Abstract
Tyrosinase is used to eliminate phenolic compounds from wastewater. Therefore, its immobilization is important to enhance catalytic efficiency. Papery materials are of particular interest for use as support for enzyme immobilization since the porous microstructure of fiber networks in papers can provide a suitable reaction environment, especially in flow-type catalytic reactions. However, immobilization of protein onto papery structure needs chemical modifications in severe conditions. To overcome this challenge, a cellulosic paper was directly amine-functionalized in moderate conditions and used for tyrosinase immobilization. The support was pretreated with HCl (0.5 N) solution and then sequentially immersed in ethylenediamine (EDA), glutaraldehyde solution (2% v/v) and the crude enzyme. In comparison with the untreated one, the immobilized enzyme on the EDA-treated support offered a 3.7-fold increase in activity. The FTIR spectra as well as EDX analysis proved the presence of amine groups in the cellulosic paper and also covalent immobilization of tyrosinase on the modified support. When considering the effect of pH on the activity at 25 °C, a maximum relative activity of 134% at pH 6 was revealed. Similarly, evaluating the effect of temperature on the activity at pH 7 displayed a maximum relative activity of 152% at 35 °C. The immobilized enzyme was suitable for use for more than four cycles to degrade a phenolic compound at severe pH and temperature conditions. Additionally, the immobilized enzyme was active after treatment of the surface at different pHs and temperatures for 105 min. The chemically modified cellulosic paper can be used as a support for enzyme immobilization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tyrosinase is a copper-containing enzyme with two copper ions that catalyzes the hydroxylation of monophenols to o-diphenols, and subsequently the reaction proceeds to yield o-quinones in the presence of oxygen (Apetrei and Apetrei 2015; Ismaya et al. 2011). The enzyme is considered an oxidase with two kinds of activities: a monophenolase activity (cresolase) and diphenolase activity (catecholase). The fruiting body of the edible mushroom Agaricus bisporus, produced in large amounts for human consumption, is one of the available and inexpensive resources of tyrosinase (Dinçer et al. 2012).
Tyrosinase is used for elimination of phenolic compounds from wastewater on the industrial scale as well as in biosensors for detecting different pollutants (Fenoll et al. 2004; Stanca and Popescu 2004). The most recognized performance of tyrosinase is the formation of melanin from l-tyrosine and l-3,4-dihydroxyphenylalanine (l-DOPA) (Labus et al. 2012). Using pure tyrosinase for phenol degradation from wastewater streams makes it expensive for large-scale application. Additionally, the enzyme activity is partially lost during the purification process. Therefore, it is acceptable to pass up the purification of the enzyme. On the other hand, the direct use of mushroom tissue may have disadvantages such as slowing down the enzymatic reaction. Therefore, using immobilization techniques can reduce this disadvantage (Kampmann et al. 2014).
Immobilization of enzymes is one of the most effective ways to enhance catalytic efficiency, which increases the stability of enzymes and makes them reusable (Mateo et al. 2007; Ensuncho et al. 2005; Bayramoglu et al. 2013; Arica and Bayramoglu 2006). The other advantages of immobilized enzymes include easy isolation of enzymes from raw materials, increasing the contact area of enzymes and substrates, and preventing the accumulation of enzymes. There are different methods for enzyme immobilization, the most common being adsorption, covalent bonding and entrapment (Datta et al. 2013; Jesionowski et al. 2014; Mohamad et al. 2015; Brena et al. 2013).
Different supports and surfaces have been used for enzyme immobilization including nano-particles (Karim et al. 2015; Soozanipour et al. 2015; Meng et al. 2014; Karim and Lee 2013; PérezLópez and Merkoçi 2011; Wu et al. 2011; Liu et al. 2011), beads (Dinçer et al. 2012; Labus et al.2011; Marín-Zamora et al. 2007), sol gel (Kampmann et al. 2015; Munjal and Sawhney 2002), carbon nano-tubes (Apetrei and Apetrei 2015; Wang and Hasebe 2011) and different surfaces (Khan et al. 2005).
Cellulose is the most common and abundant organic polymer. Considering the importance of sustainable development, the use of cellulosic resources is growing quickly, and many new cellulose-based materials with various functions are developing. Paper, as the main cellulosic product, is inexpensive, flexible, easy to handle and lightweight. In addition, the porous microstructure of fiber networks in papers can provide a suitable reaction environment, especially in flow-type catalytic reactions, allowing for the effective diffusion of heat and reactants. Hence, different proteins have been immobilized on modified cellulosic paper (Koga et al. 2012, 2015; Cao et al. 2015; Nery and Kubota 2016; Zhao et al. 2016).
However, the functionalization of cellulosic materials for enzyme immobilization is challenging and carried out in severe conditions. In this study, direct introduction of amine groups into cellulosic paper in ambient temperature is reported. Modified papers were used as supports for covalent immobilization of tyrosinase. The supports were characterized, and the catalytic properties of the immobilized enzymes were studied.
Experimental section
Reagents and materials
Whatman filter paper (grade 42, ashless) was used as a cellulosic support. It is produced from high-quality cotton linters containing 98% cellulose. Ethylenediamine (EDA, 99%), propylenediamine (PDA, 98%) and glutaraldehyde aqueous solution (GL, 25%) were from Merck. Fresh mushrooms were purchased from the local market. The other chemicals were analytical grade.
Enzyme extraction and assays
To extract tyrosinase, briefly, fresh mushrooms were cut into small pieces. Afterward, cold acetone (−10 °C) was added to the mushroom pieces, and the mixture was agitated for 30 min. The mixture was centrifuged at 7000 rpm for 20 min. The sediment was resuspended in phosphate buffer (1 M, pH 7) and stirred for 30 min. Then, the mixture was centrifuged at 10,000 rpm to remove mushroom pulps. The supernatant was saturated with ammonium sulfate to 30% and subsequently centrifuged at 10,000 rpm to remove the precipitate. The supernatant was saturated with ammonium sulfate to 60%, and the mixture was centrifuged at 10,000 rpm to harvest the precipitate. The precipitate was resuspended in phosphate buffer and stored as crude enzyme (Zynek et al. 2010).
Tyrosinase activity was determined using fresh 1 mM l-tyrosine (as specific mono-phenolic substrate) solution in phosphate buffer at pH 7 and 25 °C. Change in absorbance was recorded in 30-s intervals using a Lambda 25 UV/Vis spectrophotometer (PerkinElmer) at a wavelength of 475 nm. The enzyme activity unit (U) is defined as the amount of tyrosinase that converts l-tyrosine into colored products causing an increase in absorbance of 0.001 per min (Dinçer et al. 2012). The protein concentration of enzyme solutions was determined by the Bradford method using bovine serum albumin (BSA) as standard (Bradford 1976). The activity of the crude enzyme solution was more than 10,000 U/ml. For kinetic study, l-DOPA (1 mM) was used as substrate to measure tyrosinase activity.
Cellulosic support preparation and enzyme immobilization
Briefly, the supports (0.7 cm × 1 cm) were sequentially treated at ambient temperature as follows: pretreatment with ethanol (95% v/v) or HCl (0.5 N) solutions, washing with distilled water, immersion in EDA or PDA for 45 min to be amine-functionalized, washing out with plenty of phosphate buffer (0.1 M, pH 7) and finally immersion in 2% GL solution (as crosslinking agent) for 3 h. For covalent immobilization of the tyrosinase, the prepared surfaces were individually immersed in crude enzyme solution with an activity of 4700 U/ml (protein concentration of 3.1 mg/ml) at 4 °C for 16 h. The enzyme-immobilized surfaces were washed with plenty of buffer to remove the non-covalent attached enzyme. Activity of the immobilized enzyme was measured, similar to that of the free enzyme. Substrate solution (at pH 7 and 25 °C) was added to 0.7 cm2 of the enzyme-immobilized surface, and changes in absorbance were recorded at 475 nm in 2-min intervals (Parkhill and Gulliver 1997). The surface showing the highest activity was selected for further studies, and the other immobilized enzyme activities relative to the highest one were reported. To discover the effect of functionalizing and crosslinking agents on immobilized enzyme activity, several experiments in the absence of corresponding chemicals were carried out and the relative activities recorded.
Characterization analysis of cellulosic support
Identification of the chemical groups on the untreated and treated support was performed using KBr disks on a Fourier transform infrared (FTIR, Bruker Vector-22) spectrometer, and absorptions were reported as wave numbers (cm−1). A scanning electron microscope (SEM) was used to observe surface morphologies of the support. The instrument was used in high vacuum mode (HV/20.0 kV) at 1000× and 450× magnification. Samples were cut into small pieces for surface scanning. The surfaces were coated with 20 μm of Au. Additionally, energy-dispersive X-ray spectroscopy (EDX) was carried out for qualitative elemental analysis of the surfaces.
Enzyme activity at different pHs and temperatures
To determine the effect of pH on free and immobilized enzyme activities, individual substrate solutions at different pHs between 4 and 8 (phosphate buffer for pHs 6, 7 and 8 and citrate phosphate buffer for pHs 4 and 5) were prepared, and the immobilized enzyme activities were measured at 25 °C. For temperature effect, the activities of the immobilized enzyme at pH 7 and different temperatures from 25 to 55 °C were measured. The same experiments were conducted using the free enzyme.
Reusability of the immobilized enzyme
Reuse tests were done at different pHs (4, 5, 6, 7 and 8) and 25 °C using l-tyrosine (1 mM) as substrate, and the activities of the immobilized enzyme were measured. For each cycle, the substrate solution was sequentially replaced with a fresh one after washing the surface with plenty of buffer. The activity of each cycle was calculated and reported relative to the activity of the first cycle at pH 7. The same experiments were conducted at different temperatures (25, 35, 45 and 55 °C) and pH 7.
Stability tests of the immobilized enzyme
For the pH stability test, the enzyme-harboring samples were immersed in buffers at different pHs (5, 6 and 8) for known periods. Then, the surfaces were individually transferred to substrate solution (1 mM l-tyrosine), and the corresponding activities were measured at pH 7 and 25 °C. To investigate temperature stability, the enzyme-immobilized surfaces were incubated at different temperatures (45, 55, 65 and 75 °C) for the specified times. Subsequently, the activity of the immobilized enzymes in l-tyrosine was measured at pH 7 and 25 °C.
Kinetic study
Oxygen is necessary for conversion of phenolic substrates using tyrosinase, and the consumption of molecular oxygen is equimolar to the di-phenolic substrate conversion. However, the oxygen content of aqueous solutions is sufficient for tyrosinase activity at low substrate concentrations (Kampmann et al. 2014; Parkhill and Gulliver 1997). In high concentrations of substrate, oxygen deficiency can hinder complete conversion of the substrate unless there is a sufficient oxygen mass transfer from ambient air to the solution that can compensate for the lack of oxygen. Therefore, in the kinetic study, it was assumed that at low concentrations of l-DOPA, the enzymatic reaction was not limited by the oxygen concentration (Kampmann et al. 2015). The effect of substrate concentration on the enzyme reaction rate was described with the Michaelis-Menten kinetic model (Eq. 1):
where υ max (U/cm2), S (mM) and K m (mM) were the maximum rate of the reaction, the concentration of the substrate (l-DOPA) and the Michaelis-Menten constant. To find the constant values of the model, the activity of the immobilized enzyme was measured using different concentrations (0.75, 1, 2, 3, 4 and 5 mM) of l-DOPA at 25 °C and pH 7. Then, a graph of the activities versus concentrations was plotted, and the values of K m and υ max were calculated by non-linear fitting of the model to the experimental data.
Support loading capacity
To understand the support capacity, BSA solutions with different concentrations (0.75, 1.5, 3, 6, 9 and 12 mg/ml) were prepared and individually immobilized on the support, similar to tyrosinase immobilization. At the end of the process, the protein concentration of the solutions was determined, and protein loaded on the support was also calculated.
Results and discussion
The effect of chemicals on immobilization performance
The cellulosic support functionalization and enzyme immobilization using different chemicals were carried out, and corresponding relative activities were reported (Table 1). A maximum activity of 40.8 U/cm2 was found when the supports were pretreated with HCl (0.5 N) and functionalized with EDA. In comparison with ethanol, HCl was more suitable to prepare the support to react with amine groups of the EDA. As shown in Table 2, using EDA and GL simultaneously in the immobilization process caused a 3.7-fold increase in activity compared to the untreated support. The results proved that 27% of the maximum activity could be referred to enzyme adsorption on the untreated support. In addition, the relative activity of the enzyme-free treated papers was negligible (<3%). The results showed the appropriateness of the procedure to immobilize the enzyme.
Characterization of the samples
FTIR
The FTIR spectra were taken from untreated, EDA-functionalized and fully prepared (without enzyme) supports to determine any changes in chemical bonding states (Fig. 1). The peaks at 3347 and 2901 cm−1 in the untreated support were significantly weakened in that for the amine-functionalized sample. These peaks were referred to O–H and C–H bonds. The O–H bond was mainly related to the cellulosic structure of the support. In Fig. 1a, the bands near 560 and 610 cm−1 correspond to the stretching vibration of the C–Br and C–Cl bonds, respectively. The disappearance of these bands in Fig. 1b was related to the condensation reaction between halides and amine groups of the EDA and formation of the –C–N– bond that created the new band near 1075 cm−1. The appearance of new bands at 1580 and 825 cm−1, observed in Fig. 1b, was attributed to the bending and wagging vibrations of N–H in amine groups. Similarly, the displacement and decreasing of the band size originally observed in Fig. 1a at 3350 cm−1, which shifted to 3400 cm−1 in Fig. 1b, were referred to the stretching vibration of the N-H bond in both the primary and secondary amines. The reaction between EDA and –C-Cl of cellulosic materials and formation of –C–N– has been previously proved (da Silva Filho et al. 2006). The new band at 1640 cm−1 (as shown in Fig. 1c) was ascribed to the presence of imine (–N=C–) in the support as the result of the reaction between glutaraldehyde and the amine groups of the EDA-treated sample. However, the reaction of glutaraldehyde with primary amine groups in both the treated support and enzyme is well accepted (Migneault et al. 2004).
SEM and EDX analyses
The surface morphology of the support was studied using SEM. As shown in Fig. S1, the enzyme-immobilized support clearly had a fibrous structure with the intertwined strands. There was no major change in the structure after the immobilization process, although a little destruction could have occurred because of the pretreatment with HCl.
EDX is a qualitative analysis that identifies some of the major elements in the supports. Therefore, this analysis was performed for the untreated surface, EDA-treated paper and enzyme-harboring support to determine the elements existing in the surface (Fig. 2). Peaks of O and C, referred to the principle elements of cellulosic materials, were common between all graphs. The peaks corresponding to Br and Cl seen in Fig. 2a were related to the additives used in the support production process. The bonds of C-Cl and C-Br for the untreated surface were also confirmed in the FTIR analysis. As shown in Fig. 2b, the peaks of Br and Cl disappeared for the EDA-functionalized support because of the condensation reaction between the halides and amine groups, resulting in the appearance of the N peak in harmony with the FTIR spectra. Phosphor (P) and potassium (K) are in this figure was because of washing the surface with phosphate buffer. Figure 2c representsPhosphor major elements ofPhosphor enzyme-immobilized support. The presence of sulfur (S) and copper (Cu) in Fig. 2c was attributed toPhosphor tyrosinase structure, which contains both elements. Tyrosinase has two atoms of copper (Cu) in the center, specifically (Donato et al. 2012). The existence of copper and sulfur on the surface obviously proves the succes of the immobilization process.
Based on the results from FTIR and EDX analyses, the scheme of enzyme immobilization on the support is proposed in Fig. 3.
Effects of pH and temperature on enzyme activity
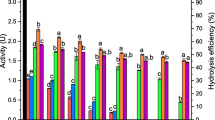
As a substantial parameter, pH influences the enzymatic activity and stability (Apetrei and Apetrei 2015). The effect of pH on the activity of immobilized and free enzymes was investigated. As displayed in Fig. 4a, the immobilized enzyme offered the maximum relative activity of 134% at pH 6, while that for the free enzyme was found at pH 7. During the immobilization process, amino groups were added to the surface, which probably interacted with enzyme functional groups around the active site causing conformational changes in the enzyme structure. As a result, the optimum pH shifted to 6.
The effect of pH and temperature on enzyme activities. a pH effects: free and immobilized enzyme activities were determined at ambient temperature using l-tyrosine (1 mM) as substrate. b Temperature effects: free and immobilized enzyme activities were determined at pH 7 using l-tyrosine (1 mM) as substrate
Moreover, free enzyme activity at pH 4 was negligible in contrast with that of the immobilized enzyme with a relative activity of 32%. The lowest activity of free and immobilized tyrosinase was related to pH 4.0, which could be associated with the net positive charge of the enzyme at this condition since the pH value at the isoelectric point (pI) of tyrosinase is about 4.7 (Rijiravanich et al. 2006).
To investigate temperature effects, the activities were measured at different temperatures (Fig. 4b). The immobilized enzyme showed the maximum relative activities of 152% (62.0 U/cm2) at 35 °C, while that for the free enzyme was 128%. Higher temperature strongly caused decreasing of the activities. In harmony with the results provided by the other researchers (Labus et al. 2011; Khan et al. 2005; Munjal and Sawhney 2002), immobilization made enzymes more stable in comparison with free enzymes.
Enzyme reusability
The experiments were individually done in four cycles at different pHs, and the activity of each cycle relative to that for the first cycle at pH 7 was reported (Fig. S2). The maximum relative activity of 128% was seen for the first cycle of the experiment at pH 6. Also, a sharp decrease in activity occurred for the experiment at pH 4, and the enzyme was only active for three cycles at this condition. In the case of temperature effect examination (Fig. S3), the process was carried out similarly to the method described above at different temperatures and pH 7, and the activity of each cycle relative to that of the first cycle at 25 °C was considered as 100% activity. The highest relative activity of 141% was found in the first cycle of the experiment at 35 °C. For higher temperatures, the relative activities decreased intensely as a result of enzyme denaturation. Relative activity losses during the second cycles and the next ones were due to enzyme inactivation as well as deposition of the reaction product (ortho-quinone) on the surfaces.
Stability of the immobilized enzyme
A lower degree of enzyme autolysis, increase in the conformational rigidity of enzyme structure and limited susceptibility to drastic conformational changes can be achieved by protein immobilization, which increases the enzyme stability (Jiang et al. 2005; Homaei et al. 2010).
In order to assess the stability of the immobilized enzyme, the sample was incubated at various pHs, and then the activity of the immobilized enzyme was measured (Fig. 5a). After incubation of the supports for 45 min, the immobilized enzyme was stable with relative activities of more than 85%, but the activities decreased after incubation of more than 75 min.
The effect of pH and temperature on enzyme stability. a pH effects: the immobilized enzyme was retained in buffer at different pHs for specified periods, and the enzyme activity was measured using l-tyrosine (1 mM) at pH 7 and ambient temperature. b Temperature effects: the immobilized enzyme was incubated in buffer at different temperatures for specified periods, and the enzyme activity was measured using l-tyrosine (1 mM) at pH 7 and ambient temperature
Thermal stability tests were done by incubating the surfaces at different temperatures (Fig. 5b), and subsequently the activities of the immobilized enzyme were determined. Keeping the surfaces at 45 and 55 °C for 30 min caused a loss of 50–60% in the relative activities. For incubating at higher temperatures and longer times, the losses in activity were more than 75%. In comparison with pH effects, temperature had severe impacts on the activity of the immobilized enzyme. An immobilized enzyme with a higher stability and a broad range of temperature profiles could be an appropriate candidate for practical applications (Homaei et al. 2010). However, the results showed that the immobilized enzyme was partially stable against the loss of enzymatic activity and catalytic efficiency during applications at the different conditions.
Kinetic study of the reaction
The kinetic studies for both immobilized and free enzymes were carried out using different concentrations of l-DOPA. In addition, non-linear fitting of the Michaelis-Menten model to the experimental data was performed (Fig. 6). The constant values, υ max and K m, were 2505 (U/cm2) and 7.66 (mM) for immobilized enzymes, while the corresponding values for free enzymes were 62,000 (U/ml) and 1.1 (mM), respectively. The increment of the K m value after the immobilization process shows lower affinity of the immobilized enzyme to its substrates in comparison with the free one as an intrinsic specification of enzyme immobilization processes. The discrepancy between the affinities of the free and immobilized enzymes to their substrates are owing to enzyme structural changes during immobilization (Bayramoglu et al. 2013), diffusional restrictions and the introduction of steric hindrance formed by the support toward the active site causing the lack of adequate enzyme flexibility for binding of the natural ligands during catalysis.
Loading capacity of the cellulosic support
Loading capacity of the carriers is an important parameter for practical applications. The results clearly showed that loading of protein on the support increased by increasing the protein concentration in solution. At the initial BSA concentration of 8 mg/ml, 35% of the protein was immobilized on the carrier, proving high affinity of the treated support to BSA. The carrier probably had high affinity to the other proteins. However, during the immobilization process adsorption and chemical bonding occurred simultaneously (Fig. 7).
Conclusions
Cellulosic paper was used as a support for immobilization of tyrosinase in a simple method. The amine groups were directly introduced into the cellulosic paper by treatment of the support using EDA. Tyrosinase was immobilized on the amine-functionalized surface by using glutaraldehyde as a crosslinking agent. EDX and FTIR characterization techniques confirmed the presence of amine groups in the support as a result of the condensation reaction between halides in the support structures and amine groups of the EDA. The covalent immobilization of tyrosinase on the modified support was also proved. Features of immobilized enzyme were studied from various aspects. The maximum activity of immobilized enzyme was 62.0 U/cm2 at pH 7 and 35 °C, while in the same experiment at pH 7 and 25 °C, the immobilized enzyme activity was 40.8 U/cm2. The immobilized enzyme was stable at different pHs and temperature conditions, and it could be reused for more than four cycles. The reaction rate of l-DOPA conversion was described using the Michaelis-Menten kinetic model, and the corresponding constants, υ max and K m, were 2505 (U/cm2) and 7.66 (mM). The treated support had high affinity to immobilize BSA. The chemically modified cellulosic paper can be used as a support for enzyme immobilization.
References
Apetrei IM, Apetrei CJ (2015) The biocomposite screen-printed biosensor based on immobilization of tyrosinase onto the carboxyl functionalised carbon nanotube for assaying tyramine in fish products. J Food Eng 149:1–8. doi:10.1016/j.jfoodeng.2014.09.036
Arıca MY, Bayramoğlu G (2006) Invertase reversibly immobilized onto polyethylenimine-grafted poly (GMA–MMA) beads for sucrose hydrolysis. J Mol Catal B Enzym 38:131–138. doi:10.1016/j.molcatb.2005.12.006
Bayramoglu G, Akbulut A, Arica MY (2013) Immobilization of tyrosinase on modified diatom biosilica: enzymatic removal of phenolic compounds from aqueous solution. J Hazard Mater 244:528–536. doi:10.1002/jctb.2743
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Brena B, González-Pombo P, Batista-Viera F (2013) Immobilization of enzymes: a literature survey. Methods Mol Biol 1051:15–31. doi:10.1007/978-1-62703-550-7_2
Cao R, Guan L, Li M, Tian J, Shen W (2015) A zero-step functionalization on paper-based biosensing platform for covalent biomolecule immobilization. Sens Bio-Sens Res 6:13–18. doi:10.1016/j.sbsr.2015.09.002
Da Silva Filho EC, de Melo JCP, Airoldi C (2006) Preparation of ethylenediamine-anchored cellulose and determination of thermochemical data for the interaction between cations and basic centers at the solid/liquid interface. Carbohydr Res 341:2842–2850. doi:10.1016/j.carres.2006.09.004
Datta S, Christena LR, Rajaram YRS (2013) Enzyme immobilization: an overview on techniques and support materials. 3 Biotech 3:1–9. doi:10.1007/s13205-012-0071-7
Dinçer A, Becerik S, Aydemir T (2012) Immobilization of tyrosinase on chitosan–clay composite beads. Int J Biol Macromol 50:815–820. doi:10.1016/j.ijbiomac.2011.11.020
Donato L, Algieri C, Miriello V, Mazzei R, Clarizia G, Giorno L, Memb J (2012) Biocatalytic zeolite membrane for the production of l-DOPA. J Membr Sci 407:86–92. doi:10.1016/j.memsci.2012.03.034
Ensuncho L, Alvarez-Cuenca M, Legge RL (2005) Removal of aqueous phenol using immobilized enzymes in a bench scale and pilot scale three-phase fluidized bed reactor. Bioprocess Biosyst Eng 27:185–191. doi:10.1007/s00449-005-0400-x
Fenoll LG, Penalver MJ, Rodrıguez-López JN, Varon R, Garcıa-Cánovas F, Tudela J (2004) Tyrosinase kinetics: discrimination between two models to explain the oxidation mechanism of monophenol and diphenol substrates. Int J Biochem Cell Biol 36:235–246. doi:10.1016/S1357-2725(03)00234-6
Homaei AA, Sajedi RH, Sariri R, Seyfzadeh S, Stevanato R (2010) Cysteine enhances activity and stability of immobilized papain. Amino Acids 38:937–942. doi:10.1007/s00726-009-0302-3
Ismaya WT, Rozeboom HJ, Weijn A, Mes JJ, Fusetti F, Wichers HJ, Dijkstra BW (2011) Crystal structure of Agaricus bisporus mushroom tyrosinase: identity of the tetramer subunits and interaction with tropolone. Biochemistry 50:5477–5486. doi:10.1021/bi200395t
Jesionowski T, Zdarta J, Krajewska B (2014) Enzyme immobilization by adsorption: a review. Adsorption 20:801–821. doi:10.1007/s10450-014-9623-y
Jiang DS, Long SY, Huang J, Xiao HY, Zhou JY (2005) Immobilization of Pycnoporus sanguineus laccase on magnetic chitosan microspheres. Biochem Eng J 25:15–23. doi:10.1016/j.bej.2005.03.007
Kampmann M, Boll S, Kossuch J, Bielecki J, Uhl S, Kleiner B, Wichmann R (2014) Efficient immobilization of mushroom tyrosinase utilizing whole cells from Agaricus bisporus and its application for degradation of bisphenol A. Water Res 57:295–303. doi:10.1016/j.watres.2014.03.054
Kampmann M, Hoffrichter AC, Stalinski D, Wichmann R (2015) Kinetic characterization of tyrosinase containing mushroom (Agaricus bisporus) cells immobilized in silica alginate. J Mol Catal B Enzym 116:124–133. doi:10.1016/j.molcatb.2015.03.013
Karim MN, Lee HJ (2013) Amperometric phenol biosensor based on covalent immobilization of tyrosinase on Au nanoparticle modified screen printed carbon electrodes. Talanta 116:991–996. doi:10.1016/j.talanta.2013.08.003
Karim Z, Khan MJ, Maskat MY, Adnan R (2015) Immobilization of horseradish peroxidase on β-cyclodextrin capped silver nanoparticles: its future aspects in biosensor application. Prep Biochem Biotechnol 46:321–327. doi:10.1080/10826068.2015.1031389
Khan AA, Akhtar S, Husain Q (2005) Simultaneous purification and immobilization of mushroom tyrosinase on an immunoaffinity support. Process Biochem 40:2379–2386. doi:10.1016/j.procbio.2004.09.020
Koga H, Kitaoka T, Isogai A (2012) Paper-immobilized enzyme as a green microstructured catalyst. J Mater Chem 22:11591–11597. doi:10.1039/C2JM30759F
Koga H, Kitaoka T, Isogai A (2015) Chemically-modified cellulose paper as a microstructured catalytic reactor. Molecules 20:1495–1508. doi:10.3390/molecules20011495
Labus K, Turek A, Liesiene J, Bryjak J (2011) Efficient Agaricus bisporus tyrosinase immobilization on cellulose-based carriers. Biochem Eng J 56:232–240. doi:10.1016/j.bej.2011.07.003
Labus K, Gancarz I, Bryjak J (2012) Immobilization of laccase and tyrosinase on untreated and plasma-treated cellulosic and polyamide membranes. Mater Sci Eng C 32:228–235. doi:10.1016/j.msec.2011.10.023
Liu T, Xu M, Yin H, Ai S, Qu X, Zong S (2011) A glassy carbon electrode modified with graphene and tyrosinase immobilized on platinum nanoparticles for sensing organophosphorus pesticides. Microchim Acta 175:129–135. doi:10.1007/s00604-011-0665-5
Marín-Zamora ME, Rojas-Melgarejo F, García-Cánovas F, García-Ruiz PA (2007) Effects of the immobilization supports on the catalytic properties of immobilized mushroom tyrosinase: a comparative study using several substrates. J Biotechnol 131:388–396. doi:10.1016/j.jbiotec.2007.05.004
Mateo C, Palomo JM, Fernandez-Lorente G, Guisan JM, Fernandez-Lafuente R (2007) Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb Technol 40:1451–1463. doi:10.1016/j.enzmictec.2007.01.018
Meng X, Xu G, Zhou QL, Wu JP, Yang LR (2014) Highly efficient solvent-free synthesis of 1,3-diacylglycerols by lipase immobilised on nano-sized magnetite particles. Food Chem 143:319–324. doi:10.1016/j.foodchem.2013.07.132
Migneault I, Dartiguenave C, Bertrand MJ, Waldron KC (2004) Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 37:790–806
Mohamad NR, Marzuki NHC, Buang NA, Huyop F, Wahab RA (2015) An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol Biotechnol Equip 29:205–220. doi:10.1080/13102818.2015.1008192
Munjal N, Sawhney SK (2002) Stability and properties of mushroom tyrosinase entrapped in alginate, polyacrylamide and gelatin gels. Enzyme Microb Technol 30:613–619. doi:10.1016/S0141-0229(02)00019-4
Nery EW, Kubota LT (2016) Evaluation of enzyme immobilization methods for paper-based devices—a glucose oxidase study. J Pharm Biomed Anal 117:551–559. doi:10.1016/j.jpba.2015.08.041
Parkhill KL, Gulliver JS (1997) Indirect measurement of oxygen solubility. Water Res 31:2564–2572. doi:10.1016/S0043-1354(97)00092-4
PérezLópez B, Merkoçi A (2011) Magnetic nanoparticles modified with carbon nanotubes for electrocatalytic magnetoswitchable biosensing applications. Adv Funct Mater 21:255–260. doi:10.1002/adfm.201001306
Rijiravanich P, Aoki K, Chen J, Surareungchai W, Somasundrum M (2006) Micro-cylinder biosensors for phenol and catechol based on layer-by-layer immobilization of tyrosinase on latex particles: theory and experiment. J Electroanal Chem 589:249–258. doi:10.1016/j.jelechem.2006.02.019
Soozanipour A, Taheri-Kafrani A, Isfahani AL (2015) Covalent attachment of xylanase on functionalized magnetic nanoparticles and determination of its activity and stability. Chem Eng J 270:235–243. doi:10.1016/j.cej.2015.02.032
Stanca SE, Popescu IC (2004) Phenols monitoring and Hill coefficient evaluation using tyrosinase-based amperometric biosensors. Bioelectrochemistry 64:47–52. doi:10.1016/j.bioelechem.2004.02.004
Wang Y, Hasebe Y (2011) Tyrosinase-modified carbon felt-based flow-biosensors: the role of ultra-sonication in shortening the enzyme immobilization time and improving the sensitivity for p-chlorophenol. J Environ Sci 23:1038–1043. doi:10.1016/S1001-0742(10)60511-6
Wu S, Wang H, Tao S, Wang C, Zhang L, Liu Z, Meng C (2011) Magnetic loading of tyrosinase-Fe3O4/mesoporous silica core/shell microspheres for high sensitive electrochemical biosensing. Anal Chim Acta 686:81–86. doi:10.1016/j.aca.2010.11.053
Zhao M, Li H, Liu W, Guo Y, Chu W (2016) Plasma treatment of paper for protein immobilization on paper-based chemiluminescence immunodevice. Biosens Bioelectron 79:581–588. doi:10.1016/j.bios.2015.12.099
Zynek K, Bryjak J, Polakovič M (2010) Effect of separation on thermal stability of tyrosinase from Agaricus bisporus. J Mol Catal B Enzym 66:172–176. doi:10.1016/j.molcatb.2010.05.003
Acknowledgments
The authors would like to thank Dr. M.M. Mojtahedi for FTIR analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
SEM micrographs of fibrous structure of (a) the untreated and (b) enzyme-immobilized cellulosic support. Cross-section micrographs of (c) the untreated and (d) enzyme-immobilized support with a magnification of 500×. For preparation of the enzyme-immobilized support, cellulosic paper was pretreated with HCl (0.5 N) solutions and then sequentially immersed in ethylenediamine, glutaraldehyde solution (2% v/v) and crude enzyme. There was no major change in the structure after the immobilization process, although a little destruction could have occurred because of pretreatment with the HCl. (DOCX 946 kb)
Fig. S2
Reusability of immobilized enzyme at different pHs. Activity of each cycle was determined at ambient temperature using L-tyrosine (1 mM). (XLSX 13 kb)
Fig. S3
Reusability of immobilized enzyme at different temperatures. Activity of each cycle was determined at pH 7 using L-tyrosine (1 mM). (XLSX 13 kb)
Rights and permissions
About this article
Cite this article
Soltani Firooz, N., Panahi, R., Mokhtarani, B. et al. Direct introduction of amine groups into cellulosic paper for covalent immobilization of tyrosinase: support characterization and enzyme properties. Cellulose 24, 1407–1416 (2017). https://doi.org/10.1007/s10570-017-1192-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1192-2