Abstract
This work studies structure and properties of cellulose ultrafiltration membrane created by coupling of TiO2 nanoparticles onto the cellulose matrix. Supported cellulose ultrafiltration membranes were cast out of cellulose-titanium dioxide-ionic liquid solution via phase inversion. The aim was to determine the effect of titanium dioxide concentration on cellulose membrane morphology and performance. 1-ethyl-3-methylimidazolium acetate ([emim][OAc]) was used to obtain cellulose-ionic liquid solutions at a concentration of 9 %. Thin polymeric films of 250 µm thickness were cast onto a non-woven PET support material with an adjustable casting knife. Pure deionized water was employed as a non-solvent agent. The obtained product morphology (cross-section) was examined with field emission scanning electron microscopy. Filtration tests were made to determine pure water flux and molar mass cut-off (MMCO). Filtration tests with humic acid solutions were carried out to provide initial indications of performance in industrial applications. The obtained results showed that addition of titanium dioxide particles in small amounts had a positive impact on virgin cellulose ultrafiltration membranes. Tested samples had good mechanical stability, stable pure water flux and a MMCO typical for commercial ultrafiltration membranes. In addition, all tested samples showed excellent fouling resistance to humic acid solutions. In general, incorporation of titanium dioxide particles into cellulose matrix membrane should be taken into account as a potential way to create ultrafiltration membranes with high operation performance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulose membranes are widely utilized in industry and research, with variety of applications in manufacturing and the pharmaceutical industry. In a view of unique cellulose properties and natural prevalence among vast range of polymer materials this material has attracted the attention of scientists since the very beginning of membrane technology development (Baker 2004; Mulder 2003). This consideration can be attributed to the low material costs, perfect hydrophilicity, non-toxicity and biodegradability of cellulose. However, this polymer is also well-known due to difficulties in dissolution and further processing, because of large amount of intra- and intermolecular hydrogen bonds in cellulose chains. Nowadays almost all existing and industrially utilized traditional ways of cellulose dissolution and shaping can be considered as hazardous or technologically complicated (Hameed et al. 2013). As an example, viscose process, the oldest and the most used one globally (Fink et al. 2014), produces not only cellulose films and fibres, but also an unreasonable amount of gaseous and aqueous by-products.
Thus, growing interest in environmentally friendly processes is encouraging scientists to find alternatives for existing methods of cellulose dissolution, and recent findings in the area of organic solvents have led ionic liquids to be considered as an alternative solvent for cellulosic materials (Kosan et al. 2008; Gericke et al. 2012). Ionic liquids (molten salts consisting of anions and cations) are non-flammable, recyclable, and chemically and thermally stable solvents. It has already been shown that at least some of them yield good results in the cellulose dissolution process (Zhang et al. 2005; Liu et al. 2013). Hence, ionic liquids can be regarded as a valid substitute for volatile organic solvents in cellulose membrane production. Their inherent properties mean that ultrafiltration and nanofiltration membranes prepared out of cellulose and ionic liquids can be used in a wide range of industrial purification applications. Therefore, usage of mentioned combination will certainly improve membrane effectiveness and lead for the new ways of membrane production.
All above mentioned membrane types, such as ultra-, nano- and microfiltration, can be prepared with phase inversion process-immersion precipitation technique. This technique is one of the most studied membrane preparation processes (Baker 2004). Immersion precipitation can be used to prepare membranes out of a wide range of different polymers. Using this approach, these polymers can be transformed into a membrane with desirable properties by appropriate choice of a suitable solvent for the casting solution and non-solvent for the coagulation bath (Mulder 2003; Reuvers et al. 1987). However, final membrane properties (morphology, pure water flux, solute rejection) can be tailored not only with basic casting conditions, but also with big variety of additional ones. So, one more option for control of membrane effectiveness is to modify the membrane properties during the preparation process by altering, for example, the coagulation bath temperature, the concentration of cellulose in the casting solution, or by incorporating metal oxide nanoparticles in the membrane matrix. Recently, the latter one has gained an interest from different scientific groups because of unique properties of metallic oxide particles. One of the most studied for now, titanium dioxide has been the focus of much research, because of its hydrophilicity, photocatalytic effects and anti-bacterial properties (Yang et al. 2006; Sotto et al. 2011). According to already done studies, there are two ways for creation of titanium dioxide/polymer membrane. One approach is to incorporate metal oxide nanoparticles in the casting solution and prepare a blended titanium dioxide-polymer membrane (Zhu et al. 2012). Another possibility for casting of metal oxide treated membranes is to focus on changing the membrane surface through self-assembly of nanoparticles (Li et al. 2009). Although, in this case, natural chemical or physical interaction should exist between titanium and the membrane material. In this study, creation of titanium dioxide-cellulose blended membranes with direct incorporation of nanoparticles into the membrane matrix was chosen as the most appropriate approach.
The objective of the work reported in this paper was to prepare cellulose and cellulose-titanium dioxide mixed matrix membranes via immersion precipitation using a new type of green solvent, 1-ethyl-3-methylimidazolium acetate, to dissolve the cellulose. To the best of our knowledge, this research is a first ever attempt to create and characterize mixed-matrix cellulose-titanium dioxide membrane, based on dissolution of cellulose in ionic liquid. The influence of the nanoparticle filler and solvent type on the morphology of the newly created ultrafiltration membranes was assessed using X-ray diffraction (XRD), atomic force microscopy (AFM) and field emission microscopy (FESEM) techniques. Investigation of cellulose membrane surface chemistry was done with contact angle measurements. In addition, a number of ultrafiltration experiments was carried out to determine the effect of the nanoparticles concentration and solvent type on the filtration performance of the membrane.
Experiments
Materials
Ionic liquid, 1-ethyl-3-methylimidazolium acetate ([emim][OAc]), was obtained from BASF (Basionics™ BC01, CAS: 143314-17-4, assay >98 %) and used as received in preparation of a membrane casting solution. Cellulose (degree of polymerization 780, α-cellulose content >93 %) for the membrane preparation was purchased from Domsjö pulp mill, Sweden. Titanium dioxide nanoparticles with a reported primary particle size of 21 nm (Degussa, Aeroxid TiO2 P25) were used as an inorganic additive in the membrane casting solution. Humic acid (HA) and dextran (with different mean molar masses of 20, 40, 70, 110 and 150 kDa) were supplied by Sigma-Aldrich and Pharmacosmos, respectively. HA (tech. cat.: H1, 675-2) water solutions with a concentration of 200 ppm were used as a model organic foulant during membrane filtration tests. Dextran water solutions of 200 ppm concentration were used to determine the cellulose membrane molar mass cut-off. Deionized water was used for preparation of all membrane samples (as a coagulant) and during membrane storing and testing.
Membrane preparation
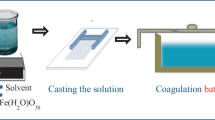
Flat sheet cellulose membranes were prepared with a phase inversion (immersion precipitation) method (Baker 2004; Reuvers et al. 1987). An appropriate amount of cellulose was ground and dissolved in the ionic liquid under vigorous stirring at 90°C for 12 h to produce a 9 % casting solution. It should be noted that insufficient mixing can lead to the formation of undissolved cellulose lumps in the solution (Cao et al. 2009).These unevenly distributed lumps may cause defects in the cellulose film during membrane casting and precipitation, which can be a cause for significant changes in the filtration and retention ability of the membrane samples.
For formation of the cellulose-titanium dioxide blended membranes, a precise amount of TiO2 nanoparticles (0.1, 0.5 or 1 wt%) was dispersed in the ionic liquid and the dispersion was placed in an ultrasound bath for 30 min to prevent possible immediate agglomeration of the metal oxide particles. The dispersion was then added to the hot cellulose-[emim][OAc] solution. The solution with the polymer and the regarded amount of nanoparticles was placed into the hot oil bath for 12 h under vigorous stirring. Finally, light-yellow and transparent cellulose or white cellulose-titanium dioxide solution was obtained. It was left for 2 h without stirring to prevent occurrence of defects in the film by air bubbles in the casting solution. The resultant casting solution was poured onto a non-woven PET support material (Ahlstrom Filtration LLC, grade 3329) and distributed with an adjustable casting knife (BYK Additives & Instruments) via an automatic film applicator (BYK Additives & Instruments) at room temperature. Casting speed was uniform throughout (50 mm/s) and nominal film casting thickness was 250 µm for all cast membranes. The samples created were immediately immersed in the coagulation bath with deionized water at 19°C for 24 h. The membranes obtained were stored in deionized water to prevent the samples from drying and to guarantee complete phase separation (solvent removal).
Characterization of membranes
All prepared flat sheet ultrafiltration cellulose-titanium dioxide membranes were investigated in terms of morphology, pure water flux, MMCO, contact angle and fouling.
Membrane morphology
Field emission scanning electron microscopy (FESEM, Philips XL30 FEG) was used for observation and characterization of the cross-sections of the membranes obtained. Wet membrane samples were cut into small pieces and semi-dried with filter paper. The half-dried pieces were then immediately immersed in liquid nitrogen prior to being cut with razor blades to get clean cross-sections for further analysis. The samples obtained were coated with a thin gold layer before scanning to improve electrical conductivity.
XRD analysis
To determine the crystal phase composition of the TiO2 nanoparticles and to ascertain formation of membranes of mixed-matrix nature, X-ray diffraction (XRD) measurements were carried out with an acceleration voltage of 40 kV and 30 mA emission at room temperature (Bruker D8 Advance).
Contact angle measurements
The membrane surface hydrophilicity was examined with optical tensiometry (Theta model, Attention) using a sessile drop method. All membrane samples were dried prior to the contact angle experiments to obtain a flat and homogeneous surface. A deionized water drop (3 µL) was automatically distributed onto the membrane by the system syringe. The contact angle between the water drop and membrane surface was measured and recorded for further calculations. For each sample, five random locations were utilized to minimize possible measurement error. The contact angle results are an average of the five trials for each membrane.
AFM analysis
The surface roughness of the blended membranes was characterized with AFM (Bruker Multimode 8) using a root mean square (RMS) roughness determination mode. All samples were dried and cleaned with pressurized air before the measurements. An area of 10 × 10 µm was taken as the determination area. All further calculations were carried out by the program software.
Pure water flux measurements
All PWF, MMCO and fouling filtration tests were carried out in an in-house made cross-flow filter equipped with two parallel cells of active membrane surface area of 40 cm2 (Fig. 1). All membrane samples were washed thoroughly with distilled water to remove possible ionic liquid residue. The washed membranes were fixed in the filtration cells and pressurized with water for 30 min under 2.5 bar and 23°C temperature. This step was taken to prevent any possible compaction effect causing errors in future PWF tests (Stade et al. 2013). The operating pressure was then reduced and all further filtration experiments were run at 1 bar and room temperature. Pure water fluxes were calculated according to the following Eq. (1):
where Q is the quantity of collected permeate (kg), A is the active membrane area (m2), and Δt is the sampling time (h).
Molar mass cut-off experiments
MMCO of all the obtained samples (cellulose and cellulose-titanium dioxide membranes) was determined using 200 ppm aqueous solutions of dextrans of different molar mass. Filtration experiments were performed in the same cross-flow set-up as the PWF experiments (Fig. 1). Concentrations of the sample feed and permeates were analyzed with a total organic carbon analyzer (TOC-L, Shimadzu). Retention (R) was calculated according to the Eq. (2):
where cf is the concentration of dextran in the feed solution, and cp is the concentration of dextran in the permeate.
Molar mass with retention higher than 90 % was considered to be the MMCO of the ultrafiltration membranes.
Fouling tests with humic acid solutions
All membranes were tested with humic acid aqueous solutions (200 ppm) to determine their anti-fouling and retention ability for humic acid compounds. Filtrations were carried out at 1 bar operational pressure and at room temperature for 24 h. PWF was measured before and after the fouling experiments to find the normalized flux ratio (NFR) that characterizes the anti-fouling ability of the membrane. NFR was calculated according to the following Eq. (3):
where F2 is the flux after the fouling experiments (kg/m2 h), and F1 is the flux before the fouling experiments (kg/m2 h).
Higher membrane NFR values indicate better antifouling properties.
Concentrations of the HA sample feed and permeates were analyzed with a UV–visible spectroscopy (Jasco V-670 spectrophotometer) to evaluate retention of humic acid from the water solutions.
Results and discussion
Morphological studies
The presence of nanoparticles and their distribution in the cellulose membrane matrix was observed with a FESEM microscope. Cross-section micrographs (Fig. 2) of the prepared samples were taken to characterize possible changes and to study the effect of titanium dioxide on the membrane structure. No information about pore size or pore size distribution could be obtained from the FESEM micrographs, which is typical for cellulose membranes precipitated from solutions of cellulose in ionic liquids (Cao et al. 2010; Li et al. 2011).
The cellulose membrane without additives (Fig. 2a) was seen to have a macrovoid-free and dense-layered structure. This observation can be explained by the mechanism of the cellulose dissolution in ionic liquid and subsequent precipitation process. During preparation of the casting solution, numerous cellulose hydroxyl groups form an association with the organic cation [emim+] and organic anion [OAc−] of the solvent. With such a tight ionic interaction, it will take a longer period of time for the ionic liquid components to overcome the chemical bonding forces and diffuse into the coagulation bath (water) during membrane precipitation. In addition, the high concentration of the casting solution (Cao et al. 2009; Pinkert et al. 2009) results in a slower penetration velocity of the coagulant (water), resulting in prolongation of the precipitation time and formation of a dense-layered membrane structure. Thus, high dope viscosity may also lead to dense membrane formation with suppression of possible macrovoid occurrence (Li et al. 2011). The FESEM micrographs (Fig. 2a–c) can be considered as demonstrating the influence of the ionic liquid chemistry on cellulose matrix during membrane formation and precipitation. The achieved membrane structure had a completely different dense-layered structure than found in commercial cellulose membranes created with utilization of traditional organic solvents.
It can be clearly observed from Fig. 2b–d, that TiO2 nanoparticles are trapped in the cellulose matrix. Also it can be seen that nanoparticles distribution increases with augmentation in TiO2 concentration. However, an agglomeration effect occurs with growth in the particle concentration distribution (from 0 to 1 %). The presence of big nanoparticle agglomerates in the cellulose membrane matrix, Fig. 2c–d, can be explained with the high viscosity of the casting solution, which prevents even distribution of the nanoparticles in the casting solution even with intensive mixing. Although nanoparticles have a natural affinity for aggregates formation in ionic liquid suspensions due to lack of electrostatic repulsion, the viscosity of the casting solution also has a significant effect. Thus, during film casting and subsequent immersion precipitation, titanium dioxide nanoparticle clusters stay trapped in the membrane matrix. The FESEM micrographs in this study indicate that the addition of titanium dioxide nanoparticles does not appear to impact membrane morphology. All created samples can be characterized as macrovoid-free and dense-layered membranes.
XRD analysis
The X-ray diffraction diagrams of the cellulose-titanium dioxide mixed matrix membrane and TiO2 nanoparticles are shown in Fig. 3. The diffraction pattern in the XRD analysis shows the nanoparticle crystal structure type and confirms the presence of TiO2 in the cellulose matrix.
Titanium dioxide particles can exist in two different crystal forms: rutile and anatase. The anatase form has photocatalytic properties and high hydrophilicity, which makes it an appealing additive for membrane manufacturing (Balta et al. 2012). In Fig. 3, the one main peak at 25.3° characteristic for the anatase form of titanium dioxide can clearly be seen, while the other peaks belong to the rutile form (about 20 % of the initial product; more detailed information can be found from the product specification).
The diffraction peaks from the mixed matrix membrane at 12.1° and 21° can be assigned to cellulose II (crystal). Though Domsjö cellulose is known to be cellulose I crystalline type, a finding of earlier studies, its dissolution in ionic liquid and further precipitation initiates crystalline structure transformation (Li et al. 2011; Liu et al. 2013). The diffraction peak for the anatase form of TiO2 remains the same, but with lower intensity, due to the nanoparticles embedded in the membrane matrix. The rutile form is almost undetectable in the analyzed membrane sample because of the small amount of this form in the pure product. Thus, the XRD results can be taken as indicating that a reasonable amount of anatase TiO2 was trapped in the cellulose membrane during the immersion precipitation process, and it can be concluded that a cellulose-TiO2 mixed matrix membrane was successfully obtained.
Contact angle measurements
The contact angle between the prepared membranes and water was measured with a sessile drop method to evaluate the hydrophilicity of the obtained samples. Surface hydrophilicity is a critical membrane property because it can directly affect the flux and fouling resistance of the membrane. Contact angle data for the different membrane samples is summarized in Table 1.
As can be seen from the data, the contact angle of the mixed matrix membranes gradually decreases with increase in TiO2 content in the cellulose membrane matrix, indicating that addition of nanoparticles to the casting solution enhances hydrophilicity. The decreasing trend in contact angle can be explained with the high natural hydrophilicity of titanium dioxide nanoparticles (Huang et al. 2012). In this case, embedded metal oxide particles, trapped too close to membrane surface, may provide more hydrophilic groups, improving membrane hydrophilicity.
However, a noticeable change in hydrophilicity can be detected for samples with a relatively small amount of TiO2 (0.1 and 0.5 %), while 1 % TiO2 mixed-matrix membranes exhibit almost the same value of contact angle as that 0.5 % TiO2 membranes. This effect can be explained with changes in nanoparticles distribution in the membrane matrix. The FESEM micrographs, Fig. 2b, show that the distribution of nanoparticles at small concentration is almost uniform, without any big aggregation effect. However, increasing the amount of nanoparticles in the membrane matrix leads to aggregate formation (Fig. 2c–d). Thus, reduced effective area of newly formed aggregates may cause the observed effect on contact angles (Oh et al. 2009).
AFM analysis
Membrane surface roughness has the same level of importance as hydrophilicity for characterization of membranes. With knowledge of certain sample roughness anti-fouling and filtration abilities for newly developed membranes can be explained (Pinkert et al. 2009). AFM images for the cellulose and cellulose-titanium dioxide membranes are presented in Fig. 4. It can be seen that the cellulose membrane surface is almost homogeneous with typical granular morphology, with the exception of some errors caused by dirt accumulation during sample preparation (Fig. 4a). The surfaces of the mixed-matrix membranes are not smooth, with obvious growth in the RMS parameter, as can be seen from Table 1.
The detected change in the RMS of the surface of the mixed matrix membranes can be explained with the presence of TiO2 nanoparticles in the membrane body. The originally homogeneous and smooth polymer matrix covers the particles and aggregates, which causes roughness growth. With increase in TiO2 concentration, the aggregation effect also increases, which results in the higher RMS values for the 1 % TiO2 mixed matrix membranes (Table 1). These observations are in agreement with similar research on membrane creation with other polymers and solvents (Zhu et al. 2012).
Ultrafiltration experiments
The influence of TiO2 nanoparticles concentration on the mixed matrix membrane PWF and retention of dextran from the model compound water solutions was studied with filtration experiments. As shown in Fig. 5, the cellulose membrane demonstrates relatively low but stable PWF of 21 kg/m2 h at 1 bar operating pressure and room temperature. The somewhat lower permeate flux compared to commercial cellulose UF membranes can be explained with differences in membrane morphology caused by unique interactions between the casting solution and coagulation media (deionized water) during the immersion precipitation process. The high viscosity of the casting solution causes lower velocity of the membrane precipitation with suppression of macrovoid occurrence and formation of a dense-layered structure for all membranes cast. This newly formed structure with minimal roughness of the membrane surface (Table 1) and a dense filtration layer may lead to prolonged sample wettability time and also significantly decreased permeation of water. The high density of the cellulose membrane thus results in relatively low PWF (Yang et al. 2006).
The PWF of the mixed matrix samples analyzed showed a clear trend of growth in PWF with increase in the amount of TiO2 nanoparticles in the membrane body. However, at the highest studied additive concentration (1 % TiO2) PWF decreased almost to the level of the 0.1 % TiO2- cellulose membrane.
From Table 1, it can be seen that the increase in the PWF values of the membranes may be caused by the growth in membrane surface roughness and decrease in contact angle (enhanced sample hydrophilicity). However, the RMS of the 1 % TiO2 membrane is the highest, while the contact angle is the lowest, yet this membrane still exhibited a quite low PWF value. This result can be explained with the combined effect of the dense membrane structure and the aggregation affinity of titanium dioxide nanoparticles. Big aggregate nanoparticle clusters may act as a bonding agent for polymer chains during the immersion precipitation process, resulting in the formation of a packed membrane structure (Arsuaga et al. 2013). The large nanoparticle aggregates observed in the membrane matrix for 1 % concentration of TiO2 nanoparticles in the casting solution may greatly affect the membrane morphology and filtration ability, suppressing all positive effects deriving from the addition of the nanoparticles to the membrane matrix.
Molar mass cut-off curves were made for all membrane samples by measuring solute retention with various dextran compounds of molar masses from 20 to 150 kDa. The results are presented in Fig. 6.
As can be seen from Fig. 6, retention for all samples increases with growth in dextran molar mass. The curves are gradual, without any noticeable peaks, which indicate similar pore size distribution for all membrane samples. The MMCO of the cellulose membrane is around 110 kDa, while that of the 0.1 and 0.5 % TiO2 membranes is around 150 kDa. The cut-off of the 1 % TiO2 membrane was found to be higher than 150 kDa (retention for 150 kDa dextran was about 81 %). It is interesting to note that the retention values of 150 kDa dextran do not exhibit any significant difference, for all membranes, which allows the assumption to be made that addition of TiO2 did not initiate any significant change in pore formation. However, retention results for smaller molar masses were found to be clearly different, with the cellulose membrane showing the best rejection for 20 kDa dextran. The results can be explained as being indicative of the membrane separation being based on the sieve effect for compounds with large molar masses (110 and 150 kDa). Additionally, the purity of the dextran should be taken into account: all dextrans used in the ultrafiltration experiments were technical grade compounds with main molar mass ratio of ≥90 %. Thus, some small weight impurities could slightly change MMCO results for the tested membrane samples.
The fact that all membranes were found to be very dense, based the FESEM images, corresponds to the PWF results but does not correlate with the MMCO results. These conflicting findings may have an obvious explanation, as discussed elsewhere (Li et al. 2011). Namely, all FESEM pictures were taken for dry membrane samples and the UF tests were carried out in a wet state; cellulose is known to change remarkably during the drying process. In the filtration experiments, all samples were treated with water until they attained their swelling equilibrium, thus the membrane sample pores were open, allowing the dextran molecules to penetrate the membrane surface. However, during the drying process, pores collapse and close, which in this case may have led to the inability of FESEM to detect pores.
Humic acid filtration
Humic acid compounds are known to be one of the most abundant contaminants in natural waters. Combination of these substances with other non-humic chemicals (also called natural organic matter or NOM) are considered a major fouling agent for surface water membrane purification processes, especially ultrafiltration (Costa and de Pinho 2005; Gullinkala and Escobar 2010). All the prepared membranes were tested with a 200 ppm water solution of HA in a cross-flow set-up (flow velocity = 1.33 m/s and Re = 2520) for 24 h. PWF was measured before and after the humic acid filtrations and rinsing to calculate the NFR values. All effluents were analyzed with UV–visible spectroscopy to evaluate retention of humic acid from the water solutions. Results are presented in Table 2. It can be seen that the NFR values are 100 % for all tested membranes, both mixed-matrix and pure cellulose. In practice, this practically means that all the prepared membrane samples had very good anti-fouling ability for the humic acid compound.
Such a high NFR can be explained with excellent membrane hydrophilicity. Furthermore, cellulose is known to be a non-charged (or only slightly negatively charged) polymer, which can help prevent molecules of HA accumulating on the membrane surface to form a surface cake. No direct influence of the titanium dioxide nanoparticles could be noted, since all the membranes demonstrated perfect anti-fouling properties.
The retention values for the HA water solutions show the same trend as with the MMCO measurements. HA is a mixture of differently sized molecules, so it is expected that retention values do not reach 100 %.
Conclusions
Cellulose and mixed matrix (cellulose-titanium dioxide) ultrafiltration membranes were successfully prepared via immersion precipitation from cellulose dissolved in an ionic liquid, 1-ethyl-3-methylimidazolium acetate (C8H14N2O2), a new type of green solvent. The effect of the nanoparticle concentration in the casting solution on the membrane morphology and filtration performance was studied. The morphologies and properties of the resulting membranes were investigated with XRD, FESEM and AFM characterization techniques. The study showed that the addition of small concentrations of TiO2 to the membrane casting solution had a negligible effect on the membrane structure but led to an increase in PWF. Larger amounts of nanoparticles caused slight changes in membrane structure (titanium aggregates formation) but no marked change in the filtration properties. All the prepared membranes showed perfect anti-fouling abilities for humic acid water solutions, (NFR = 100 %). Differences in retention between the cellulose membrane and mixed matrix membranes with a small amount of TiO2 (0.1–0.5 % TiO2) were found to be very moderate. The 1 % TiO2 mixed matrix membranes showed the lowest retention results. The results suggest that the addition of larger amounts of nanoparticles to the membrane matrix is not beneficial, because the PWF and model compound retention values for these samples exhibited almost no improvement. It can be concluded that the addition of only small amounts of nanoparticles to the cellulose matrix is sufficient for significant improvement in membrane filtration performance.
References
Arsuaga JM, Sotto A, del Rosario G, Martinez A, Molina S, Teli SB, de Abajo J (2013) Influence of the type, size, and distribution of metal oxide particles on the properties of nanocomposite ultrafiltration membranes. J Membr Sci 428:131–141
Baker RW (2004) Membrane technology and applications. Wiley, Chichester
Balta S, Sotto A, Luis P, Benea L, Van der Bruggen B, Kim J (2012) A new outlook on membrane enhancement with nanoparticles: the alternative of ZnO. J Memb Sci 389:155–161
Cao Y, Wu J, Zhang J, Li H, Zhang Y, He J (2009) Room temperature ionic liquids (RTILs): a new and versatile platform for cellulose processing and derivatization. Chem Eng J 147:13–21
Cao Y, Li H, Zhang Y, Zhang J, He J (2010) Structure and properties of novel regenerated cellulose films prepared from cornhusk cellulose in room temperature ionic liquids. J Appl Polym Sci 116:547–554
Costa AR, de Pinho MN (2005) Effect of membrane pore size and solution chemistry on the ultrafiltration of humic substances solutions. J Membr Sci 255:49–56
Fink HP, Ganster J, Lehmann A (2014) Progress in cellulose shaping: 20 years industrial case studies at Fraunhofer IAP. Cellulose 21:31–51
Gericke M, Fardim P, Heinze T (2012) Ionic liquids—promising but challenging solvents for homogeneous derivatization of cellulose. Molecules 17:7458–7502
Gullinkala T, Escobar I (2010) A green membrane functionalization method to decrease natural organic matter fouling. J Membr Sci 360:155–164
Hameed N, Xiong R, Salim NV, Guo Q (2013) Fabrication and characterization of transparent and biodegradable cellulose/poly(vinyl alcohol) blend films using an ionic liquid. Cellulose 20:2517–2527
Huang J, Zhang K, Wang K, Xie Z, Ladewig B, Wang H (2012) Fabrication of polyethersulfone-mesoporous silica nanocomposite ultrafiltration membranes with antifouling properties. J Membr Sci 423–424:362–370
Kosan B, Michels C, Meister F (2008) Dissolution and forming of cellulose with ionic liquids. Cellulose 15:59–66
Li JH, Xu YY, Zhu LP, Wang JH, Du GH (2009) Fabrication and characterization of a novel TiO2 nanoparticle self-assembly membrane with improved fouling resistance. J Membr Sci 326:659–666
Li XL, Zhu LP, Zhu BK, Xu YY (2011) High-flux and anti-fouling cellulose nanofiltration membranes prepared via phase inversion with ionic liquid as solvent. Sep Purif Technol 83:66–73
Liu X, Pang J, Zhang X, Wu Y, Sun R (2013) Regenerated cellulose film with enhanced tensile strength prepared with ionic liquid 1-ethyl-3-methylimidazolium acetate (EMIMAc). Cellulose 20:1391–1399
Mulder M (2003) Basic principles of membrane technology. Kluwer, Dordrecht
Oh SJ, Kim N, Lee YT (2009) Preparation and characterization of PVDF/TiO2 organic-inorganic composite membranes for fouling resistance improvement. J Membr Sci 345:13–20
Pinkert A, Marsh KN, Pang S, Staiger MP (2009) Ionic liquids and their interaction with cellulose. Chem Rev 109:6712–6728
Reuvers AJ, van den Berg JWA, Smolders CA (1987) Formation of membranes by means of immersion precipitation: part I. A model to describe mass transfer during immersion precipitation. J Membr Sci 34:45–65
Sotto A, Boromand A, Balta S, Kim J, Van der Bruggen B (2011) Doping of polyethersulfone nanofiltration membranes: antifouling effect observed at ultralow concentrations of TiO2 nanoparticles. J Mater Chem 21:10311–10320
Stade S, Kallioinen M, Mikkola A, Tuuva T, Mänttäri M (2013) Reversible and irreversible compaction of ultrafiltration membranes. Sep Purif Technol 118:127–134
Yang Y, Wang P, Zheng Q (2006) Preparation and properties of polysulfone/TiO2 composite ultrafiltration membranes. J Polym Sci 44:879–887
Zhang H, Wu J, Zhang J, He J (2005) 1-Allyl-3-methylimidazolium chloride room temperature ionic liquid: a new and powerful nonderivatizing solvent for cellulose. Macromolecules 38:8272–8277
Zhu T, Lin Y, Luo Y, Hu X, Lin W, Yu P, Huang C (2012) Preparation and characterization of TiO2-regenerated cellulose inorganic-polymer hybrid membranes for dehydration of caprolactam. Carbohydr Polym 87(2012):901–909
Acknowledgments
The authors would like to thank Ahlstrom Filtration LLC (USA) for donation of the membrane support material, Dr. Judy Lee (Melbourne University) for the FESEM micrographs of the prepared membranes, and the Finnish Bioeconomy Cluster (FIBIC) and TEKES—the Finnish Funding Agency for Innovation (FuBio JR2 project) for financial support. Peter G. Jones is thanked for language checking.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nevstrueva, D., Pihlajamäki, A. & Mänttäri, M. Effect of a TiO2 additive on the morphology and permeability of cellulose ultrafiltration membranes prepared via immersion precipitation with ionic liquid as a solvent. Cellulose 22, 3865–3876 (2015). https://doi.org/10.1007/s10570-015-0746-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-015-0746-4