Abstract
Hydrochars in situ functionalized with –SO3H groups were generated from kenaf core via a low-temperature hydrothermal carbonization process of 105 °C with a consecutive catalysis of H2SO4. The micro-morphology of the hydrochars was strongly affected by the sulfuric acid concentration. Sphere-like particles with size varying between 200 nm and 1 μm were obtained when the acid concentration was 52 wt%. Acid density of the hydrochar increased with the H2SO4 concentration increasing. The presence of considerable acidic groups of –SO3H, –COOH, and –OH on the surface of hydrochars was evidenced by Fourier-transform infrared spectroscopy and X-ray photoelectron spectroscopy. The hydrochar obtained can be used directly for effective catalytic hydrolysis of cellulose without any post-modification. This study proposed a promising sustainable and cost-effective route for facile production of acidic hydrochar from crude plant with tunable properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to a growing challenge from global energy and environmental crises, the effective conversion of renewable lignocellulosic biomass to valuable materials is of great importance in strategy for sustainable consumption to reduce the excessive dependence on fossil resources (Hu et al. 2010; Titirici and Antonietti 2010; Libra et al. 2011; Kambo and Dutta 2015). In this respect, researchers in many disciplines are currently driven to develop new technologies which could alleviate the energy shortage and environmental deterioration. Carbon materials fabricated from natural biomass have shown broad practical applications in a variety of areas, such as environmental, catalytic, electronic, sensing, and biological applications (Titirici et al. 2012). Unfortunately, the ‘‘biomass recalcitrance’’ property holds back a general and satisfactory process for the production of valuable products from crude biomass to date (Li et al. 2008; Hu et al. 2010). Amongst various techniques, the hydrothermal carbonization (HTC) process is an attractive promising way for highly efficient biomass conversion, owing to the fact that it represents an easy, green, energy-saving and kg-scalable process. It could allow the production of various functional hydrochars and hybrid nanostructures, which have already found broad applications including soil enrichment, catalysis, water purification, energy storage, and CO2 sequestration (Hu et al. 2010; Titirici et al. 2012; Oliveira et al. 2013; Dai et al. 2014; Zhang et al. 2014).
The exploitation of non-food biomass as a biomass resource for the manufacture of useful materials and chemicals is crucial to avoid competition with an increasing food demand. Kenaf (Hibiscus cannabinus L.) is well known as a valuable cellulosic source, a good carbon sequester, and a potential bioenergy crop with both economic and ecological advantages because of its fast growth rate and large biomass production (Akil et al. 2011; Chen et al. 2013; Jeun et al. 2015). Various uses of the long and strong bark fibers of the kenaf plant have been explored. Whereas, the predominant inner core part, comprising around 65% of the entire kenaf plant, has few applications due to its short and spongy fiber, and large amounts of cores were discarded (Hasegawa et al. 2009; Sajab et al. 2011). Since kenaf core is characteristic of high yield yet low lignin and low ash content (Wi et al. 2015), it could be a promising biomass resource for hydrochar production to develop alternative profitable outlets.
So far, an extensive number of studies have been reported on synthesis carbonaceous materials via the HTC process of pure xylose, glucose, cyclodextrin, sucrose, starch and so on (Wang et al. 2010; Titirici et al. 2012). Only a little attention has been paid to generate hydrochars directly from crude plants (Braghiroli et al. 2014), primarily due to the harsh hydrothermal conditions of high temperatures and pressures required during such a thermo-chemical process (Onda et al. 2009; Guiotoku et al. 2012; Goswami et al. 2015). Namely, direct HTC of real biomass for the efficient production of hydrochars, especially with regard to reducing the reaction temperature, is still in its early stage of development. Therefore, there are many aspects that require additional research (Hu et al. 2015; Kambo and Dutta 2015).
Recently, carbonaceous solid acids have received more and more attention since they are capable of easy separation from reaction products together with excellent catalytic performance and recyclability for cellulose hydrolysis or other acid-catalyzed reactions (Guo et al. 2012; Qi et al. 2014; Yabushita et al. 2014; Goswami et al. 2015). Up to date, carbonaceous solid acid catalysts with –SO3H groups have been mostly designed for the efficient hydrolysis of cellulose (Qi et al. 2014; Hu et al. 2015). These sulfonated carbonaceous materials are generally prepared in two steps with tedious and energy-intensive procedures. The first step involves the pyrolysis of sugars, cellulose or crude biomass at temperatures higher than 673 K for several hours to obtain a solid carbon material. To introduce –SO3H groups, a sulfonation reaction is subsequently carried out by mixing the resulting carbonaceous precursors with largely excessive amount of concentrated or fuming sulfuric acid at around 473 K for 8–10 h under inert atmosphere (Bai et al. 2014; Zeng et al. 2014). Evidently, such a sulfonation step generates some toxic gases and a large amount of acidic washing waste. Thus, a more simple, energy-efficient approach for the preparation of functional carbonaceous materials are essential for the catalysis and sustainable biomass utilization (Titirici et al. 2012). Besides, it should be mentioned that much effort has been taken in seeking the neoteric carbonaceous solid acid catalysts, while the effects of preparation conditions on these carbonaceous solid acid catalysts have rarely been reported.
In this work, sulfuric acid was utilized in the starting digestion step to denature the whole crude plant rapidly for efficient reproducible hydrolysis of cell-wall components in kenaf core. In addition, sulfuric acid has already been proved highly active to facilitate the dehydration and polymerization reactions of the hydrolyzed precursors in the formation of carbonaceous structures under ideal conditions (Wang et al. 2010). Therefore, we proposed herein a more facile, sustainable and benign process to combine the good reactive and catalytic performance of sulfuric acid with mild hydrothermal conditions for the preparation of functional hydrochars from kenaf core. This was done over a range of different concentrations of sulfuric acid, and pure water was used as the reaction medium without any additives. In this way, H2SO4 was utilized consecutively, so it can not only improve the recycling efficiency of sulfuric acid with less possible handling hazards during the whole process, but also can in situ produce sulfonated hydrochars with tunable properties in one step. The schematic diagram of the preparation processes of sulfonated hydrochars derived from kenaf core was illustrated in Fig. 1. Moreover, the effects of different acid concentrations on the characteristics of hydrochar catalysts produced via low-temperature HTC process were investigated for the first time. The as-prepared functional hydrochar was used to catalyze the hydrolysis of microcrystalline cellulose (MCC) into reducing sugars. As a potentially promising scale-up production strategy of functional hydrochars with flexible application performance, the consecutive method presented in this work can be extended for direct conversion of various types of lignocellulosic biomass or with some minor modifications.
Experimental
Materials
Kenaf was provided from the Kenaf Experimental Plantation Base of Fujian Agriculture & Forestry University (FAFU) in Fuzhou, China. The kenaf core obtained was first washed with distilled water to remove dirt, dried at 100 °C for 24 h, ground and sieved to pass through 60–80 mesh size (0.18–0.25 mm) prior to all further treatments. Microcrystalline cellulose was purchased from Sinapharm Chemical Reagent Co., Ltd (Shanghai, China). The cellulose content was above 98 wt%. All the chemicals/reagents used in this work were analytical grade. The kenaf core was mainly composed of 20.1 wt% lignin, 48.3 wt% cellulose, 19.6 wt% hemicellulose and 1.8 wt% ash. The elemental analysis of kenaf core was 45.99 wt% C, 5.82 wt% H, < 0.3% wt% N and 46.58 wt% O.
Sulfuric acid hydrolyzation of kenaf core and synthesis of sulfonated hydrochars
In a typical experiment, 10.0 g (oven dried weight) of kenaf core powder was mixed with 72 wt% sulfuric acid solution (1:10 g/mL) in a 250 mL beaker. The beaker was then put into a water bath for acid hydrolysis. After being continuously stirred for 10 min at a temperature of 50 °C, distilled water was added to the resultant mixture to produce solution with different acid concentrations of 42, 52, 62 and 72 wt%, respectively. After filtration, the corresponding hydrolysate solution (60 mL) was transferred into a 100 mL Teflon-lined stainless steel autoclave for hydrothermal carbonization (HTC). The sealed autoclave was directly placed in a ventilated oven and heated up to 105 °C and maintained for 6 h under autogenic pressure. After the synthesis, the autoclave was cooled by ambient air. The hydrochars were recovered as a black brown solid residue by vacuum filtration, washed thoroughly with distilled water and ethanol, and finally dried overnight in an oven at 105 °C. The dried hydrochars were ground into powders and denoted as HC-42, HC-52, HC-62, and HC-72 in accordance with different sulfuric acid concentrations. Hydrochar yield was calculated using the following equation.
where W1 is the weight of dry hydrochar solid, W0 is the input weight of dry kenaf core.
Typical experimental procedure for cellulose hydrolysis
The hydrolysis reaction was carried out in a 100 mL Teflon-lined stainless steel autoclave. 40 mL of distilled water, 0.10 g of microcrystalline cellulose, and 0.30 g of as-prepared sulfonated hydrochar (HC-52) was introduced into the autoclave. The reactor was heated to the desired treatment temperature (140 ≤ T ≤ 200 °C) and maintained at that temperature for a given period of time (6 ≤ t ≤ 14 h). When the reaction was finished, the autoclave was taken out to cool down by natural convection. The resulting mixture was filtered through 0.45 μm membrane to remove the catalyst and the unreacted cellulose. After that, the amount of reducing sugars in the liquid products was estimated by means of dinitrosalicylic acid (DNS) method (Li et al. 2008). Cellulose conversions (wt%) were determined by the change in cellulose weight before and after the reaction. The yield of total reducing sugars (TRS) was calculated from the equation: yield (%) = (weight of reducing sugar in the products)/(weight of cellulose in the loading sample) × 100%.
Analytical methods
The elemental composition (C, H, N, S) of hydrochars was determined using a Vario EL cube elemental analyzer with oxygen content calculated by the difference. The microscopic features of the hydrochar were observed by Hitachi SU-8010 field-emission scanning electron microscope. Infrared spectra were recorded with a Nicolet Avatar 360 spectrometer over the wavenumber range of 4000–400 at 4 cm−1 resolution. X-ray photoelectron spectroscopy (XPS) spectra were measured on an ESCALAB 250Xi system (Thermo Fisher) with a vacuum generator using Mg Kα radiation (hν = 1253.6 eV). The C1 s peak at 284.6 eV was used for binding energy correction. The concentration of acid sites on the hydrochar was quantified by an acid–base titration method in aqueous solution. Briefly, one gram of the sample was placed in 50 mL of 0.05 M NaOH solution. The vials were sealed and shaken for 24 h and then 5 mL of the filtrate was pipetted and the excess of base was titrated with HCl. The numbers of acidic sites were calculated from the amount of used NaOH solution (Tong et al. 2013; Bai et al. 2014).
Results and discussion
Yield and chemical characteristics of the hydrochars
After highly effective hydrolysis with sulfuric acid followed by HTC, pale yellow kenaf core powder was converted into black brown solid chars. The chemical composition of the hydrochar samples prepared under different H2SO4 concentrations along with their yields is presented in Table 1. Slight difference in elemental compositions in the solids obtained after HTC was observed by the elemental analysis. Therefore, it can be assumed that the composition of hydrothermal carbons derived from kenaf core under the experimental conditions reported in this work was nearly constant, and that higher acid concentration only led to higher amounts of solid products. When temperature and time were fixed at 105 °C for 6 h, the yield reached a maximum value of 37.8% with the acid concentration of 72 wt%. The reason was that sulfuric acid facilitated the reaction of dehydration and carbonization of the carbon precursors. Similar findings were presented from other authors (Wang et al. 2010, 2011; Braghiroli et al. 2014). Likewise, no clear change in the weight percentage of carbon content between pristine kenaf core (46.0 wt% C) and the hydrochars (48.7–49.9% wt% C) could be observed. The reason may be that the HTC process was conducted under low temperature and the raw materials might not be fully carbonized (Sun et al. 2014; Zhang et al. 2014). Compared with that of kenaf core, significant reduction in hydrogen content of hydrochar products was observed, resulting in lower H/C ratios, which could lead to elevated higher heating values (HHV) as expected after HTC (He et al. 2013; Ghanim et al. 2016). In addition, it was found in Table 1 that sulfur content in the produced hydrochars increased gradually with the increase of acid concentration. To be more specific, the higher the sulfur content was, the total acid density accordingly increased from 0.46 to 1.93 mmol g−1. Therefore, it can be speculated that more –SO3H groups along with other acidic functional groups such as phenolic –OH groups and/or –COOH groups were introduced to the hydrochars in the presence of higher concentrations of sulfuric acid (Shen et al. 2014; Hu et al. 2015). As a result, the hydrochar samples bearing rich oxygen-containing functional groups gave rise to very little difference of oxygen content as compared to that of the feedstock listed in Table 1. Thus, the as-synthesized sulfonated hydrochars could be expected to possess effective catalytic activities for hydrolysis of cellulose to reducing sugars (or glucose) as proposed by various authors (Shen et al. 2014; Yabushita et al. 2014; Hu et al. 2015).
Evolution of functional groups in hydrochars
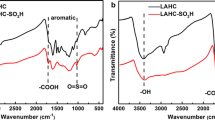
FT-IR spectra were employed to characterize the functional groups on the hydrochars. As is shown in Fig. 2, all the hydrochars have major bands at 3405 cm−1 (O–H), 2930 and 2850 cm−1 (C–H), 1710 cm−1 (C=O), 1610 cm−1 (C=C), 1460 cm−1 (C–H) and 1030 cm−1 (C–O) (He et al. 2013; Zhang et al. 2014). Two bands at around 1210 and 1030 cm−1 in the spectra can be assigned to S=O stretching modes of –SO3H, illustrating the successful introduction of sulfonic groups to the hydrochars (Bai et al. 2014; Zeng et al. 2014). The bands at 1620, 1510 cm−1, together with the band at 1460 cm−1, represent –C=C vibrations in aromatic ring carbons (Tong et al. 2013). In addition, the bands at 875–750 cm−1 are attributed to aromatic C–H out-of-plane bending vibrations, which also suggest the occurrence of aromatization of the hydrochars (Wang et al. 2010; Zhu et al. 2014).
Additionally, XPS analysis was carried out to indentify the elemental composition and chemical bonding state on the sample surface. An example of hydrochar XPS spectra from HC-52 is given in Fig. 3. As expected, dominant photoelectron peaks of C ls (about 285 eV) and O ls (about 532 eV) peaks were clearly resolved in the survey spectrum. The distribution of C and O structures could be derived from C1 s and O1 s spectra. The C1 s spectrum was fitted into three separate peaks. The peak at 284.8 eV is assigned to the C–C bond, the peak at 286.4 eV corresponds to C–O bond, and the peak at 288.8 eV is associated with the carbon atoms in –COOH (Wu et al. 2009; Xue et al. 2012; Gao et al. 2013; Bai et al. 2014). O1 s spectrum of hydrochar could both be fitted into three peaks at 532.2, 533.3 and 534.2 eV, which are attributed to C=O, C–OH, –COOR, respectively (Wu et al. 2009; Bai et al. 2014). Moreover, the successful introduction of –SO3H groups was further confirmed by the peak of S2p with binding energy of 168.7 eV (Bai et al. 2014). The sulfur content of H-52 was determined to be 0.34% on a mass basis by elemental analysis, which infers an acid site concentration of about 0.11 mmol/g for –SO3H. Then, based on the total acid density determined as 1.29 mmol/g for H-52, the rest acid density originated from carboxylic groups, phenolic groups and lactonic groups was thus calculated to be 1.18 mmol/g, assuming that the total acid sites of the samples were made up of the above acid groups such as –COOH, –OH, –COOR and –SO3H only. Furthermore, according to the analyzed integral area ratio of the three fitted peaks shown in O1 s XPS spectrum, the surface acidity distributions of –COOH, –OH and –COOR groups on H-52 could thus be calculated as about 0.69, 0.34 and 0.15 mmol/g, respectively. Overall, XPS results indicate the presence of rich surface oxygenic functional groups on the surface of HC-52, which agrees well with FT-IR spectra.
SEM images of hydrochar microstructure
A morphological change of the solid products after HTC was detected by SEM observation. Under hydrothermal conditions at relatively low sulfuric acid concentration, hydrochar samples consist mainly of aggregated spheroids whose size varies between 200 nm and 1 μm (Fig. 4a, b). The formation of these spherical particles with polydispersity can be associated with the difference in the kinetic of nucleation and growth as spheres, which may involve different intramolecular reactions of dehydration, condensation, polymerization, and aromatization of various pentoses, hexoses, as well as other hydrolysis fragments generated in the acid-catalyzed hydrolysate of the feedstock (Hu et al. 2010; Guiotoku et al. 2012; Titirici et al. 2012; Zhu et al. 2014). It is obvious that more severe irregular granular agglomerates (Fig. 4c, d) can be observed with the increased concentration of sulfuric acid. That is, the nucleation and linkage of the spherical microparticles could be promoted under the higher acid concentration. In particular, more compact and stacked surface texture of lumped carbon clusters with noticeable mesopores (Fig. 4d) is eventually developed in HC-72 sample as a result of higher degree of polymerization and carbonization. It might be that, with the higher concentration of sulfuric acid added, the fragmented products of sugars may undergo a series of more vigorous dehydration and polymerization reactions (Tong et al. 2013; Qi et al. 2014).
Catalytic hydrolysis of MCC to reducing sugars over sulfonated hydrochar
Effect of the concentration of acid sites on cellulose conversion
The hydrolysis efficiency of MCC and yield of reducing sugars over hydrochars with different total acid density are shown in Table 2. It can be seen that all the as-prepared hydrochars are active for cellulose hydrolysis, and the yield of total reducing sugars (TRS) synchronously increases as the conversion of cellulose rises. Namely, the concentration of acid sites has a significant positive effect on catalytic activity for MCC hydrolysis and the TRS yield remarkably increases with the amount of acid sites on hydrochars. Since carbonaceous solid acid catalysts possess carboxylic (–COOH), lactone (–COOR), phenolic hydroxyl (–OH) groups as well as –SO3H groups are widely proved to have efficient catalytic activities for cellulose hydrolysis in previous studies (Bai et al. 2014; Qi et al. 2014). As a consequence, the synergic catalytic effect of various acidic oxygen-containing functional groups bearing in the hydrochars, as verified from the FT-IR and XPS spectra, should be the reason for the improved catalytic efficiency of the as-prepared hydrochar with higher acid density (Hu et al. 2015).
Effect of reaction temperature and time
The hydrolysis of MCC to reducing sugars catalyzed by HC-52 in aqueous solution at different reaction temperatures and times is listed in Table 3. The results showed that the yield of TRS increased notably upon increasing the reaction temperature from 140 up to 180 °C. It is generally known that the yield of TRS depends on the equilibrium between cellulose hydrolysis and sugars decomposition (Guo et al. 2012). The produced reducing sugars are normally not stable at high temperature and readily degraded to the water soluble and insoluble byproducts (Shen et al. 2014). Therefore, the effectiveness of catalyst has a suitable temperature range. The TRS yield reached 27.7% at 180 °C and then remained almost constant by further increasing the temperature to 200 °C in this work, and this is probably due to the fact that the rate of cellulose hydrolysis to reducing sugars was close to that of sugars decomposition (Onda et al. 2009; Wu et al. 2009; Bai et al. 2014). When the hydrolysis was conducted at 150 °C, prolonging the hydrolysis time favored the conversion of cellulose into reducing sugars, while the hydrolysis time has little or no effect on the TRS yield after 12 h. Although the total acidity of HC-52 is relatively low amongst the samples, a highest TRS yield of 34.2% can be achieved when the reaction time was extended to 12 h, indicative of its effective catalytic ability for MCC hydrolysis. Overall, the TRS yields observed in this case are very comparable with or better than quite a few recent pioneering results on the hydrolysis of cellulose in aqueous solution over a variety of solid acid catalysts with high catalyst loading ratios (especially without any pretreatment or ionic liquids as reaction media) as reported by Tong et al. (2013) and by Hu et al. (2015). The desirable results achieved herein could be ascribed to the synergistic effect of considerable phenolic –OH, –COOH groups derived by HTC treatment through formation of hydrogen bonds with OH groups of cellulose, which can make cellulose readily hydrolyzed by –SO3H groups grafted on the sulfonated hydrochar (Shen et al. 2014; Hu et al. 2015).
Recycling of the catalyst
To examine the reusability of as-prepared sulfonated hydrochar catalyst in the reaction system, recycling of the HC-52 catalyst was investigated by carrying out four subsequent reaction cycles (Fig. 5). After the first reaction run was finished at 150 °C for 12 h, the solid residue was recovered from the hydrolytic solution by filtration, washed with hot water repeatedly and then dried before reusing in the next run at identical conditions. It can be seen that the TRS yield decreased slightly from 34.2 to 32.6% in the first two cycles and then dropped gradually to 29.2% in the 3rd run, indicating a good activity of the catalyst for cellulose hydrolysis owing to the stability of the functional groups on the catalyst (Qi et al. 2014). The results showed that the catalyst was still active in the 4th run, although the reducing sugar yield obviously decreased to 19.1%. This is probably due to not only the mass loss of catalyst mass and the leaching of some sulfonic groups during the filtration and washing steps (Bai et al. 2014), but also the coverage of some active acid sites of the catalyst by adsorbed intermediates. Even so, the covered or the loss of active acid sites might be recovered by simple regeneration process as described by Zeng et al. (2014).
Conclusions
With the consecutively reasonable utilization of sulfuric acid proposed in this work, a successful facile production of acidic hydrochars with tunable properties from crude kenaf core via a low-temperature HTC process was achieved. The yield, micro-morphology and acid sites of the hydrochars considerably depend on sulfuric acid concentrations. More importantly, the as-prepared sulfonated hydrochars functionalized in situ can be used directly without any post-modification for effective cellulose hydrolysis in aqueous solution. In summary, the route proposed in this work is characterized with simple, mild operation conditions, controllable, and high efficiency, thereby probably suitable for easy mass production of real biomass-derived sulfonated hydrochar solid acids with tunable catalytic performance for industrial application.
References
Akil HM, Omar MF, Mazuki AAM et al (2011) Kenaf fiber reinforced composites: a review. Mater Des 32:4107–4121. doi:10.1016/j.matdes.2011.04.008
Bai YY, Xiao LP, Sun RC (2014) Efficient hydrolyzation of cellulose in ionic liquid by novel sulfonated biomass-based catalysts. Cellulose 21:2327–2336. doi:10.1007/s10570-014-0287-2
Braghiroli FL, Fierro V, Izquierdo MT et al (2014) Kinetics of the hydrothermal treatment of tannin for producing carbonaceous microspheres. Bioresour Technol 151:271–277. doi:10.1016/j.biortech.2013.10.045
Chen YD, Chen WQ, Huang B, Huang MJ (2013) Process optimization of K2C2O4-activated carbon from kenaf core using Box-Behnken design. Chem Eng Res Des 91:1783–1789. doi:10.1016/j.cherd.2013.02.024
Dai L, Wu B, Tan F et al (2014) Engineered hydrochar composites for phosphorus removal/recovery: lanthanum doped hydrochar prepared by hydrothermal carbonization of lanthanum pretreated rice straw. Bioresour Technol 161:327–332. doi:10.1016/j.biortech.2014.03.086
Gao Y, Wang X, Wang J et al (2013) Effect of residence time on chemical and structural properties of hydrochar obtained by hydrothermal carbonization of water hyacinth. Energy 58:376–383. doi:10.1016/j.energy.2013.06.023
Ghanim BM, Pandey DS, Kwapinski W, Leahy JJ (2016) Hydrothermal carbonisation of poultry litter: effects of treatment temperature and residence time on yields and chemical properties of hydrochars. Bioresour Technol 216:373–380. doi:10.1016/j.biortech.2016.05.087
Goswami M, Meena S, Navatha S et al (2015) Hydrolysis of biomass using a reusable solid carbon acid catalyst and fermentation of the catalytic hydrolysate to ethanol. Bioresour Technol 188:99–102. doi:10.1016/j.biortech.2015.03.012
Guiotoku M, Hansel FA, Novotny EH, de Freitas Maia CMB (2012) Molecular and morphological characterization of hydrochar produced by microwave-assisted hydrothermal carbonization of cellulose. Pesqui Agropecu Bras 47:687–692. doi:10.1590/S0100-204X2012000500008
Guo H, Qi X, Li L, Smith RL (2012) Hydrolysis of cellulose over functionalized glucose-derived carbon catalyst in ionic liquid. Bioresour Technol 116:355–359. doi:10.1016/j.biortech.2012.03.098
Hasegawa T, Iwasaki S, Shibutani Y, Abe I (2009) Preparation of superior humidity-control materials from kenaf. J Porous Mater 16:129–134. doi:10.1007/s10934-007-9176-5
He C, Giannis A, Wang JY (2013) Conversion of sewage sludge to clean solid fuel using hydrothermal carbonization: hydrochar fuel characteristics and combustion behavior. Appl Energy 111:257–266. doi:10.1016/j.apenergy.2013.04.084
Hu B, Wang K, Wu L et al (2010) Engineering carbon materials from the hydrothermal carbonization process of biomass. Adv Mater 22:813–828. doi:10.1002/adma.200902812
Hu L, Lin L, Wu Z et al (2015) Chemocatalytic hydrolysis of cellulose into glucose over solid acid catalysts. Appl Catal B Environ 174–175:225–243. doi:10.1016/j.apcatb.2015.03.003
Jeun JP, Lee BM, Lee JY et al (2015) An irradiation-alkaline pretreatment of kenaf core for improving the sugar yield. Renew Energy 79:51–55. doi:10.1016/j.renene.2014.10.030
Kambo HS, Dutta A (2015) A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew Sustain Energy Rev 45:359–378. doi:10.1016/j.rser.2015.01.050
Li C, Wang Q, Zhao ZK (2008) Acid in ionic liquid: an efficient system for hydrolysis of lignocellulose. Green Chem 10:177. doi:10.1039/b711512a
Libra JA, Ro KS, Kammann C et al (2011) Hydrothermal carbonization of biomass residuals: a comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2:71–106. doi:10.4155/bfs.10.81
Oliveira I, Blöhse D, Ramke HG (2013) Hydrothermal carbonization of agricultural residues. Bioresour Technol 142:138–146. doi:10.1016/j.biortech.2013.04.125
Onda A, Ochi T, Yanagisawa K (2009) Hydrolysis of cellulose selectively into glucose over sulfonated activated-carbon catalyst under hydrothermal conditions. Top Catal 52:801–807. doi:10.1007/s11244-009-9237-x
Qi X, Lian Y, Yan L, Smith RL (2014) One-step preparation of carbonaceous solid acid catalysts by hydrothermal carbonization of glucose for cellulose hydrolysis. Catal Commun 57:50–54. doi:10.1016/j.catcom.2014.07.035
Sajab MS, Chia CH, Zakaria S et al (2011) Citric acid modified kenaf core fibres for removal of methylene blue from aqueous solution. Bioresour Technol 102:7237–7243. doi:10.1016/j.biortech.2011.05.011
Shen S, Wang C, Han Y et al (2014) Influence of reaction conditions on heterogeneous hydrolysis of cellulose over phenolic residue-derived solid acid. Fuel 134:573–578. doi:10.1016/j.fuel.2014.06.023
Sun Y, Gao B, Yao Y et al (2014) Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties. Chem Eng J 240:574–578. doi:10.1016/j.cej.2013.10.081
Titirici M-M, Antonietti M (2010) Chemistry and materials options of sustainable carbon materials made by hydrothermal carbonization. Chem Soc Rev 39:103–116. doi:10.1039/b819318p
Titirici M-M, White RJ, Falco C, Sevilla M (2012) Black perspectives for a green future: hydrothermal carbons for environment protection and energy storage. Energy Environ Sci 5:6796. doi:10.1039/c2ee21166a
Tong DS, Xia X, Luo XP et al (2013) Catalytic hydrolysis of cellulose to reducing sugar over acid-activated montmorillonite catalysts. Appl Clay Sci 74:147–153. doi:10.1016/j.clay.2012.09.002
Wang L, Guo Y, Zhu Y et al (2010) A new route for preparation of hydrochars from rice husk. Bioresour Technol 101:9807–9810. doi:10.1016/j.biortech.2010.07.031
Wang L, Guo Y, Zou B et al (2011) High surface area porous carbons prepared from hydrochars by phosphoric acid activation. Bioresour Technol 102:1947–1950. doi:10.1016/j.biortech.2010.08.100
Wi SG, Kim SB, Lee DS et al (2015) A comparative study on enzymatic hydrolysis of kenaf from two different harvest time-points, with- and without pretreatment. Ind Crops Prod 76:237–243. doi:10.1016/j.indcrop.2015.06.054
Wu B, Hu H, Zhao Y et al (2009) XPS analysis and combustibility of residues from two coals extraction with sub- and supercritical water. J Fuel Chem Technol 37:385–392. doi:10.1016/S1872-5813(10)60001-1
Xue Y, Gao B, Yao Y et al (2012) Hydrogen peroxide modification enhances the ability of biochar (hydrochar) produced from hydrothermal carbonization of peanut hull to remove aqueous heavy metals: batch and column tests. Chem Eng J 200–202:673–680. doi:10.1016/j.cej.2012.06.116
Yabushita M, Kobayashi H, Fukuoka A (2014) Catalytic transformation of cellulose into platform chemicals. Appl Catal B Environ 145:1–9. doi:10.1016/j.apcatb.2013.01.052
Zeng D, Liu S, Gong W et al (2014) Synthesis, characterization and acid catalysis of solid acid from peanut shell. Appl Catal A Gen 469:284–289. doi:10.1016/j.apcata.2013.09.038
Zhang JH, Lin QM, Zhao XR (2014) The hydrochar characters of municipal sewage sludge under different hydrothermal temperatures and durations. J Integr Agric 13:471–482. doi:10.1016/S2095-3119(13)60702-9
Zhu X, Liu Y, Qian F et al (2014) Preparation of magnetic porous carbon from waste hydrochar by simultaneous activation and magnetization for tetracycline removal. Bioresour Technol 154:209–214. doi:10.1016/j.biortech.2013.12.019
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 31000276 and 31370560), Natural Science Foundation of Fujian Province, China (No. 2015J01073), Natural Science Foundation of Fujian Province Education Department (No. JAT160183).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Y., Ai, X., Huang, B. et al. Consecutive preparation of hydrochar catalyst functionalized in situ with sulfonic groups for efficient cellulose hydrolysis. Cellulose 24, 2743–2752 (2017). https://doi.org/10.1007/s10570-017-1306-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1306-x