Abstract
Cellulose-Ag@AgCl composites have been directly fabricated by electrospinning of cellulose/LiCl/dimethylacetamide solution with AgNO3 under visible light. AgCl is formed when AgNO3 is added into a cellulose solution, and then the sufficient Cl− interacts with AgCl, which leads to the complete dissolution of AgCl. Meanwhile, AgCl is easily precipitated when the jet encounters water during electrospinning. Finally, cellulose-Ag@AgCl composites are formed because of the visible light irradiation during the whole electrospinning process. X-ray diffraction, scanning electron microscopy, X-ray photoelectron spectroscopy measurements, thermogravimetric analysis and ultra-violet-visible diffuse reflectance spectra are used to characterize the crystal structure, morphology, composition, thermal stability and ability of cellulose-Ag@AgCl composites to absorb visible light, respectively. In addition, the photocatalytic properties of cellulose-Ag@AgCl composites are examined by a model experiment of the degradation of methyl orange under visible light. The photocatalyst still exhibits a good catalytic ability in the process of reuse.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Photocatalysis has recently attracted attention because it is becoming promising in many applications with the utilization of solar energy (Fujishima 1972; Carey et al. 1976; Frank and Bard 1977). Especially for Ag@AgCl, it shows highly efficient photocatalytic activity under visible light because of the strong surface plasmon resonance of Ag nanoparticles (Wang et al. 2008; An et al. 2010; Zhu et al. 2012; Jiang and Zhang 2011; Lou et al. 2011; Cai et al. 2014; An et al. 2011; Zhu et al. 2011; Xu et al. 2010). Ag@AgCl is a very promising photocatalyst, but the efficiency of photocatalytic materials is greatly influenced by their size and morphology (Wang et al. 2008; An et al. 2010; Zhu et al. 2012). Nanoparticles of Ag@AgCl, which can achieve high efficiency during photoreaction, have recently been fabricated (Wang et al. 2008; Zhu et al. 2012; An et al. 2011). However, some new problems appear concerning Ag@AgCl nanoparticles: (1) easy aggregation of particles; (2) difficulty in the recovery process; (3) difficulty in application to continuous flow systems. To solve these problems, some studies adopt a method to immobilize Ag@AgCl onto a support (Ma et al. 2011; Zhou et al. 2016; Lei et al. 2011a, b; Zhu et al. 2013; Zhang et al. 2011; Lei et al. 2011a, b; Shah et al. 2015).
Inorganic materials and polymers are used as the support for Ag@AgCl, which includes Ag@AgCl–graphene oxide (Zhu et al. 2013), Ag@AgCl-cotton (Ma et al. 2011), Ag@AgCl-polyacrylonitrile (Lei et al. 2011a, b) and Ag@AgCl–cellulose acetate (CA) (Zhou et al. 2016). According to the studies on polymer supports, electrospun polymer fibers are a good choice. Its specific properties of a large specific surface area and length to diameter guarantee highly photocatalytic efficiency. Moreover, these supports can be obtained easily by means of electrospinning, which is a facile way to fabricate ultra-fine fibers with sub-microscale diameters from a polymer solution or melts (Subbiah et al. 2005; Teo and Ramakrishna 2006; Burger et al. 2006; Huang et al. 2003).
Cellulosic-based fibers have been used as a support for Ag@AgCl (Ma et al. 2011; Zhou et al. 2016) because the cellulose and cellulosic base show excellent advantages of biodegradability, accessibility, bio-compatibility and high mechanical properties (John and Thomas 2008; Rodríguez et al. 2012). However, there have been no reports about native cellulose nanofibers as the support directly through electrospinning. In addition, the synthetic process of Ag@AgCl-cellulosic-based nanofibers reported in the literature is complicated. The method is that cellulosic-based nanofibers are fabricated first by electrospinning, and then AgCl is deposited on the electrospun fibers through an ion-exchange reaction. Finally, the composite containing Ag@AgCl is obtained under ultraviolet light (UV) or visible light illumination (Zhou et al. 2016).
In the present study, we find a novel and direct way to produce cellulose-Ag@AgCl composites based on electrospinning under ambient conditions. With the addition of AgNO3 in cellulose/DMAc/LiCl solution, AgCl is formed when Ag+ meets with Cl−, and then AgCl dissolves in DMAc/LiCl because of the sufficient Cl−. Furthermore, the composite is obtained directly from electrospinnning because AgCl is precipitated when the jet contacts water in ambient conditions, and Ag@AgCl is formed under visible light illumination. The detailed mechanism of cellulose-Ag@AgCl composite formation will be studied, and the photocatalytic activities of cellulose-Ag@AgCl will be evaluated by degrading MO under visible light irradiation.
Experiments
Materials
Absorbent cotton cellulose was used as a cellulose source (DP ~1400). It was purchased from Xuzhou Lierkang Sanitary Material Co., Xuzhou, China. DMAc (anhydrous, 99.8 %, CP), MO (dye content 96 %) and silver nitrate (AgNO3, 99 %, AP) were obtained from the Guoyao Group. LiCl, AP, 97 %, was purchased from Shanghai Jufeng Chemical Co.
Fabrication of cellulose-Ag@AgCl composites
Preparation of cellulose solution: The cellulose was soaked in DMAc at 160 °C for 30 min, and then the activated cellulose was obtained when DMAc was squeezed at about 180 °C. Cellulose solution with a concentration of 1.5 wt% was prepared by adding 1.5 g activated cellulose into 98.5 g DMAc/LiCl solution (91.5/8.5, weight ratio) and stirred at 100 °C for 3 h until the gel was formed. A transparent cellulose solution was obtained after the above gel solution cooled at room temperature for some days. The solution was prepared on a weight basis.
Preparation of the mixed solution: A different quantity of AgNO3 was added to 9.8 g cellulose solution (1.5 wt%), and then the mixed solution was stirred until the white precipitate was dissolved. The homogeneous solution was kept at room temperature for 24 h before electrospinning. The solution was prepared on a weight basis.
Electrospinning for the preparation of cellulose-Ag@AgCl composites: The mixed solution was electrospun into ultrafine fibers by using an electrospinning equipment (HD-2335, Beijing Ucalery Technology Development Co.). The applied voltage was under 18–20 kV, the feed rate of the solution was controlled at 0.03 ml/min, and the rotating collector wrapped with aluminum foil was placed 15 cm away from the tip of the nozzle to collect fibers. Electrospinning was carried out at room temperature and at 60 % relative humidity. The obtained fibers were soaked in water for an hour and removed from the aluminum foil and then completely dried under vacuum at 60 °C. The whole process was carried out under a visible light.
Solubility of AgNO3 in DMAc and DMAc–LiCl
The determination of solubility of AgNO3 in DMAc and DMAc–LiCl was based on the equilibrium method; 0.5 g AgNO3 was added to a bottle, and then DMAc or DMAc–LiCl was added dropwise until the solid was dissolved completely. This experiment was repeated three times, and the solubility of AgNO3 in DMAc and DMAc–LiCl was determined by the average value.
Evaluation of photocatalytic activities
The photocatalytic experiments were conducted by visible light irradiation. In the study, MO was chosen as a model for degradation. The cellulose-Ag@AgCl photocatalyst (50 mg) was immersed in a 30 ml aqueous solution of MO (10 mg/l) in a 50-ml flask used as the reactor, and the visible light source was a 500-W Xe arc lamp equipped with an ultraviolet cutoff filter (λ > 420 nm). To ensure the accuracy of the experiment, the support of polyethylene terephthalate (PET) was set in the flask for better interaction between the photocatalyst and MO, and the flask was kept in the dark for 30 min to achieve an equilibrium adsorption state before visible-light irradiation. During the photoreaction, the aliquot of the sample was taken out for real-time investigations at certain time intervals. The efficiency of the degradation reaction was monitored by measuring the real time for the UV-Vis absorption of MO at 464 nm where C and C0 refer to the concentrations of MO at real time and initial time (10 mg l−1), respectively.
Characterization
The cellulose-Ag@AgCl composites were examined by X-ray diffraction (XRD), scanning electron microscopy (SEM), X-ray photoelectron spectroscopy measurements (XPS), thermogravimetric analysis (TGA) and ultra-violet-visible (UV-Vis) diffuse reflectance spectra. XRD was used to characterize the composition and crystal structure of the obtained cellulose-Ag@AgCl composites, and their patterns were collected by a D8 ADVANCE (Bruker AXS) with Ni-filtered Cu-Ka radiation (λ = 0.154 nm) from 5° to 80°. The morphology of the electrospun fibers was observed by a field emission scanning electron microscope (S-4800, JEOL, Japan). The measurement of XPS was use to characterize the composition and chemical state of elements of the cellulose-Ag@AgCl composites, and it was conducted on a Kratos spectrometer (AXIS Ultra DLD, Shimadzu/Kratos Analytical, Hadano, Kanagawa, Japan) with monochromatic Al Ka radiation (hν = 1486.69 eV) and with a concentric hemispherical analyzer. TGA was characterized by STA-409PC (NETZSCH Co., Germany) for analysis of the thermal stability of composites and Ag@AgCl content determination. The sample was heated from 25 to 800 °C at a rate of 10 °C/min under nitrogen atmosphere; set C0 (%) as the residual weight of cellulose fibers, C x (%) as the residual weight of composite fibers and C (%) as the Ag@AgCl content in composite fibers (C = (C x −C0)/(1−C0)). UV-Vis diffuse reflectance spectra were obtained with a spectrophotometer (3310, Hitachi, Japan), and they were used to prove the absorption of the cellulose-Ag@AgCl composites in the visible region.
Results and discussion
Preparation and mechanisms of cellulose-Ag@AgCl composites
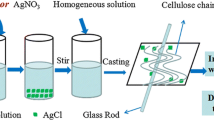
In this article, we have found a novel way to directly fabricate cellulose-Ag@AgCl composites (Scheme l). In the process of fabrication, AgCl is initially formed when AgNO3 is added to cellulose/DMAc/LiCl cellulose. Then, AgCl is dissolved completely under stirring because of the reaction between AgCl and Cl−, but the mixed solution is susceptible to water. According to the literature (Parker et al. 1979), AgCl2 − is easy to transform into AgCl when it contacts water. Cellulose is also sensitive to water because of the hydrogen bonding, and the water is beneficial to the solidification of the jet of the cellulose solution during electrospinning. Therefore, AgCl is precipitated on electrospun cellulose fibers during electrospinning in ambient conditions. Moreover, the process of electrospinning is always accompanied by visible light that partially reduces Ag+ into Ag0. Finally, the cellulose-Ag@AgCl composites are successfully fabricated. Compared with other methods, the unique characteristic of this method is that the cellulose-Ag@AgCl composites are prepared through in situ electrospinning. All of the operations are completed in the process of electrospinning, which immensely simplifies the process of preparing the cellulose-Ag@AgCl composites.
In order to prove the above mechanism, the solubility of AgNO3 in DMAc and DMAc–LiCl and the effects of water on mixed solutions are examined. The solubility results show that 1 g AgNO3 can be dissolved in 1 g DMAc (the summit solubility is not characterized), but the solubility of AgNO3 in DMAc–LiCl exhibits a power-law behavior with the LiCl content (see in Fig. 1). With the enrichment of the concentration of LiCl, the solubility of AgNO3 in DMAc–LiCl increases. The molar ratio of Ag+ and Cl− is 1–2.15 when the solubility of AgNO3 in DMAc–LiCl reaches the summit, and this is similar to the precipitation-solubility equilibrium of AgCl (see Eq. 1, 2). Because the maximum solubility of LiCl in absolute DMAc at 25 °C is 8.46 wt% (Potthast et al. 2002), the quantity of AgNO3 dissolved in DMAc–LiCl is limited. In addition, the solution of AgNO3 dissolved in DMAc–LiCl is sensitive to water, and the white precipitate (AgCl) is precipitated when a little water is added to this solution. These results indicate that the mechanism for the preparation of cellulose-Ag@AgCl composites is reasonable.
Characterization of cellulose-Ag@AgCl composites
The morphology of composites
The morphology of cellulose-Ag@AgCl composites is shown in Fig. 2. As for the electrospun cellulose-Ag@AgCl composites, the electrospun cellulose fibers randomly orient and distribute well. However, the size and dispersion of Ag@AgCl particles are related to the Ag@AgCl content in composites. As shown in Fig. 2a and b, it can be clearly seen that cube-like nanoparticles disperse uniformly on the outside of cellulose fibers, and the diameters of nanoparticles range from 100 to 300 nm when the percentage of Ag@AgCl in composite fibers is at 2 wt%. The composites that contain the Ag@AgCl below 2 wt% have the same results, but the capacity of particles is less. In addition, the Ag@AgCl particles start to agglomerate, and the sizes of particles become larger when the percentage of Ag@AgCl exceeds 2 wt% as shown in Fig. 2c and d. The Ag@AgCl particles with different contents have different sizes and dispersion states, which has an influence on the photocatalytic activity. Therefore, all of the next characterizations are based on the composites containing 2 wt% Ag@AgCl.
As for the cellulose-Ag@AgCl composites, AgCl can grow in the fibers because of the reaction between AgCl2 − and water when the cellulose jets solidify during electrospinning. Moreover, the large quantity of hydroxyl on the surface of cellulose fibers is good for controlling the growth and aggregation of particles when the content of AgCl is not above 2 wt%. The nanoscale and good dispersity of Ag@AgCl particles will improve the photocatalytic activity of the composites.
XRD analysis
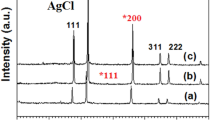
The crystal structure of the obtained cellulose-Ag@AgCl composites is examined by XRD patterns. As shown in Fig. 3, the XRD patterns of composites display distinct diffraction peaks (2θ) at 38.1° (111), 44.5° (200), 64.7° (220), 77.8° (311), 27.8° (111), 32.2° (200), 46.3° (220), 54.7° (311), 57.4° (222), 67.5° (400), 74.5° (331) and 76.6° (420), which correspond to the typical cubic phase of metallic Ag (JCPDS file: 65-2871) and that of AgCl (JCPDS file: 31-1238), respectively. These results indicate that Ag and AgCl exist in the composites, and Ag nanoparticles are successfully produced on the surface of AgCl crystals under visible light illumination during electrospinning. Moreover, the two diffraction peaks in the range from 2θ = 20° to 22° are the typical diffraction peaks of cellulose II, which is consistent with the early work (Kim et al. 2006). The XRD results prove that the samples are composed of Ag, AgCl and cellulose, and the cellulose-Ag@AgCl composites are successfully produced through electrospinning.
XPS Analysis
The chemical state of Ag and Cl elements is investigated by XPS. It shows the XPS peaks of Ag 3d and Cl 2p in Fig. 4. It is clear that the peaks at 367.2 and 373.2 eV are attributed to the Ag 3d5/2 and Ag 3d3/2 binding energies, respectively. Furthermore, the peaks at 368.2 and 374.2 eV appear through deconvoluting the peaks of Ag 3d5/2 and Ag 3d3/2, and this is the peak of metallic silver (Du et al. 2006). The binding energies located at 197.9 and 199.6 eV are fitted with Cl 2p1 and 2p3 binding energies, respectively, indicating the existence of Cl−. These results show that Ag and AgCl exist in the composites, like the XRD analysis results. Therefore, the XPS and XRD analysis indicates that the sample is the cellulose-Ag@AgCl composite.
UV-Vis analysis
As shown in Fig. 5, the UV-Vis diffuse reflectance spectra show the absorption of composites in the visible region. Compared with the sample of cellulose, the cellulose-Ag@AgCl composite has distinct absorption at wavelengths of 400–800 nm. According to the literature (Wang et al. 2008; An et al. 2010), AgCl nanospecies could indicate negligible absorption in the visible region, but Ag nanospecies have strong absorption at 435 nm. However, the band of cellulose-Ag@AgCl composites shows a large red shift compared with Ag, which is due to the recombination of Ag and AgCl. A similar phenomenon has been reported in other literature (Jiang and Zhang 2011; Wang et al. 2008). Consequently, this result reveals the existence of metallic Ag nanospecies in composites, which is in agreement with the results of XRD and XPS. The visible light absorption ability of cellulose-Ag@AgCl composites has a great effect on the photocatalytic activity in the visible region.
Thermal stability
The thermal stability of the cellulose fibers and cellulose-Ag@AgCl composite fibers under nitrogen atmosphere is characterized. From Fig. 6, the TGA curves show that the main weight loss of the cellulose fibers and cellulose-Ag@AgCl composite fibers is in the range of 300–350 °C. Moreover, the thermal degradation temperature of the cellulose-Ag@AgCl composite fibers is lower than that of cellulose fibers, which indicates that the addition of Ag@AgCl nanoparticles slightly reduces the thermal stability of the materials. This is because AgCl has an interaction with the –OH of cellulose, and then the hydrogen bonding of cellulose is partly destroyed. This result is similar to the composites based on electrospun cellulose fibers (Shu and Li 2011; Li et al. 2013). In addition, the Ag@AgCl content in the composite fibers is 46 %.
Photocatalytic Activity
Photocatalytic behaviors of cellulose-Ag@AgCl composites for the degradation of MO are explored under visible light irradiation. As shown in Fig. 7, the composites can completely degrade MO in 180 min. The composites exhibited excellent photocatalytic activity for MO degradation, and it can be easily retrieved without centrifugation and filtration processes. The degradation of MO under visible light is due to Ag@AgCl, which has a visible light absorption ability and produces active species for MO decomposition. The mechanism of the photocatalytic properties of Ag@AgCl has been reported in other literature (Wang et al. 2008; Hu et al. 2014; Zhou et al. 2016). Moreover, the repeated photocatalytic experiments of cellulose-Ag@AgCl are also examined. The results show that the catalytic activity remained 75 % at the third cycle in Fig. 8.
Conclusion
In summary, cellulose-Ag@AgCl composites have been successfully prepared through a one-step method based on electrospinning. This novel method is attributed to DMAc–LiCl being able to dissolve AgCl, and AgCl easily precipitates from the mixed solution with the appearance of water. Experimental results show that the cellulose-Ag@AgCl composite exhibits highly efficient photodegradation of MO and good catalytic ability in repeated use. This work not only provides a novel method for preparing the composite cellulose-Ag@AgCl, but it also can stimulate the development of photocatalysts based on silver halide for the utilization of solar energy.
References
An C, Peng S, Sun Y (2010) Facile synthesis of sunlight-driven AgCl: ag plasmonic nanophotocatalyst. Adv Mater 22(23):2570–2574
An C, Wang R, Wang S et al (2011) Converting AgCl nanocubes to sunlight-driven plasmonic AgCl: Ag nanophotocatalyst with high activity and durability. J Mater Chem 21(31):11532–11536
Burger C, Hsiao BS, Chu B (2006) Nanofibrous materials and their applications. Annu Rev Mater Res 36:333–368
Cai B, Wang J, Gan S et al (2014) A distinctive red Ag/AgCl photocatalyst with efficient photocatalytic oxidative and reductive activities. J Mater Chem A 2(15):5280–5286
Carey JH, Lawrence J, Tosine HM (1976) Photodechlorination of PCB’s in the presence of titanium dioxide in aqueous suspensions. Bull Environ Contam Toxicol 16(6):697–701
Du J, Zhang J, Liu Z et al (2006) Controlled synthesis of Ag/TiO2 core-shell nanowires with smooth and bristled surfaces via a one-step solution route. Langmuir 22(3):1307–1312
Frank SN, Bard AJ (1977) Photoassisted oxidations and photoelectrosynthesis at polycrystalline titanium dioxide electrodes. J Am Chem Soc 99(14):4467–4475
Fujishima A (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238:37–38
Hu P, Hu X, Chen C et al (2014) Biomaterial-assisted synthesis of AgCl@Ag concave cubes with efficient visible-light-driven photocatalytic activity. CrystEngComm 16(4):649–653
Huang ZM, Zhang YZ, Kotaki M et al (2003) A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol 63(15):2223–2253
Jiang J, Zhang L (2011) Rapid microwave-assisted nonaqueous synthesis and growth mechanism of AgCl/Ag, and its daylight-driven plasmonic photocatalysis. Chem A Eur J 17(13):3710–3717
John MJ, Thomas S (2008) Biofibres and biocomposites. Carbohydr Polym 71(3):343–364
Kim CW, Kim DS, Kang SY (2006) Structural studies of electrospun cellulose nanofibers. Polymer 47:5097–5107
Lei J, Wang W, Song M et al (2011a) Ag/AgCl coated polyacrylonitrile nanofiber membranes: synthesis and photocatalytic properties. React Funct Polym 71(11):1071–1076
Lei J, Wang W, Song M et al (2011b) Ag/AgCl coated polyacrylonitrile nanofiber membranes: synthesis and photocatalytic properties. React Funct Polym 71(11):1071–1076
Li C, Shu S, Chen R et al (2013) Functionalization of electrospun nanofibers of natural cotton cellulose by cerium dioxide nanoparticles for ultraviolet protection. J Appl Polym Sci 130(3):1524–1529
Lou Z, Huang B, Wang P et al (2011) The synthesis of the near-spherical AgCl crystal for visible light photocatalytic applications. Dalton Trans 40(16):4104–4110
Ma B, Guo J, Zou L et al (2011) Ag/AgCl@Cotton-fabric: a highly stable and easy-recycling plasmonic photocatalyst under visible light irradiation. Chin J Chem 29(4):857–859
Parker AJ, Clare BW, Smith RP (1979) Solvation of ions. Some applications. IV A novel process for the recovery of pure silver from impure silver chloride. Hydrometallurgy 4(3):233–245
Potthast A, Rosenau T, Buchner R et al (2002) The cellulose solvent system N, N-dimethylacetamide/lithium chloride revisited: the effect of water on physicochemical properties and chemical stability. Cellulose 9(1):41–53
Rodríguez K, Gatenholm P, Renneckar S (2012) Electrospinning cellulosic nanofibers for biomedical applications: structure and in vitro biocompatibility. Cellulose 19(5):1583–1598
Shah M, Sher SA, Kim YH et al (2015) Self-supported Ag/AgCl nanoparticles incorporated polymeric multilayer films for reusable electrophotocatalyst. Mater Expr 5(5):401–409
Shu SX, Li CR (2011) Fabrication and characterization of regenerated cellulose/TiO2 nanocomposite hybrid fibers. Adv Mater Res 418:237–241
Subbiah T, Bhat GS, Tock RW et al (2005) Electrospinning of nanofibers. J Appl Polym Sci 96(2):557–569
Teo WE, Ramakrishna S (2006) A review on electrospinning design and nanofibre assemblies. Nanotechnology 17(14):R89–R106
Wang P, Huang B, Qin X et al (2008) Ag@AgCl: a highly efficient and stable photocatalyst active under visible light. Angew Chem Int Ed 47(41):7931–7933
Xu H, Li H, Xia J et al (2010) One-pot synthesis of visible-light-driven plasmonic photocatalyst Ag/AgCl in ionic liquid. ACS Appl Mater Interfaces 3(1):22–29
Zhang H, Fan X, Quan X et al (2011) Graphene sheets grafted Ag@AgCl hybrid with enhanced plasmonic photocatalytic activity under visible light. Environ Sci Technol 45(13):5731–5736
Zhou Z, Peng X, Zhong L et al (2016) Electrospun cellulose acetate supported Ag@AgCl composites with facet-dependent photocatalytic properties on degradation of organic dyes under visible-light irradiation. Carbohydr Polym 136:322–328
Zhu M, Chen P, Liu M (2011) Sunlight-driven plasmonic photocatalysts based on Ag/AgCl nanostructures synthesized via an oil-in-water medium: enhanced catalytic performance by morphology selection. J Mater Chem 21(41):16413–16419
Zhu M, Chen P, Ma W et al (2012) Template-free synthesis of cube-like Ag/AgCl nanostructures via a direct-precipitation protocol: highly efficient sunlight-driven plasmonic photocatalysts. ACS Appl Mater Interfaces 4(11):6386–6392
Zhu M, Chen P, Liu M (2013) High-performance visible-light-driven plasmonic photocatalysts Ag/AgCl with controlled size and shape using graphene oxide as capping agent and catalyst promoter. Langmuir 29(29):9259–9268
Acknowledgments
The authors are grateful to the Scientific Research Foundation of the Hunan Provincial Education Department, China, for financial support (Grant no. 15K080). We thank the Ningbo Key Laboratory of Polymer Materials, Ningbo Institute of Material Technology and Engineering, Chinese Academy of Sciences, for providing access to instrumentation.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, S., Luo, T., Zhu, J. et al. A facile way to fabricate cellulose-Ag@AgCl composites with photocatalytic properties. Cellulose 23, 3737–3745 (2016). https://doi.org/10.1007/s10570-016-1064-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-016-1064-1