Abstract
Cellulose is a major biopolymer on the earth that is produced by cellulose synthase in the cell membrane of living organisms. Cellulose synthase is a hetero-subunit complex composed of several different protein subunits, and is visualized as a supermolecular complex called a “terminal complex” by electron microscopy. Such supermolecular organization of an enzyme complex is believed to be important for the fiber formation or crystallization of cellulose microfibrils in cellulose biosynthesis. In the case of the cellulose-producing bacterium Acetobacter, it is hypothesized that the enzyme complex includes at least six subunits given its genetic constitution. However, to date, only three of these molecules have been experimentally confirmed as the subunits included in the terminal complex: CesB, CesD, and ccp2. In this study, we used fluorescence immuno-microscopy to show that CesA protein, the catalytic subunit, is included in the terminal complex of Acetobacter. Furthermore we discuss the obtained microscopic data for improving our understanding of the molecular organization of the bacterial cellulose synthase complex.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulose is one of the major biopolymers on Earth. Despite its mass production on Earth by plants and other living organisms, destructive accumulation of cellulose on Earth has never been identified, although this has been observed for some synthetic plastics. This indicates that the cycle of synthesis and degradation shows a good balance for maintaining the cellulose content on Earth, which is actually a striking feature of cellulose, suggesting it as a promising material for sustainable human life.
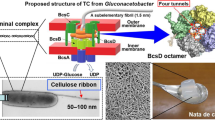
All of the cellulose on Earth is produced by living organisms, and originates from the cellulose synthase complex (CSC) in living cells. CSC is a hetero-subunit complex in the cell membrane (Somerville 2006). Electron microscopy with the freeze-fracture technique has been used to visualize CSC as a terminal complex (TC), which is a characteristic array of particles found on the cell membrane at the terminal of cellulose microfibrils (Kimura et al. 1999, 2001). Since the 1970s, TCs have been found in many of the cellulose-producing organisms (a tunicate, algae, plants, and a bacterium), although the arrangement of the TC particles showed a variety of patterns (Itoh et al. 2007): linear type (for a bacterium, tunicate, and algae producing a giant microfibril) and rosette-type (for higher plants and an alga of the order Zygnematale). Regardless of the specific pattern, such a regular array of a cellulose-synthesizing enzyme is considered to be important for cellulose microfibril formation by assembling many cellulose chains into a cellulose microfibril.
To date, several studies have identified the molecules included in the CSC based on biochemical and molecular/cell biological analyses. For Acetobacter (recently renamed for some strains as Gluconacetobacter, Komagataeibacter, and so on), a popular model for studying cellulose biosynthesis, six subunits are proposed to be included in the CSC given the constitution of the genes related to cellulose synthesis (McNamara et al. 2015): GH-8 (also known as carboxymethyl cellulase (CMC)) (Standal et al. 1994), cellulose complementing factor (ccp) (Standal et al. 1994), CesA, CesB, CesC, and CesD (Wong et al. 1990; Saxena et al. 1994). Among these, CesA is the catalytic subunit harboring the glycosyltransferase domain of the GT-2 family in the cytosolic part (Morgan et al. 2013), and CesA and CesB are the minimally required subunits for cellulose-synthesizing activity (Wong et al. 1990; Saxena et al. 1994; Omadjela et al. 2013). CesD is considered to control the crystallization process of cellulose microfibrils (Saxena et al. 1994; Hu et al. 2010), and four chains are found inside the ring structure formed by the octamer of CesD protein (Hu et al. 2010). The functions of the other subunits have not yet been clarified despite their clear relevance to cellulose-synthesizing activity, as experimentally reported for GH-8 (Kawano et al. 2002, 2008; Nakai et al. 2013), ccp (Sunagawa et al. 2013), CesC (Saxena et al. 1994), and CesD (Saxena et al. 1994; Hu et al. 2010; Sunagawa et al. 2013).
The SDS-freeze replica labeling (SDS-FRL) method (Fujimoto 1995), an immuno-labeling technique combined with the freeze-replica technique, is a direct method to localize a specific protein in the TCs, and its application has shown that CesB protein is found in the linear TC of Acetobacter (Kimura et al. 2001). In addition, fluorescence microscopy could also be used to successfully visualize the linear localization of GFP-fused CesD and ccp protein in the cells of Acetobacter (Sunagawa et al. 2013), which confirmed that these two proteins are also included in the linear TC of Acetobacter. These microscopic studies showed that CesB, CesD, and ccp proteins form a part of the TC or CSC of Acetobacter. However, no report has provided concrete evidence that CesA protein is included in the linear TC of Acetobacter, although this is the widely accepted hypothesis given that CesA is the core subunit of the CSC. In the present study, CesA protein was successfully visualized as a linear array in the cell, and experimental evidence was obtained to show that CesA protein is included in the TC of Acetobacter.
Materials and methods
Chemicals
Peptone and yeast extract for the culture medium were purchased from Becton, Dickinson and Company Inc. (USA). Paraformaldehyde (PFA) for cell fixation was paraformaldehyde EM from TAAB Inc. (UK). Poly-l-lysine solution, lysozyme and BSA were purchased from Sigma-Aldrich Inc. The other chemicals used in this study were purchased from Wako Pure Chemicals Inc. (Japan) unless described.
Cell culture
Three different strains of Acetobacter were used in this study: ATCC53264, ATCC53524, and JCM9730. For convenience, the former name Acetobacter is used for these strains herein, although these are actually considered to be different species based on the current taxonomy (Gluconacetobacter xylinus for ATCC53264, Komagataeibacter xylinus for ATCC53524, and Komagataeibacter sucrofermentans for JCM9730). The two ATCC strains were provided by the American Type Culture Collection and the last strain was obtained from the Japan Collection Microorganisms at BRC-RIKEN, Japan. Each strain was grown in Schramm-Hestrin medium (Schramm and Hestrin 1954) at 30 °C in a static condition for 3–5 days, until a sufficient amount of cellulose was produced. The cells were detached from the cellulose pellicle by shaking the culture medium and pressing the pellicle with a spatula, and then filtered by 37- or 50-µm pore-sized nylon mesh. The filtrated cells were collected by centrifugation (2000×g for 10 min at RT).
Antibody evaluation by western blot analysis
Western blot analysis was performed to evaluate whether the primary antibody has cross-reactivity with the proteins in the strains ATCC53264 and JCM9730, as well as strain ATCC53524 for which cross-reactivity has already been shown (Hashimoto et al. 2011). The primary antibodies used in this study were the same as those used in our previous studies (Hashimoto et al. 2011; Imai et al. 2014; Sun et al. 2016). In brief, each antibody is a polyclonal antibody against the synthetic peptide corresponding to a part of CesA (carboxyl terminal), CesB (a loop in the CBD2 domain), CesC (the part between the last six-TPR repeat and the carboxyl terminal region), and the CesD subunit (the loop between the β3 and β4 strands). The antigen peptide sequence for each of the proteins was designed from the sequence of the strain BPR2001 (Nakai et al. 1998) or JCM9730 (GenBank: AB010645) as reported in our previous study (Hashimoto et al. 2011). As shown in Table 1, high sequence similarity was found for each of the proteins between this strain and ATCC53264 or 1306-03 (GenBank: AAA21884 – 21887), and probably ATCC53524 or 1306-21, which is a derivative strain of ATCC53264 (Wong et al. 1990).
The centrifuged cells described above were resuspended in a buffer of 10 mM Tris–HCl (pH 8.0), 5 mM EDTA, 0.02% NaN3, 50 μg/mL chloramphenicol. Then, the cell suspension was mixed with the SDS-PAGE sample buffer. After incubating at 4 °C for overnight, the sample was analyzed with a precast gel with a gradient of 5–20% acrylamide (SuperSep Ace, Wako Pure Chemicals Industries Ltd., Japan). The band pattern was transferred from the gel to a PVDF membrane (Immobilon-P, Millipore Inc.), and then the membrane was incubated with each of the primary antibodies against CesA, CesB, CesC, and CesD protein. Finally, the protein band was visualized on the PVDF membrane by a chemical luminescence method with ECL select (GE Healthcare Inc.) and recorded by a CCD camera (EZ-capture, ATTO Inc., Japan).
Preparation of the cells for immunolabeling
The centrifuged cells were resuspended in CBS (citrate buffered saline: 40 mM sodium citrate buffer (pH 5.0), 110 mM NaCl, 2.2 mM KCl) and then incubated in 2% PFA in CBS at 4 °C overnight to chemically fix the cells. Then, the gently centrifuged cells (1000×g for 10 min at RT) were resuspended in PBS (phosphate buffered saline: 10 mM phosphate buffer (pH7.4), 136 mM NaCl, 2.7 mM KCl) with 0.1 M glycine for quenching the PFA. The cell suspension was dropped on the glass coverslip, which was made to be hydrophilic in advance by dipping in 1 mg/mL poly-l-lysine solution at RT for 30 min. The coverslip carrying the cells was processed with the following procedures of lysozyme treatment and permeabilizing treatment, prior to the antibody treatment.
The cells on the coverslip were treated with 1 mg/mL lysozyme in TE buffer (100 mM Tris–HCl (pH 6.7), 5 mM EDTA) at 37 °C for 1 h. After four repeated washes with PBS, the cells were permeabilized with 1% IGEPAL CA-630 (MP Biomedicals LLC; equivalent to a detergent Nonidet P-40) in PBS at 30 °C for 30 min. The cells were then washed four times with PBS for 5 min each time. Some of these treatments were skipped to explore the subunit localization in the cell.
Immunolabeling of the cells
Prior to the antibody treatment, the cells on the coverslip were incubated in 1% BSA and 1% Blocking Reagent (Roche Inc.) in PBS at RT for 1 h for blocking. Then, the cells were treated with the primary antibody solution, which was diluted 500-fold in the blocking buffer, at 4 °C for overnight with gentle shaking. After four washes with PBS every 5 min, the cells were treated with 5 µg/mL of the fluorophore-conjugated anti-rabbit IgG (Alexa fluor 488, Thermo Fisher Scientific Inc.) in the blocking buffer at RT for 2 h in the dark. The cells on the coverslip were washed with PBS four times every 5 min. The coverslip was taken out to wash the side without the cells in pure water, and then placed on the slide glass to seal the cells in the anti-fading reagent (SlowFade, Invitrogen Inc.). Control experiments were done for the fixed Acetobacter cells by using either no primary antibody or fluorophore-conjugated anti-mouse IgG (Alexa fluor 488, Thermo Fisher Scientific Inc.) for the secondary antibody.
The cells on the slide glass were observed on an IX71 microscope (Olympus Inc., Japan) with an oil immersion lens. Epi-fluorescence mode with a mercury lamp and the filter set FITC-2024B (Semrock Inc., USA) was used for recording the fluorescence image by a CCD camera (DP73, Olympus Inc., Japan). The same region of interest was also recorded with the phase-contrast mode for subsequent merging with the fluorescence image.
SDS-freeze replica labeling
The cells collected from the pellicle as described above were quickly frozen on the gold sample career by dipping into liquid ethane at −175 °C with a Leichert KF-80 system (Leica Inc.). The freeze-fracture replica of these cells without chemical fixation was prepared using a BAF-400D system (Balzers Inc.). The fracture was performed at −113 °C, and then platinum/carbon was evaporated on the fractured surface at an angle of 45° followed by rotary carbon coating to support the platinum replica. The prepared replica was treated in the lysozyme solution (1 mg/mL lysozyme in 25 mM Tris–HCl (pH 8.0), 10 mM EDTA) for 4 h at RT, and subsequently in the lysis solution (2.5% SDS, 10 mM Tris–HCl (pH 8.0)) for 2 h at RT. After washing three times with PBS, the replica was then incubated in the blocking solution (1% BSA in PBS) for 30 min at RT, and then treated with the primary antibody diluted in the blocking buffer overnight at 4 °C. The replica was then washed in PBS with 0.05% Tween-20 (PBST) and treated with the secondary antibody (anti-rabbit IgG conjugated with 15-nm colloidal gold, British BioCell International, UK) for 1.5 h at RT. Finally, the replica was treated with 0.5% glutaraldehyde in PBS for 15 min at RT, and then transferred on the carbon-coated copper grid after washing with water.
The replica on the grid was observed by a JEM-2000EXII (Jeol Inc., Japan) electron microscope and the images were recorded with photo-emulsion (FG film, FujiFilm Inc., Japan), which was developed by Korectol (FujiFilm Inc., Japan) for 4 min at 20 °C.
Results
CesA is present in the linear array in the bacterial cells
The results of the western blot analysis with the antibodies used in this study are shown in Fig. 1. These antibodies basically showed cross-reactivity to the proteins extracted from Acetobacter in the three different strains used in this study (ATCC 53524, ATCC53264, and JCM9730). We then used these antibodies for immunolabeling fluorescence microscopy, as shown in Fig. 2.
Western blot analysis with SDS-PAGE for the whole cell sample of Acetobacter cells. A–D The results with the antibody against CesA, CesB, CesC, and CesD protein, respectively. The sample of ATCC53524, ATCC53264, and JCM9730 was loaded into the lane 1, 2, and 3, respectively. Roughly the same number of cells, measured by the optical density at 600 nm, were loaded. The arrow indicates the band of interest
Fluorescence micrographs of Acetobacter cells with immunolabeling by the antibodies against CesA and CesD proteins. The phase-contrast images and the epi-fluorescence image are merged. A Three different strains (ATCC53524, ATCC53264, and JCM9730) were labeled using the identical protocol with a correct choice of the antibodies. The inset shows the image at a higher magnification. B Control experiments with a strain ATCC53524. Combination of the primary/secondary antibodies was used as indicated. Almost no labeling was found in neither conditions
As a result of optimizing the pretreatment of the cells (fixation, lysozyme treatment, and detergent treatment) as well as the antibody treatment, we could successfully label CesA and CesD proteins as a linear array in the cell (Fig. 2A) whereas no labeling was found in negative controls (Fig. 2B). Such a linear labeling pattern was not found for the immunolabeling of CesB and CesC. The linear labeling pattern of CesA and CesD was observed for all of the strains used in this study. This clearly indicates that CesA and CesD proteins are the subunits included in the linear TC of Acetobacter. Furthermore the linear signal was sometimes found at the lateral edge of the cell on the micrograph, indicating that the labeled protein is not on the inside but rather at the boundary of the Acetobacter cell. Therefore, the linear immunolabeling pattern shown in Fig. 2A provides the experimental evidence that CesA and CesD are included in the linear TC on the cell membrane, the bacterial CSC.
Change of the immunolabeling efficiency for CesA and CesD protein
For efficient immunolabeling, the cells are usually treated with an adequate procedure prior to labeling. In the case of the bacterial cell, lysozyme treatment is commonly used for disintegrating the peptidoglycan layer beneath the outer membrane, and detergent treatment is used for permeabilizing the outer and inner membrane. Therefore, in principle, the protein exposed to the outside of the cell will be labeled without any pretreatment. We then surveyed the change in the immunolabeling efficiency depending on the pretreatment applied to explore the location of CesA and CesD proteins, which were successfully immunolabeled in this study. The strain ATCC53524 was used for this purpose given the fact that this strain showed the highest immunolabeling efficiency.
We tested five different pretreatments, in addition to the optimized condition shown above (Figs. 3A, 4A): (1) no treatments, (2) EDTA treatment, (3) detergent treatment, (4) EDTA treatment followed by detergent treatment, and (5) lysozyme treatment (Figs. 3, 4, and summarized in Table 2). First, the cells with no pretreatment showed almost no immunolabeling for neither CesA nor CesD (Figs. 3B, 4B), indicating that CesA and CesD are not exposed to the outside of the cell. Notably, EDTA treatment alone allowed for the immunolabeling of CesD but not CesA (Figs. 3C, 4C). Given the relatively mild disturbance of the outer membrane only by depletion of divalent cations with EDTA, CesD is probably located in the periplasmic space and was immunolabeled due to access of the antibody. By contrast, CesA protein is a transmembrane protein, with its carboxyl terminal (the epitope of the antibody used in this study) facing to the cytoplasm. Therefore, it is reasonable that CesA was not immunolabeled for cells whose outer membrane is mildly disturbed by EDTA alone, which is not harsh enough to allow for cell lysis.
Fluorescence micrographs with immunolabeling of the strain ATCC53524 by the antibody against CesA protein, merged on the phase-contrast image. Pretreatment of the cell prior to the primary antibody treatment was as follows: A lysozyme treatment followed by detergent treatment (the optimized condition in this study), B no pretreatment, C EDTA treatment, D detergent treatment, E EDTA treatment followed by detergent treatment, F lysozyme treatment. The inset shows the image at a higher magnification
Fluorescence micrographs with immunolabeling of the strain ATCC53524 by the antibody against CesD protein, merged on the phase-contrast image. Pretreatment of the cell prior to the primary antibody treatment was as follows: A lysozyme treatment followed by detergent treatment (the optimized condition in this study), B no pretreatment, C EDTA treatment, D detergent treatment, E EDTA treatment followed by detergent treatment, F lysozyme treatment. The inset shows the image at a higher magnification
Detergent treatment alone did not allow for the immunolabeling of CesA and CesD, in contrast to the expectation (Figs. 3D, 4D). However, EDTA treatment prior to detergent treatment dramatically improved the immunolabeling efficiency for both CesA and CesD (Figs. 3E, 4E). This indicates that the permeabilization by the detergent is not sufficient for disturbing the outer membrane of Acetobacter to introduce the antibody to the inside of the cell (periplasm and cytoplasm).
A substantial number of cells were immunolabeled when treated with lysozyme alone for both CesA and CesD (Figs. 3F, 4F). Given that CesD is localized in the periplasm, as shown above, the lysozyme treatment without permeabilizing the inner membrane was sufficient to immunolabel CesD protein. However the substantial immunolabeling of CesA protein from such pretreatment requires a speculative interpretation given that the carboxyl terminal of CesA protein (the epitope of the antibody used in this study) is on the cytoplasmic side and prevents access of the antibody unless the inner membrane is permeabilized. We consider that this observation reflects weak but nevertheless significant cell lysis due to the lysozyme treatment.
Discussion
This study provides evidence that CesA is included in the TC of bacterial cells, which had already been reported for plant cells (Kimura et al. 1999). Based on fluorescence immuno-microscopy, this study also showed that CesD is included in the linear TC, as reported previously (Sunagawa et al. 2013). These observations are not sufficient to conclude that the CesA and CesD proteins are colocalized in the TC. Direct immunolabeling with a fluorescence dye-labeled primary antibody should provide a clearer conclusion for the colocalization of CesA and CesD proteins in the TC. Neverthelss, the linear labeling pattern observed for CesA and CesD in this study is striking enough to propose that CesA and CesD are colocalized in the TC of Acetobacter, regardless of whether their interaction is direct or indirect.
The structural models for the CesA/CesB complex (Morgan et al. 2013, 2014, 2016) and CesD (Hu et al. 2010) also support the functional link between CesA and CesD, given that the former generates cellulose from UDP-glucose and the latter includes cellulose chains in the channel formed by its homo-octamer. It is then proposed that CesD functions downstream of the CesA/CesB complex in the process of cellulose biosynthesis, and that they are spatially close. This hypothesis is consistent with the observation that immunolabeling of CesA and CesD proteins showed a linear pattern in the cells in this study.
We also attempted the immunolabeling of CesB and CesC protein in this study although no successful data were obtained. For CesC protein, which is currently the most enigmatic subunit, the reason for the failure is unclear. A possible reason could be related to access of the antibody to the epitope, which is significantly influenced by the stereo arrangement of this subunit in the cell. However, it was unexpected that the immunolabeling of CesB protein did not show a linear labeling pattern as previously observed by SDS-FRL (Kimura et al. 2001). It is noticeable that the antibody against CesB used in this study allowed for the linear TC to be labeled by SDS-FRL (Fig. 5), despite a relatively high non-specific labeling probably owing to that it is a polyclonal antibody. This result indicates that this antibody is able to label CesB protein in the SDS-treated freeze-replica prepared from the cells fixed by flash-freezing with no chemicals. Therefore, a possible interpretation for this unexpected result is that the PFA fixation might kill the epitope activity of the CesB protein, for example by changing the protein itself and/or its surrounding environment, so as to inhibit binding of the antibody in immunofluorescence labeling.
Electron micrograph of SDS-FRL for the strain ATCC53524 with the antibody against CesB protein. The antibody location was visualized by colloidal gold of a 15 nm diameter. Linear pattern of the labeling was clearly found despite a relatively high non-specific antibody labeling, which is probably due to that the antibody is a polyclonal antibody against peptide
The antibody against CesA protein, which gave successful immunolabeling as shown in Fig. 2A, was also used for SDS-FRL to visualize CesA protein in Acetobacter TC. However to date, no labeling for CesA protein was found on the replica despite successful labeling for CesB protein as shown above. This is probably due to that CesA protein in the inner membrane was detached from replica of the outer membrane by solubilization with SDS. Significant improvement will be required for successful SDS-FRL with the antibody against CesA protein.
Compiling the results of this and previous studies, we propose a hypothetical model for the TC of Acetobacter as shown in Fig. 6. The CesA/CesB complex is embedded in the inner membrane, given that the ligands (UDP-glucose and c-di-GMP) are cytosolic molecules and the product cellulose is extruded outside through the membrane-spanning channel (Morgan et al. 2013). CesC is depicted as the cellulose-translocating channel in the outer membrane according to the currently accepted model (Saxena et al. 1994; McNamara et al. 2015). No immunolabeling from the lack of pretreatment, and weak immunolabeling from EDTA treatment alone for CesD protein indicated that CesD is located in the periplasmic space. The result of a biochemical study using marker enzyme assays also support this hypothesis (Iyer et al. 2011). Given that the function of CesD is carried out downstream of CesA as discussed above, CesD protein is located close to the exit of the cellulose-translocation channel of CesA protein in the periplasm, as proposed based on a previous structural analysis of the Acetobacter CesA/CesB complex with electron microscopy (Du et al. 2016).
A schematic model for the cellulose synthase complex of Acetobacter. In the schematic diagram of the subunit location in one complex (a), CesA and CesB are depicted as monomers while CesD is illustrated as an octamer through which four cellulose chains pass, as reported previously (Hu et al. 2010). CesC is located in the outer membrane according to the currently accepted model (McNamara et al. 2015; Saxena et al. 1994). The terminal complexes are probably formed by the linear array of these complexes as shown in (b). OM outer membrane, IM inner membrane
Given that one CesA/CesB complex produces one cellulose chain (Morgan et al. 2013), and the CesD oligomer includes four chains in its inner pore (Hu et al. 2010), the model in Fig. 6a represents only one CesA/CesB complex, and the other three complexes are not shown for visual clarity. A combination of these molecules could be the functional unit to produce the primary assembly of the polymerized cellulose chains prior to microfibril formation, which has been proposed as a “mini-sheet” in a previous study (Cousins and Brown 1995). The linear array of this whole complex should be visualized as the linear type TC in Acetobacter (Fig. 6b).
The SDS-FRL experiment also provided insight about CesB protein. Given the smoothness of the fractured surface, the linear TC of Acetobacter is found in the P-face (the extracellular surface of the inner leaflet of the lipid bilayer) of the outer membrane (Kimura et al. 2001). The successful immunolabeling of CesB protein by SDS-FRL indicates that this protein remains with the replica even after SDS-treatment, indicating that CesB protein significantly interacts with the outer membrane from the periplasmic side. This interaction is likely important for guiding the cellulose chain to the extracellular side and/or the crystallization of cellulose chains into a microfibril. Further SDS-FRL experiment with other antibodies will shed light on the locations of the other subunits.
Concluding remarks
This study demonstrated that CesA, the core catalytic subunit of cellulose synthase, is the molecule included in the linear-type TC or the CSC of Acetobacter. Structural analysis of these proteins has recently started providing many insights about the enzymatic mechanism of cellulose synthase as well as other well-known membrane proteins such as ion/water channels and transporters. However, for cellulose synthase, which functions in the assembly of polymer chains into a supermolecular aggregation, the structural analysis of the protein complex at a cellular/subcellular scale is important for understanding the underlying mechanism. Further studies with microscopy will play an important role for shedding light on the mechanism of cellulose chains assembly into the microfibril.
Abbreviations
- PFA:
-
Paraformaldehyde
- EDTA:
-
Ethylenediamine tetra-acetic acid
- SDS:
-
Sodium dodecyl sulfate
- PAGE:
-
Poly-acrylamide gel electrophoresis
- PVDF:
-
Poly-vinylidene difluoride
- RT:
-
Room temperature
- BSA:
-
Bovine serum albumin
References
Cousins SK, Brown RM Jr (1995) Cellulose I microfibril assembly: computational molecular mechanics energy analysis favours bonding by van der Waals forces as the initial step in crystallization. Polymer 36:3885–3888
Du J, Vepachedu V, Cho SH, Kumar M, Nixon BT (2016) Structure of the cellulose synthase complex of Gluconacetobacter hansenii at 23.4 Å resolution. PLoS ONE 11:3e0155886. doi:10.1371/journal.pone.0155886
Fujimoto K (1995) Freeze-fracture replica electron microscopy combined with SDS digestion for cytochemical labeling of integral membrane proteins—application to the immunogold labeling of intercellular junctional complexes. J Cell Sci 108:3443–3449
Hashimoto A, Shimono K, Horikawa Y, Ichikawa T, Wada M, Imai T, Sugiyama J (2011) Extraction of cellulose-synthesizing activity of Gluconacetobacter xylinus by alkylmaltoside. Carbohydr Res 346:2760–2768
Hu S-Q, Gao Y-G, Tajima K, Sunagawa N, Zhou Y, Kawano S, Fujiwara T, Yoda T, Shimura D, Satoh Y, Munekata M, Tanaka I, Yao M (2010) Structure of bacterial cellulose synthase subunit D octamer with four inner passageways. Proc Natl Acad Sci USA 107:17957–17961
Imai T, S-j Sun, Horikawa Y, Wada M, Sugiyama J (2014) Functional reconstitution of cellulose synthase in Escherichia coli. Biomacromolecules 15:4206–4213
Itoh T, Kimura S, Brown RM Jr (2007) Immunogold labeling of cellulose-synthesizing terminal complexes. In: Brown RM Jr, Saxena IM (eds) Cellulose: molecular and structural biology. Springer, Amsterdam, pp 237–255
Iyer PR, Catchmark J, Brown NR, Tien M (2011) Biochemical localization of a protein involved in synthesis of Gluconacetobacter hansenii cellulose. Cellulose 18:739–747
Kawano S, Tajima K, Kono H, Erata T, Munekata M, Takai M (2002) Effects of endogenous endo-β-1,4-glucanase on cellulose biosynthesis in Acetobacter xylinum ATCC23769. J Biosci Bioeng 94:275–281
Kawano S, Tajima K, Kono H, Numata Y, Yamashita H, Satoh Y, Munekata M (2008) Regulation of endoglucanase gene (cmcax) expression in Acetobacter xylinum. J Biosci Bioeng 106:88–94
Kimura S, Laosinchai W, Itoh T, Cui X, Linder CR, Brown MR Jr (1999) Immunogold labeling of rosette terminal cellulose-synthesizing complexes in the vascular plant Vigna angularis. Plant Cell 11:2075–2085
Kimura S, Chen HP, Saxena IM, Brown RMJ, Itoh T (2001) Localization of c-di-GMP-binding protein with the linear terminal complexes of Acetobacter xylinum. J Bacteriol 183:5668–5674
McNamara JT, Morgan JLW, Zimmer J (2015) A molecular description of cellulose biosynthesis. Ann Rev Biochem 84:895–921
Morgan JLW, Strumillo J, Zimmer J (2013) Crystallographic snapshot of cellulose synthesis and membrane translocation. Nature 493:181–186
Morgan JLW, McNamara JT, Zimmer J (2014) Mechanism of activation of bacterial cellulose synthase by cyclic di-GMP. Nat Struct Mol Biol 21:489–496
Morgan JL, McNamara JT, Fischer M, Rich J, Chen HM, Withers SG, Zimmer J (2016) Observing cellulose biosynthesis and membrane translocation in crystallo. Nature 531:329–334
Nakai T, Moriya A, Tonouchi N, Tsuchida T, Yoshinaga F, Horinouchi S, Sone Y, Mori H, Sakai F, Hayashi T (1998) Control of expression by the cellulose synthase (bcsA) promoter region from Acetobacter xylinum BPR 2001. Gene 213:93–100
Nakai T, Sugano Y, Shoda M, Sakakibara H, Oiwa K, Tuzi S, Imai T, Sugiyama J, Takeuchi M, Yamauchi D, Mineyukia Y (2013) Formation of highly twisted ribbons in a carboxymethylcellulase gene-disrupted strain of a cellulose-producing bacterium. J Bacteriol 195:958–964
Omadjela O, Narahari A, Strumillo J, Mélida H, Mazur O, Bulone V, Zimmer J (2013) BcsA and BcsB form the catalytically active core of bacterial cellulose synthase sufficient for in vitro cellulose synthesis. Proc Natl Acad Sci USA 110:17856–17861
Saxena IM, Kudlicka K, Okuda K, Brown RM Jr (1994) Characterization of genes in the cellulose-synthesizing operon (acs operon) of Acetobacter xylinum: implications for cellulose crystallization. J Bacteriol 176:5735–5752
Schramm M, Hestrin S (1954) Factors affecting production of cellulose at the air/liquid interface of a culture of Acetobacter xylinum. J Gen Microbiol 11:123–129
Somerville C (2006) Cellulose synthesis in higher plants. Ann Rev Cell Dev Biol 22:53–78
Standal R, Iversen T-G, Coucheron DH, Fjaervik E, Blatny JM, Valla S (1994) A new gene required for cellulose production and a gene encoding cellulolytic activity in Acetobacter xylinum are colocalized with the bcs operon. J Bacteriol 176:665–672
Sun S-J, Horikawa Y, Wada M, Sugiyama J, Imai T (2016) Site-directed mutagenesis of bacterial cellulose synthase highlights sulfur–arene interaction as key to catalysis. Carbohydr Res 434:99–106. doi:10.1016/j.carres.2016.08.009
Sunagawa N, Fujiwara T, Yoda T, Kawano S, Satoh Y, Yao M, Tajima K, Dairi T (2013) Cellulose complementing factor (Ccp) is a new member of the cellulose synthase complex (terminal complex) in Acetobacter xylinum. J Biosci Bioeng 115:607–612
Wong HC, Fear AL, Calhoon RD, Eichinger GH, Mayer R, Amikam D, Benziman M, Gelfand DH, Meade JH, Emerick AW, Bruner R, Ben-Bassat A, Tal R (1990) Genetic organization of the cellulose synthase operon in Acetobacter xylinum. Proc Natl Acad Sci USA 87:8130–8134
Acknowledgments
The authors declare no conflicts of interest. This study was supported by CREST, JST to JS, KAKENHI [25650033; 15H04530] from the Japan Society for the Promotion of Science (JSPS) to TI, and the Humanosphere Mission Research (2009, RISH, Kyoto University) to TI and SK. Electron microscopic observations were performed by the Analysis and Development System for Advanced Materials (ADAM), in RISH, Kyoto University. We would like to thank Editage (www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, Sj., Imai, T., Sugiyama, J. et al. CesA protein is included in the terminal complex of Acetobacter . Cellulose 24, 2017–2027 (2017). https://doi.org/10.1007/s10570-017-1237-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1237-6