.
The value of R L can be used to divide adsorption systems into unfavorable (R L > 1), linear (R L = 1), irreversible (R L = 0), and favorable (0 < R L < 1) (Ghorai et al. 2012). As seen in Fig. S6, R L ranged from 0 to 1, indicating favorable adsorption of heavy metals onto all the adsorbents.
Adsorption thermodynamics
The adsorption thermodynamics was investigated in the temperature range of 293–323 K. The effect of temperature on the adsorption capacity is shown in Fig. 8, revealing a slight decrease in equilibrium adsorption capacity with increasing temperature from 293 to 323 K. The estimated thermodynamic parameters are presented in Table S1 (Supplementary Information). ∆G 0 became more negative as temperature was decreased, indicating a spontaneous nature of the process, being more favorable at low temperature. In addition, its more negative value with decrease of temperature indicates that the adsorption process was promoted by decrease in temperature (Bardajee et al. 2015). The ∆H 0 values were found to lie below zero, indicating exothermic nature of the adsorption process. Negative ∆S 0 indicates decreasing randomness at the solid–liquid interface during the adsorption process, suggesting affinity of the adsorbent for the heavy metals and some structural changes and irreversibility in the adsorption process (Yadav et al. 2013).
Effect of pH
The adsorption capacity for heavy metals was investigated over the pH range of 2.0–6.0. For the three heavy metals, the removal efficiency of the porous adsorbents was greatly affected by the pH value of the solution. As shown in Fig. 9, at low pH, the adsorption capacity of S-1 was insignificant, whereas the removal efficiency increased with increase of the pH value. This is due to alteration of the surface charge on the hydrated heavy metals, because pH affects protonation or deprotonation of surface functional groups (Cao et al. 2017). At low pH, H+ ions occupy a limited number of possible binding sites on the adsorbent and heavy-metal ions must compete with them for adsorption sites. Increasing the H+ density restricts the approach of heavy metals to functional groups on the adsorbent because of the repulsive force. With increasing pH, competition from hydrogen ions decreases, more functional groups become negatively charged, and electrostatic repulsion decreases, so adsorption of heavy metals increases (Tseng et al. 2007). In conclusion, adsorption of heavy metals was more favorable at high pH values.
Effect of background electrolytes
Alkaline and alkaline-earth metal cations, such as Na+, K+, and Mg2+, are often present together with heavy-metal cation pollutants in wastewater (Ge et al. 2012). The effects of Na+, K+, and Mg2+ on the removal efficiency of heavy metals are shown in Fig. S8. Although the removal efficiency for all three heavy-metal ions significantly decreased with increasing background electrolyte concentration in the range of 0–0.08 mol L−1, only slight decrease was observed when the concentration of each background electrolyte was above 0.08 mol L−1. Figure 10 shows the removal efficiency for the three heavy-metal ions when the concentration of background electrolytes was 0.1 mol L−1. One can see that the removal efficiency decreased slightly with increasing concentration of coexisting ions. Mg2+ exhibited the most obvious impact on the removal efficiency, while the effects of Na+ and K+ ions were negligible. Mg2+ shows a greater suppressive effect on adsorption of heavy metals because of the stronger complexation between carboxyl groups and Mg2+ ions.
Desorption studies
A series of regeneration experiments were performed to investigate the reutilization performance of the adsorbents. HCl, ethylenediaminetetraacetic acid (EDTA), KCl, and CaCl2 were used as eluents for S-1. The results are shown in Fig. 11a. For Cu2+ desorption, desorption ratio of 97.9 % was obtained after the adsorbents were treated by HCl (0.1 mol L−1). This indicates almost complete desorption from the loaded adsorbent. However, after being treated by KCl (0.1 mol L−1), the desorption percentage was only 12.7 %. HCl showed the highest desorption ratio for Cu2+ and was thus selected for regeneration of S-1. Figure 11b shows the performance of S-1 during the adsorption–desorption cycles. This porous material could be used for several cycles without remarkable changes in performance. The adsorption capacity decreased slightly, which is due to irreversible combination between some active sites on the adsorbent and heavy-metal ions. In addition, according to Fig. 9, the adsorption capability was very weak at low pH, so it is speculated that some carboxyl groups may be protonated during the regeneration process. These results confirm that this porous material could be recycled for adsorption of heavy-metal ions.
Conclusions
We synthesized and characterized a modified microcrystalline cellulose-based porous material. It showed excellent capacity for rapid and efficient removal of low-concentration heavy-metal ions from aqueous solution. Adsorption occurred on the homogeneous surface of the adsorbent with identical binding sites. Thermodynamic analysis revealed that the adsorption process was thermodynamically feasible, spontaneous, and exothermic. Additionally, this adsorbent exhibited good reusability. This novel porous adsorbent is a promising material for wastewater treatment.
References
Alsbaiee A, Smith BJ, Xiao L, Ling Y, Helbling DE, Dichtel WR (2016) Rapid removal of organic micropollutants from water by a porous β-cyclodextrin polymer. Nature 529:190–194
Anirudhan TS, Rauf TA, Rejeena SR (2012) Removal and recovery of phosphate ions from aqueous solutions by amine functionalized epichlorohydrin-grafted cellulose. Desalination 285:277–284
Anwar J, Shafique U, Salman M, Waheed-uz-Zaman AS, Anzano JM (2009) Removal of chromium (III) by using coal as adsorbent. J Hazard Mater 171:797–801
Bardajee GR, Hooshyar Z, Shahidi FE (2015) Synthesis and characterization of a novel Schiff-base/SBA-15 nanoadsorbent for removal of methylene blue from aqueous solutions. Int J Environ Sci Technol 12:1737–1748
Budd PM, Ghanem BS, Makhseed S, Mckeown NB, Msayib KJ, Tattershall CE (2004) Polymers of intrinsic microporosity (PIMs): robust, solution-processable, organic nanoporous materials. Chem Commun 2:230–231
Cao J, Cao H, Zhu Y, Wang S, Qian D, Chen G, Sun M, Huang W (2017) Rapid and effective removal of Cu2+ from aqueous solution using novel chitosan and Laponite-based nanocomposite as adsorbent. Polymers 9:5
Carta M, Msayib KJ, Mckeown NB (2009) Novel polymers of intrinsic microporosity (PIMs) derived from 1,1-spiro-bis(1,2,3,4- tetrahydronaphthalene)-based monomers. Tetrahedron Lett 50:5954–5957
Chen D, Wang L, Ma Y, Yang W (2016) Super-adsorbent material based on functional polymer particles with a multilevel porous structure. NPG Asia Mater 8:e301
Duan C, Zhao N, Yu X, Zhang X, Xu J (2013) Chemically modified kapok fiber for fast adsorption of Pb2+, Cd2+, Cu2+ from aqueous solution. Cellulose 20:849–860
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manag 92:407–418
Ge F, Li M, Ye H, Zhao B (2012) Effective removal of heavy metal ions Cd2+, Zn2+, Pb2+, Cu2+ from aqueous solution by polymer-modified magnetic nanoparticles. J Hazard Mater 211–212:366–372
Ge H, Huang H, Xu M, Chen Q (2016) Cellulose/poly(ethylene imine) composites as efficient and reusable adsorbents for heavy metal ions. Cellulose 23:2527–2537
Ghorai S, Sinhamahpatra A, Sarkar A, Panda AB, Pal S (2012) Novel biodegradable nanocomposite based on XG-g-PAM/SiO2: application of an efficient adsorbent for Pb2+ ions from aqueous solution. Bioresour Technol 119:181–190
Gollavelli G, Chang C, Ling Y (2013) Facile synthesis of smart magnetic graphene for safe drinking water: heavy metal removal and disinfection control. ACS Sustain Chem Eng 1:462–472
Guo L, Sun C, Li G, Liu C, Ji C (2009) Thermodynamics and kinetics of Zn(II) adsorption on crosslinked starch phosphates. J Hazard Mater 161:510–515
Hokkanen S, Bhatnagar A, Sillanpää M (2016) A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res 91:156–173
Ji F, Li C, Tang B, Xu J, Lu G, Liu P (2012) Preparation of cellulose acetate/zeolite composite fiber and its adsorption behavior for heavy metal ions in aqueous solution. Chem Eng J 209:325–333
Kikuchi T, Tanaka S (2012) Biological removal and recovery of toxic heavy metals in water environment. Crit Rev Environ Sci Technol 42:1007–1057
Kim UJ, Kuga S (2001) Ion-exchange chromatography by dicarboxyl cellulose gel. J Chromatogr A 919:29–37
Liimatainen H, Visanko M, Sirviö J, Hormi O, Niinimäki J (2012) Enhancement of the nanofibrillation of wood cellulose through sequential periodat-chlorite oxidation. Biomacromolecules 13:1592–1597
Lin S, Juang R (2009) Adsorption of phenol and its derivatives from water using synthetic resins and low-cost natural adsorbents: a review. J Environ Manag 90:1336–1349
Lowell S, Shields JE, Thomas MA, Thommes M (2004) Characterization of porous solids and powders: surface area, pore size and density. Springer, New York
Ma S, Zhan S, Jia Y, Zhou Q (2015) Highly efficient antibacterial and Pb(II) removal effects of AgCoFe2O4-GO nanocomposite. ACS Appl Mater Interfaces 7:10576–10586
Miranda MIG, Bica CID, Nachtigall SMB, Rehman N, Rosa SML (2013) Kinetical thermal degradation study of maize straw and soybean hull celluloses by simultaneous DSC-TGA and MDSC techniques. Thermochim Acta 565:65–71
Mohammed N, Grishkewich N, Berry RM, Tam KC (2015) Cellulose nanocrystal-alginate hydrogel beads as novel adsorbents for organic dyes in aqueous solutions. Cellulose 22:3725–3738
Ozturk A, Artan T, Ayar A (2004) Biosorption of nickel(II) and copper(II) ions from aqueous solution by Streptomyces coelicolor A3(2). Colloid Surf B Biointerfaces 34:105–111
Seifi L, Torabian A, Kazemian H, Bidhendi GN, Azimi AA, Farhadi F, Nazmara S (2011) Kinetic study of BTEX removal using granulated surfactant-modified natural zeolites nanoparticles. Water Air Soil Pollut 219:443–457
Singh A, Sharma RK, Agrawal M, Marshall FM (2010) Health risk assessment of heavy metals via dietary intake of foodstuffs from the wastewater irrigated site of a dry tropical area of India. Food Chem Toxicol 48:611–619
Sirviö J, Liimatainen H, Niinimäki J, Hormi O (2011) Dialdehyde cellulose microfibers generated from wood pulp by milling-induced periodate oxidation. Carbohydr Polym 86:260–265
Slater AG, Cooper AI (2015) Function-led design of new porous materials. Science 348:988–998
Soler-Illia GJAA, Azzaroni O (2011) Multifunctional hybrids by combining ordered mesoporous materials and macromolecular building blocks. Chem Soc Rev 40:1107–1150
Tseng J, Chang C, Chen Y, Chang C, Chiang P (2007) Synthesis of micro-size magnetic polymer adsorbent and its application for the removal of Cu(II) ion. Colloid Surf A Physicochem Eng Asp 295:209–216
Udoetok IA, Dimmick RM, Wilson LD, Headley JV (2016) Adsorption properties of cross-linked cellulose-epichlorohydrin polymers in aqueous solution. Carbohydr Polym 136:329–340
Varma AJ, Chavan VB (1994) Cellulosic diamines as reaction-incorporated fillers in epoxy composites. Cellulose 1:215–219
Visanko M, Liimatainen H, Sirviö J, Hormi O (2015) A cross-linked 2,3-dicarboxylic acid cellulose nanofibril network: a nanoporous thin-film layer with tailored pore size for composite membranes. Sep Purif Technol 154:44–50
Wang B, Lin H, Guo X, Bai P (2014) Boron removal using chelating resins with pyrocatechol functional groups. Desalination 347:138–143
Wei W, Wang Q, Li A, Yang J, Ma F, Pi S, Wu D (2016) Biosorption of Pb(II) from aqueous solution by extracellular polymeric substances extracted from Klebsiella sp. J1: adsorption behavior and mechanism assessment. Sci Rep 6:31575
Xu X, Gao B, Tang X, Yue Q, Zhong Q, Li Q (2011) Characteristics of cellulosic amine-crosslinked copolymer and its sorption properties for Cr(VI) from aqueous solutions. J Hazard Mater 189:420–426
Xu X, Gao B, Tan X, Zhang X, Yue Q, Wang Y, Li Q (2013) Nitrate adsorption by stratified wheat straw resin in lab-scale columns. Chem Eng J 226:1–6
Yadav S, Srivastava V, Banerjee S, Weng C, Sharma YC (2013) Adsorption characteristics of modified sand for the removal of hexavalent chromium ions from aqueous solutions: kinetic, thermodynamic and equilibrium studies. CATENA 100:120–127
Yao Y, Xu F, Chen M, Xu Z, Zhu Z (2010) Adsorption behavior of methylene blue on carbon nanotubes. Bioresour Technol 101:3040–3046
Yi J, Zhang L (2008) Removal of methylene blue dye from aqueous solution by adsorption onto sodium humate/polyacrylamide/clay hybrid hydrogels. Bioresour Technol 99:2182–2186
Zhang Y, Yang M, Huang X (2003) Arsenic(V) removal with a Ce(IV)-doped iron oxide adsorbent. Chemosphere 51:945–952
Zhang C, Li P, Huang W, Cao B (2016a) Selective adsorption and separation of organic dyes in aqueous solutions by hydrolyzed PIM-1 microfibers. Chem Eng Res Des 109:76–85
Zhang N, Zang G, Shi C, Yu H, Sheng G (2016b) A novel adsorbent TEMPO-mediated oxidized cellulose nanofibrils modified with PEI: preparation, characterization, and application for Cu(II) removal. J Hazard Mater 316:11–18
Zhou Y, Fu S, Zhang L, Zhan H (2013) Superabsorbent nanocomposite hydrogels made of carboxylated cellulose nanofibrils and CMC-g-p(AA-co-AM). Carbohydr Polym 97:429–435
Acknowledgments
We appreciate financial support from the Open Fund of State Key Laboratory of Offshore Oil Exploitation (CCL2015RCPS0222RNN), National Natural Science Foundation of China (51203187), Fundamental Research Funds for the Central Universities (17CX02053), and Program for Changjiang Scholars and Innovative Research Team in University (IRT_14R58).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cao, J., Fei, D., Tian, X. et al. Novel modified microcrystalline cellulose-based porous material for fast and effective heavy-metal removal from aqueous solution. Cellulose 24, 5565–5577 (2017). https://doi.org/10.1007/s10570-017-1504-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1504-6