Abstract
Cellulosic material is chemically modified to impart crease-resistant properties to textile products. Due to health considerations formaldehyde-free chemicals are preferred. For this purpose, (3-glycidyloxy)propyltrimethoxysilane (GPTMS) in combination with metal alkoxides aluminium isopropoxide (AIP), titanium tetraisopropoxide (TTP), zircon tetrabutoxide (ZTB) were applied to cotton raw material and cotton fabrics which were pre-treated with butane-1,2,3,4-tetracarboxylic acid (BTCA)/sodium hypophosphite. The as-prepared samples were tested for dry crease recovery angle, tensile strength, tear strength, air permeability, contact angle and whiteness index. The application of GPTMS/AIP resulted in excellent crease resistance values, whereas TTP and ZTB provided a moderate improvement of the wrinkle resistance. The application of the hydrophobic methyltriethoxysilane, octyltriethoxysilane and Dynasylan F8815 (fluoroalkylfunctional water-borne oligosiloxane) caused a significant increase in the contact angle. Fourier-transform-infrared spectroscopy proved the formation of an ester-linkage between BTCA and the cellulose.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Textile materials (fiber, yarn, woven and knitted fabric, nonwoven) are applied in various areas of the textile industry (apparel, household and technical textiles). To fit the requirements of the final customers, textiles products are subjected to a wide range of processes (mechanical, chemical) in an attempt to convey specific properties or functionalities to the textile systems (Wei 2009; Schindler and Hauser 2004; Tao 2001; Shishoo 2005; Chapman 2010). Most of the world’s apparel is made of cotton due to the fact that cellulosic material has good strength, and it is considered to provide comfortable textile fabrics because of the fiber’s excellent moisture absorption and wicking properties (Bashar and Khan 2013).
Cotton fabrics, however, are prone to wrinkling and exhibit poor smooth drying properties after laundering. In an attempt to minimize the tendency to wrinkle cotton fabrics are chemically modified. The most effective and low cost cross-linking agent, dimethyloldihydroxyethyleneurea (DMDHEU), tends to release formaldehyde, a potential human carcinogen substance (Hewson 1994). Consequently, various difunctional chemicals, including epoxides, aldehydes, isocyanates, vinylsulfones, and aziridines, have been investigated as possible alternatives for DMDHEU. They failed to provide the desired performance owing to the higher cost and lower effectiveness (Yamamoto 1982; Frick and Harper 1981). Over the last decades various attempts have been undertaken to find novel non-formaldehyde cross-linking systems for cellulosic material to provide wrinkle-resistant cotton (Bajaj 2002; Harifi and Montazer 2012; Dehabadi et al. 2013).
Thus, polycarboxylic acids (PCA), such as butane-1,2,3,4-tetracarboxylic acid (BTCA) or citric acid, in conjunction with phosphorus-containing catalysts have been thoroughly investigated as a possible formaldehyde-free chemical agents. During the curing process the PCA is converted to a five-membered anhydride which is capable of reacting with the hydroxyl groups of the cellulose thus forming ester linkages which impart crease-resistant properties to the cotton material (Welch 1990, 1992, 1994). A new approach based on an ionic cross-linking mechanism was also developed. This method is effected by subjecting cotton fabric to a carboxymethylation using monochloroacetic acid and sodium hydroxide, then by cationization using 3-chloro-2-hydroxypropyl trimethyl ammonium chloride (Nazari et al. 2012). Cotton fabrics also have been modified by means of the sol–gel process to convey crease-resistant properties (Schramm et al. 2014, 2004; Schramm and Rinderer 2011). The sol–gel process is based on the hydrolysis and condensation of metal or semimetal alkoxides (e.g. Al, Si, Ti, Zr, Hf etc.). These reactions which are conducted at relatively low temperatures (chimie douce) result in the formation of an amorphous three-dimensional metal oxide network (Wright and Sommerdijk 2001; Hench and West 1990; Brinker and Scherer 1990). Textile materials can be impregnated with the hydrolyzed metal alkoxide solution. Subsequently, the samples are annealed at a specified temperature. The sol–gel method has been applied to impart different properties to cellulosic material, such as flame retardancy (Alongi and Malucelli 2013a, b), wettability (Bahners et al. 2008; Mahltig and Böttcher 2003), abrasion resistance (Schramm et al. 2004), photocatalytic activity (Montazer and Hashemikia 2012; Qi et al. 2006), or UV protection (Kan and Au 2014; Pan et al. 2012; Mahltig and Textor 2008). As a result, new or improved surface properties are obtained. The application of a finishing solution consisting of BTCA and hydrolyzed 3-aminopropyltriethoxysilane APTES to a cotton fabric at elevated temperatures resulted in the formation of a crease-resistant agent that is based on a polyimide-bridged polysilsesquioxane (Schramm et al. 2014).
The objective of the present study is the application of the sol–gel technology to convey crease-resistant properties to cotton fabrics. For this purpose the silicon alkoxides (3-glycidyloxy)propyltrimethoxysilane (GPTMS) in conjunction with various metal alkoxides (AIP, TTP, ZTB) were applied to cellulosic material and treated at elevated temperatures.
Experimental
Materials
(3-Glycidyloxy)propyltrimethoxysilane (98 %, GPTMS), methyltriethoxysilane (98 %, MTEOS), octyltriethoxysilane (100 %, OTEOS) were obtained from Wacker Silicone, Burghausen, Germany. Dynasylan F8815 (fluoroalkylfunctional water-borne oligosiloxane) was supplied by Evonik Industries AG, Essen, Germany. Aluminum isopropoxide (98 %, AIP), titanium tetraisopropoxide (98 %, TTP), zircon tetrabutoxide (80 % in 1-butanol, ZTB) were purchased from Sigma-Aldrich, Vienna, Austria. Butane-1,2,3,4-tetracarboxylic acid (>99 %, BTCA) and ethanol >96 %, EtOH) were purchased from Merck GmbH, Germany. Sodium hypophosphite (98 %, SHP) was obtained from VWR, Vienna, Austria. The chemicals were applied without further purification. Deionized water (DI) was used throughout the investigation. Desized, scoured, bleached and mercerized 100 % cotton fabric (weave type: plain, density warp: 52 yarn/cm, weft: 24 yarn/cm, weight: 109 g/m2) was supplied by Getzner Textil GmbH, Bludenz, Austria and was utilized throughout the study.
Preparation of the finishing solutions
BTCA/SHP solution
BTCA (3.00 g, 12.81 mmol) and SHP (1.36 g, 12.81 mmol) were dissolved in 50 mL DI.
Alkoxide solutions
11.27 mL (50 mmol) GPTMS were dissolved in 10 mL EtOH. 2.70 mL HCl (c = 0.5 mol/L) were added. The solution was stirred for 15 h at room temperature (RT). The metal alkoxide solutions were separately prepared: AIP (0.51 g, 2.5 mmol, 5 mL isopropanol, 0.12 mL DI); TTP (0.74 mL, 2.5 mmol, 5 mL isopropanol, 0.18 mL DI); ZTB (0.74 mL, 2.5 mmol, 5 mL 1-butanol, 0.18 mL DI); The solutions were also stirred for 15 h at RT. The solutions of the hydrophobic organotrialkoxysilanes (MTEOS, OTEOS) were prepared as follows: MTEOS (2.04 mL, 10 mmol) or OTEOS (3.18 mL, 10 mmol) were dissolved in 5 mL EtOH and 0.54 mL HCl (c = 0.5 mol/L) were added. The mixture was stirred for 15 h at RT. Since Dynasylan F8815 is an aqueous solution of a hydrophobic agent 5 mL were added. The finishing baths were produced by mixing the corresponding components in a 50 mL volumetric flask which was filled to the mark with EtOH.
Preparation of the cotton fabrics
The modification of the cellulosic specimens is shown in Fig. 1. The BTCA pre-treated cotton fabrics were prepared by impregnating pre-weighed cotton fabrics with the corresponding BTCA/SHP solution applying a two-roll laboratory padder (HVL 500 Mathis AG, Niederhasli, Switzerland; air pressure 1 bar, rotary speed 3 m/min). After condensation (180 °C, 1.5 min) in a lab dryer (LTE, W. Mathis AG, Switzerland) the fabrics were washed and dried at 105 °C for 2 min. Subsequently, the cotton raw material and the BTCA pre-treated cotton samples were impregnated with the alkoxide solutions utilizing the two-roll laboratory padder, dried in air, and cured at 140 °C for 15 min. Then the finished samples were washed and dried at 105 °C for 2 min.
Characterization
The add-on of the fabric weight was calculated as follows: add-on (%) = [(W2 − W1)/W1] × 100; W1 and W2 are the weights of the fabric specimens before and after treatment, respectively. Dry crease recovery angle (DCRA) was measured according to ISO 2312: 10 test specimens were creased and compressed under controlled conditions of time and load. After removal of the creasing load the angle formed between the two limbs was measured. The DCRA values render possible the evaluation of a cross-linking reaction between a crease-resistant finishing agent and the cellulosic material. Tensile strength (TS) was determined with the Material Testing System Z010 (Zwick/Roell, Ulm, Germany) according to DIN EN ISO13934. Tear strength (Elmendorf) was evaluated according to DIN EN ISO 13937 using an Elmendorf tear strength tester (Hans Baer AG, Zurich, Switzerland). The measurement of the air permeability was conducted according to DIN EN ISO 9237 using the air permeability tester Textest FX 3030 LDM, Textest Ldt, Zurich, Switzerland.
Colorimetric data measurements were conducted with the Spectrophotometer CM-3610d from Konica Minolta, Japan. The color data software CM-S100w Spectra Magic NX V1.9 was used for data acquisition. The whiteness index (WI) was calculated according to CIE.
Fourier transform infrared spectroscopy (FT-IR) spectra were recorded with a Bruker Vector 22 spectrometer using a DTGS detector. Since the FT-IR/ATR technique provided poor spectra, the KBr method was applied. Prior to the preparation of the KBr pellets the cotton samples were cut into small pieces and ground with a rotor mill ZM-1 (Retsch, Haan, Germany). The spectra were the result of 200 scans. The spectral resolution was 4 cm−1.
The contact angles (CA) were measured using a home-made CA measuring instrument, which consists of a digital microscope camera (DigiMicro 2.0 Scale; dnt GMBH, Dietzenbach, Germany). The data acquisition was performed by means of the software Microcapture. A water droplet (15 µL) was deposited on the cotton sample with a microliter syringe (Hamilton, Bonaduz, Switzerland), and a precise measurement of the CA was conducted using the software ImageJ (Research Services Branch of the National Institute of Mental Health, Bethesda, Maryland, USA), and the plugin Drop Analysis (Biomedical Imaging Group, Ecole Polytechnique Federale de Lausanne, Switzerland) (Stalder et al. 2006).
Results and discussion
As mentioned above GPTMS is an organotrialkoxysilane that can undergo both the sol–gel polymerization of the alkoxy functionality and the ring opening reaction of the epoxy group, thus producing a hybrid network that comprises organic and inorganic components. To study the impact of finishing solutions that contain GPTMS in combination with metal alkoxides (AIP, TTP or ZTB), pristine and BTCA pre-treated cotton samples were impregnated with various finishing baths, dried and subjected to a curing process at elevated temperatures. The hydrophobic organotrialkoxysilanes F8815, MT and OT were also incorporated into the treatment solution in an attempt to modify the wettability of the cotton samples. BTCA pre-treated cotton specimens were included in the study to evaluate, if the application of GPTMS results in an alteration of the physical properties (add-on, DCRA, TS, tear strength, air permeability, CA and WI).
The cotton samples are denoted as follow: RM: raw material; M: cotton sample that were solely treated with a hydrolyzed metal alkoxide solution; Al, Ti, or Zr is referred to as cotton fabrics being cured with a hydrolyzed AIP, TTP, or ZTB solution; M-F8815, M-MT, M-OT: cotton samples being treated with a mixture of metal alkoxide and a hydrophobic trialkoxysilane; GP: cotton sample finished with a GPTMS solution. GP-F8815, GP-MT, GP-OT, GP-M: cotton fabrics impregnated with mixtures comprising GPTMS and either a hydrophobic organotrialkoxysilane or a metal alkoxide. GP-M-F8815, GP-M-MT, GP-M-OT: cotton samples which were treated with a mixture of GPTMS, metal alkoxide and a hydrophobic organotrialkoxysilane. The BTCA pre-treated cotton samples are indexed with the term B-.
Cotton fabric finished with GPTMS/metal alkoxides
Table 1 presents the add-on and the physical–mechanical findings of the cotton fabrics which were treated with the different finishing solutions containing GPTMS, MT, OT, the metal alkoxides AIP, TTP, ZTB, and mixtures thereof.
Add-on
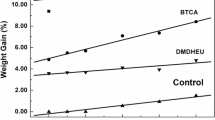
The add-on render possible the determination of the amount of supplied chemicals added to the fabric. The data reveal that the treatment of cotton fabrics with finishing baths comprising only the hydrolyzed metal alkoxide or a combination of the metal alkoxide with a hydrophobic organotrialkoxysilane resulted in add-ons of 1.0–2.1 %. The incorporation of GPTMS resulted in a significant increase of the add-on (10.6–13.2 %). This phenomenon is due to the fact that 50 mmol GPTMS/50 mL were applied to the cotton fabrics, whereas the concentrations of the metal alkoxide were 10 or 4 mmol/50 mmol. Furthermore, the hydrolyzed metal alkoxides possess a lower molecular mass than GPTMS. With respect to the metal alkoxide applied no significant difference of the add-on can be observed.
Dry crease recovery angle
Cellulose-based materials demonstrate the undesirable effect of creasing. If a cellulosic material is wetted the hydrogen bonds linking the glucan chains will be broken, and consequently the polymer chains can be easily moved under stress. In the course of a subsequent drying process the hydrogen bonds will realign in a different arrangement, such leading to the formation of creases which are defined as folds being introduced in a fabric unintentionally. To evaluate the propensity of a cellulosic fabric to crease the crease resistance is measured. The latter is defined as the ability to recover from creasing and is specified in terms of the DCRA (Froix and Nelson 1975; Huang et al. 2007). The values of the DCRA measurements are given in Table 1. Compared to RM no crease resistance effect can be observed, when the cotton fabrics were finished with metal alkoxide solutions indicating that no cross-linking reaction between the cellulosic chains has occurred, whereas the application of a finishing bath containing GPTMS results in an improvement of the anti-wrinkle properties. The combination of GPTMS and a metal alkoxide additionally increases the DCRA values. This phenomenon can be observed only in the case of AIP. The incorporation of either TTP or ZTB considerably decreases the DCRA values.
Tensile strength
The TS describes the response of a textile material when an external force is exerted on the cellulosic specimen until it breaks. This mechanical property is influenced by numerous parameters such as molecular structure, fiber properties as well as the structure of the fabric. Especially, cross-linking is a very contributing factor to tensile properties, since cross-linking prevents the dissipation of the strain energy and thus worse tensile properties are obtained. The findings in Table 1 make evident that almost no effect can be observed when the cotton samples were finished with metal alkoxide solutions. In contrast, the TS values of GPTMS treated samples are reduced by 10 %. The most significant decrease in TS can be observed for GP-Al. All the TS results give evidence that a cross-linking reaction has occurred. In the case of GP-Ti and GP-Zr almost the same level in terms of the TS values are obtained.
Tear strength
While TS of a fabric is useful as a quality characteristic, tear strength, in contrast, is more directly involved in the assessment of serviceability. The reason for this is that in order to break a fabric by a tensile stress, the applied force must be capable of breaking many threads simultaneously, whereas to tear a cloth, the threads are broken singly. The inspection of the tear strength values reveal that a reduction is observed when GPTMS is incorporated into the finishing bath. The application of GPTMS in combination with metal alkoxides results in a significant loss of tear strength. These findings demonstrate that the application of GPTMS/metal alkoxide solutions is much more effective.
Air permeability
A textile fabric is a porous material which consists of interlaced yarns or fibers. Various investigations have shown that the structure as well as the surface of the fabric affects the air permeability which is an important parameter for a number of fabric end uses such as industrial filters, tents and wind-resistant textiles. The data of the air permeability are listed in Table 2 and reflect that the values of the GPTMS/metal alkoxide treated fabrics are significantly reduced indicating that the wind-resistant properties are improved. For those cotton samples which were finished with GP-Al the highest decrease in air permeability can be observed.
Wettability
The values of the CA measurements provide information about the wettability properties of a textile material. Functional hybrid materials exhibit various surface properties such as hydrophilic (contact angle, CA < 90°), hydrophobic (CA ≥ 90°) and super-hydrophobic surface (CA ≥ 150°). However, when a water droplet is deposited on a hydrophobic surface which has a rough or heterogeneous texture the measured CA is increased. This phenomenon is described by the theory of Wenzel or Cassie and Baxter which takes into account the roughness of the surface (Wenzel 1936; Cassie and Baxter 1944; Kwok and Neumann 1999). The CAs measured are shown in Table 2. The findings make evident that the treatment with the hydrolyzed metal alkoxides leads to an enhancement of the hydrophobicity of the cotton textile. The incorporation of a hydrophobic alkoxysilane (F8815, MT, OT) into the finishing solution causes a further increase in the CA values. Also excellent hydrophobic properties are obtained when the cotton samples are treated with a treating bath which contains all three components. As expected, the best CA values can be observed with respect to the fluorinated alkoxysilane F8815. Figure 2 demonstrates the water droplet being deposited GP-Al-MT (2a) on GP-Al-F8815 (2b).
Whiteness index
When cellulosic material is exposed to heat thermal yellowing occurs. This phenomenon is ascribed to degradation processes. Three reactions are supposed to cause this yellowing effect: the hydrolysis of the glycosidic bond, the oxidation of the hydroxyl groups and the dehydration of the cellulose backbone. These undesired modifications can be accelerated by the addition of additives. Yellowing through absorption is caused by the presence of unsaturated conjugated groups in the cellulosic material. The data shown in Table 2 make evident that a significant decrease in WI can be observed when the cotton fabrics were treated with metal alkoxide solutions. An intensive reduction is obtained when TTP is present.
BTCA pre-treated cotton fabric finished with GPTMS/metal alkoxide solutions
The treatment of cellulose-based material with BTCA in conjunction with SHP as catalyst improves wrinkle recovery through an ester-linkage and it results in different mechanical properties of the cotton fabric. However, BTCA is too expensive to be used in commercial scale operations, and therefore its content in the formulation should be reduced. Therefore, BTCA pre-treated cotton samples were finished with various GPTMS/metal alkoxide solutions to study their combined effect on the cotton fabric. In terms of crease resistance the optimum results are attained when the concentration of BTCA is 6 % (w/w) and that of SHP is 3 % (w/w) (Welch 1988, 1990). Thus, in the pre-sent study the portion of BTCA/SHP was reduced by 50 %. First, the cotton specimens were treated with a solution containing 3 % (w/w) BTCA and 1.5 % (w/w) SHP. Then, the modified samples were finished with various GPTMS/metal alkoxide formulations (Fig. 1). The results of the add-on, DCRA, TS and tear strength are shown in Table 3. The findings make evident that the two-step process results in an increase of about 4 % in the add-on values compared to the values shown in Table 1. This observation indicates that the capacity of BTCA pre-treated fabrics to absorb GPTMS/metal alkoxide solution is slightly increased.
In comparison to RM as well as B-RM the treatment of the BTCA-finished cotton fabrics with hydrolyzed metal alkoxide solutions leads to an improvement of the DCRA values. The same phenomenon can be observed when BTCA pre-treated cotton samples were cured with finishing baths containing solely GPTMS. Significantly poorer DCRA values are obtained when the BTCA pre-treated cotton samples were finished with GPTMS/metal alkoxide solutions indicating that the cross-linking reaction between the cellulosic chains is inhibited. The TS properties are strongly diminished when metal alkoxides were incorporated into the treatment bath. The same tendency can be observed with respect to the tear strength values.
The findings in terms of air permeability, CA and WI are presented in Table 4. A comparison of the air permeability measurements make evident that the values of all finished cotton samples are reduced, indicating that the porosity in treated fabrics was reduced. When the BTCA pre-treated samples were treated solely with GPTMS the values of the air permeability are slightly increased compared to the BTCA untreated samples (Table 3). The data of the CA measurements reveal that all cotton samples exhibit excellent wettability properties. In comparison to the values of the BTCA-untreated samples (Table 3) no significant difference can be observed. An inspection of the WI data demonstrates that the finishing of the BTCA pre-treated specimens also results in an increase in the yellowing effect.
FT-IR
Since FT-IR spectroscopy has proven an excellent tool to provide valuable structural information, FT-IR spectra of the treated cotton samples were recorded. Figure 3a–c show the FT-IR spectra of the cotton samples which were treated with an AIP solution (3a), with a GPTMS solution (3b), and a finishing bath containing GPTMS and AIP (3c). The absorption bands which appear in the region of 1400–1200 cm−1 can be assigned to –CH– wagging and deformation modes of the cellulose. It is important to notice that no absorption band can be observed at 1255 cm−1 indicating that no epoxide ring is present (Carboni et al. 2014). The vibration modes in the region of 1200–800 cm−1 can be attributed to the asymmetric stretching vibrations of the C–O–C unit, the in plane ring stretching modes as well as the –C–O– stretching modes of the cellulose. In addition, the vibration mode –Si–O–Si– (1100 cm−1) can be observed in this region. The Al–O bond also absorbs in the same region. Therefore it cannot be distinguished from C–O vibration mode (Bradley et al. 2001). The corresponding FT-IR spectra of the BTCA pre-treated samples are shown in Fig. 3d–f. In all three spectra one additional peak at 1728 cm−1 can be seen, which is ascribed to the ester carbonyl vibration mode deriving from the reaction of the carboxylic group of the BTCA with the hydroxyl groups of the cellulose.
Cellulose consists of long molecular chains of anhydroglucose units, each of which bears one primary and two secondary hydroxyl groups which are capable of forming hydrogen bonds. Therefore, a great deal of studies has been focused on the discussion of hydrogen bonds. OH absorption bands which appear higher than 3400 cm−1 are assigned to intramolecular hydrogen bonds, whereas those at lower wavenumbers than 3400 cm−1 appear due to intermolecular hydrogen bonds (Kondo and Sawatari 1996; Hishikawa et al. 2005, 1999; Kondo 1997). Thus, the shoulder at 3440 cm−1 can be ascribed to the intramolecular hydrogen bonds, whereas the absorption bands at 3334 and 3278 cm−1 confirm the presence of intermolecular hydrogen bonds (Fig. 3).
Effect of the process parameters
All the above-mentioned studies were focused on the investigation of GPTMS as cross-linking agent in combination with various metal alkoxides. Additionally, the influence of hydrophobic agents (F8815, MT and OT) was tested and the impact of the GPTMS/metal alkoxide system has been assessed. The findings clearly make evident that the finishing system GPTMS/AIP is the most effective one under identical operating parameters. Therefore, we investigated the impact of the alteration of various process parameters on the crease-resistant properties of sol–gel modified cotton samples.
Concentration of GPTMS and AIP
The cotton samples were treated with a solution containing 50 mmol/100 mL GPTMS and 2.5 mmol/100 mL AIP. To evaluate the impact of the GPTMS concentration cotton samples were finished with finishing bath consisting of 2.5 mmol AIP and different portions of GPTMS (5, 10, 20, 30, 40 mmol). The samples were cured at 140 °C for 20 min. The results are shown in Fig. 4a. The data verify that an increase in the GPTMS concentration gives rise to a considerable improvement of the DCRA values. No significant change with respect to the WI can be detected. Therefore, it can be concluded that the reduction of the WI does not stem from a degradation reaction of GPTMS during the thermal treatment. As can be seen from Fig. 4b the alteration of the AIP concentration does not show a significant effect upon the DCRA values and the WI values.
Effect of curing time and curing temperature
Figure 5a shows the influence of the curing temperature on the DCRA as well as on the WI. The cotton samples were treated with a 100 mL solution containing 50 mmol GPTMS and 2.5 mmol AIP. Subsequently, the specimens were subjected to a thermal treatment at various temperatures (110–200 °C, 10 °C intervals) for 20 min. The results make evident that an improvement of the DCRA values can be observed as the temperature is increased indicating that the extent of the cross-linking reaction increases at higher curing temperature. In contrast the WI values are decreased when the curing temperature is increased. Figure 5b reflects the alteration of the DCRA and WI values when the curing time is gradually increased. By inspection of the DCRA values it becomes evident that the DCRA values are reaching almost the same level when the curing time is 20 min or higher. The WI drops after the treatment of 10 min and then remains constant. The results reveal that the degradation of the cellulosic material at elevated temperature causes the reduction of the WI values.
Sequential application of GPTMS and AIP
To evaluate the impact of a step-wise application of GPTMS and AIP, cotton samples were first impregnated with the GPTMS solution (50 mmol/100 mL), dried and cured at 140 °C for 20 min. Subsequently, the as-prepared cotton sample was treated with an AIP solution (2.5 mmol/100 mL) and cured under identical conditions. In a second experimental run the sequence of the impregnation steps were reversed.
The data listed in Table 5 reveal that the two-step treatment does not affect the DCRA values. However, the cellulosic specimens do not reach the same DCRA level as the sample which was treated with a finishing bath containing both components (DCRA: 289°, Table 1). The TS is reduced when BTCA pre-treated samples were modified. The same effect can be observed with respect to the values of tear strength and WI.
Cross-linking mechanism
As shown above, the best wrinkle resistance effect can be achieved when both GPTMS and AIP are in the same recipe. The GPTMS monomer contains three hydrolysable methoxy groups and an epoxy-functional organic unit. GPTMS is not miscible with water; however, in the presence of a small amount of acid or base, the methoxy groups start to hydrolyze and are converted to silanol groups which results in the formation of a single-phase solution. The hydrolyzed and partially hydrolyzed GPTMS monomers then undergo condensation reactions to form dimers and larger oligomers which are connected via siloxane bonds. The condensation rate to dimers is low at pH 4, and high in solutions with a pH > 8 (Chu et al. 1997; Gabrielli et al. 2013). It was found that the metal alkoxides are able to catalyze the epoxide ring-opening and the condensation reaction of GPTMS (Jedlinski et al. 1979; Hoebbel et al. 2000; Lee et al. 2003; Williams and Lawton 2005). The hydrolysis of the epoxide ring results in the formation of a diol which can react with organic compounds (Parker and Isaacs 1959). The extents of both reactions are not affected by the metal atom. However, the amount of the metal alkoxide strongly influences the degree of the reactions (Hoebbel et al. 2001). At strong acidic conditions the hydrolysis of the methoxy groups as well as the epoxide ring begins whereas at basic pH values the methoxy hydrolysis is slow and the epoxide ring remains stable (Gabrielli et al. 2013).
From the results shown in Table 1 it is evident that no crease-resistant effect can be observed, when the cotton sample was treated with a finishing bath containing only AIP (DCRA: 195°). An increase in the DCRA value was obtained when GPTMS was applied as cross-linking agent (DCRA: 243°). The combination of both chemicals results in the best crease resistance performance (DCRA: 286°). Therefore, it can be concluded that GPTMS is the effective cross-linking agent. Since the hydrolysis reaction of GPTMS has been conducted under acidic conditions it is reasonable to assume that the cross-linking is accomplished by monomeric or dimeric species of GPTMS. Further, one can consider that the epoxide ring has been opened in both species.
Taking into account all these, it may be hypothesized that the thermal-induced cross-linking reaction is effected either by the monomeric species (Fig. 6a) or by a dimeric species (Fig. 6b).
Conclusions
Cotton material and BTCA pre-treated cotton materials were treated with hydrolyzed GPTMS in conjunction with AIP, TTP and ZTB in an attempt to provide cellulosic specimens with crease-resistant properties. The results of the DCRA measurements revealed that the best values were obtained when GPTMS was applied in combination with AIP. TTP and ZTB were less effective. TS and tear strength are reduced. The thermal treatment brings about a yellowing of the cotton fabric. The incorporation of MTEOS, OTEOS and F8815 leads to an increase of the CA.
The finishing of BTCA pre-treated cotton fabrics with a GPTMS solution or with metal alkoxide solution gives rise to an increase in the DCRA values, whereas the application of a GPTMS/metal alkoxide finishing bath results in a reduction of the anti-wrinkle properties.
The investigation of the process parameters make evident that the curing temperature as well as the curing time slightly increase the DCRA values, whereas the WI is significantly lowered.
The application of DMDHEU-based cross-linking agents provides DCRA values of about 300°, whereas the treatment with BTCA/SHP causes DCRA values of about 290 °C (Welch 1992). When cotton fabrics were finished with BTCA and APTES, DCRA values of 274° could be observed (Schramm et al. 2014). The present study reveals that the finishing of cotton fabrics with GPTMS/AIP solutions yields DCRA values of 289°.
References
Alongi J, Malucelli G (2013a) Heat and moisture transfer in sol–gel treated cotton fabrics. J Therm Anal Calorim 111(1):459–465. doi:10.1007/s10973-012-2462-8
Alongi J, Malucelli G (2013b) Thermal stability, flame retardancy and abrasion resistance of cotton and cotton–linen blends treated by sol–gel silica coatings containing alumina micro- or nano-particles. Polym Degrad Stab 98(8):1428–1438. doi:10.1016/j.polymdegradstab.2013.05.002
Bahners T, Textor T, Opwis K, Schollmeyer E (2008) Recent approaches to highly hydrophobic textile surfaces. J Adhes Sci Technol 22(3–4):285–309
Bajaj P (2002) Finishing of textile materials. J Appl Polym Sci 83(3):631–659. doi:10.1002/app.2262
Bashar MM, Khan M (2013) An overview on surface modification of cotton fiber for apparel use. J Polym Environ 21(1):181–190. doi:10.1007/s10924-012-0476-8
Bradley DC, Mehrotra RC, Rothwell IP, Singh A (2001) Alkoxo and aryloxo derivatives of metals. Academic Press, London
Brinker CJ, Scherer GW (1990) Sol–gel science, the physics and chemistry of sol–gel processing. Academic Press, San Diego
Carboni D, Pinna A, Malfatti L, Innocenzi P (2014) Smart tailoring of the surface chemistry in GPTMS hybrid organic–inorganic films. New J Chem 38(4):1635. doi:10.1039/c3nj01385e
Cassie ABD, Baxter S (1944) Wettability of porous surfaces. Trans Faraday Soc 40:546–551. doi:10.1039/TF9444000546
Chapman RA (2010) Applications of nonwovens in technical textiles, vol 102. Woodhead Publishing Limited. doi:10.1533/9781845699741
Chu L, Daniels MW, Francis LF (1997) Use of (glycidylpropyl)trimethoxysilane as a binder in colloidal silica coatings. Chem Mater 9:2577–2582
Dehabadi VA, Buschmann HJ, Gutmann JS (2013) Durable press finishing of cotton fabrics: an overview. Text Res J 83(18):1974–1995. doi:10.1177/0040517513483857
Frick JG Jr, Harper RJ Jr (1981) Adducts of glyoxal and amides as finishing agent for cotton. Text Res J 51(9):601–606
Froix MF, Nelson R (1975) The interaction of water with cellulose from nuclear magnetic resonance relaxation times. Macromolecules 8(6):726–730. doi:10.1021/ma60048a011
Gabrielli L, Russo L, Poveda A, Jones JR, Nicotra F, Jimenez-Barbero J, Cipolla L (2013) Epoxide opening versus silica condensation during sol–gel hybrid biomaterial synthesis. Chem Eur J 19(24):7856–7864. doi:10.1002/chem.201204326
Harifi T, Montazer M (2012) Past, present and future prospects of cotton cross-linking: new insight into nano particles. Carbohydr Polym 88(4):1125–1140. doi:10.1016/j.carbpol.2012.02.017
Hench LL, West JK (1990) The sol–gel process. Chem Rev 90:33–72
Hewson M (1994) Formaldehyde in textiles. J Soc Dyer Color 110(4):140–142
Hishikawa Y, Togawa E, Kataoka Y, Kondo T (1999) Characterization of amorphous domains in cellulosic materials using a FT-IR deuteration monitoring analysis. Polymer 40(25):7117–7124. doi:10.1016/S0032-3861(99)00120-2
Hishikawa Y, S-i Inoue, Magoshi J, Kondo T (2005) Novel tool for characterization of noncrystalline regions in cellulose: a FT-IR deuteration monitoring and generalized two-dimensional correlation spectroscopy. Biomacromolecules 6(5):2468–2473. doi:10.1021/bm050032k
Hoebbel D, Nacken M, Schmidt H (2000) The effect of nanoscaled metal oxide sols on the structure and properties of glycidoxypropyltrimethoxysilane derived sols and gels. J Sol-Gel Sci Technol 19:305–309
Hoebbel D, Nacken M, Schmidt H (2001) On the influence of metal alkoxides on the epoxide ring-opening and condensation reactions of 3-glycidoxypropyltrimethoxysilane. J Sol-Gel Sci Technol 21:177–187
Huang KS, Hwang MC, Chen JS, Lin SJ, Wang SP (2007) Application of mixed gel solution in the anti-wrinkle finishing of cotton fabrics. J Text Inst 98(2):169–176. doi:10.1533/joti.2005.0300
Jedlinski Z, Dworak A, Bero M (1979) Ring opening in the polymerization of propylene oxide by the initiator systems aluminium isopropoxide or aluminium isopropoxide/zinc chloride (1:1) and the microstructure of the resulting polymers. Die Makromolekulare Chemie 180(4):949–952. doi:10.1002/macp.1979.021800410
Kan CW, Au CH (2014) Effect of biopolishing and UV absorber treatment on the UV protection properties of cotton knitted fabrics. Carbohydr Polym 101:451–456. doi:10.1016/j.carbpol.2013.09.044
Kondo T (1997) The assignment of IR absorption bands due to free hydroxyl groups in cellulose. Cellulose 4(4):281–292. doi:10.1023/A:1018448109214
Kondo T, Sawatari C (1996) A Fourier transform infra-red spectroscopic analysis of the character of hydrogen bonds in amorphous cellulose. Polymer 31(3):293–399
Kwok DY, Neumann AW (1999) Contact angle measurement and contact angle interpretation. Adv Colloid Interface Sci 81(3):167–249. doi:10.1016/s0001-8686(98)00087-6
Lee TH, Kang ES, Bae BS (2003) Catalytic effects of aluminium butoxyethoxide in sol–gel hybrid hard coatings. J Sol-Gel Sci Technol 27:23–29
Mahltig B, Böttcher H (2003) Modified silica sol coatings for water-repellent textiles. J Sol-Gel Sci Technol 27:43–52
Mahltig B, Textor T (2008) Nanosols and textiles. World Scientific Publishing Co. Pte. Ltd., Singapore
Montazer M, Hashemikia S (2012) Textile with immobilised nano titanium dioxide for repeated discoloration of CI Reactive Black 5 under UV-A. Color Technol 128(5):403–409. doi:10.1111/j.1478-4408.2012.00394.x
Nazari A, Montazer M, Moghadam MB (2012) Introducing covalent and ionic cross-linking into cotton through polycarboxylic acids and nano TiO2. J Text Inst 103(9):985–996. doi:10.1080/00405000.2011.646678
Pan C, Shen L, Shang S, Xing Y (2012) Preparation of superhydrophobic and UV blocking cotton fabric via sol–gel method and self-assembly. Appl Surf Sci 259:110–117. doi:10.1016/j.apsusc.2012.07.001
Parker RE, Isaacs NS (1959) Mechanisms of epoxide reactions. Chem Rev 59(4):737–799. doi:10.1021/cr50028a006
Qi KH, Daoud WA, Xin JH, Mak CL, Tang WZ, Cheung WP (2006) Self-cleaning cotton. J Mater Chem 16(47):4567–4574. doi:10.1039/b610861j
Schindler WD, Hauser PJ (2004) Chemical finishing of textiles. Woodhead Publishing Limited, Cambridge
Schramm C, Rinderer B (2011) Dyeing and DP treatment of sol–gel pre-treated cotton fabrics. Fibers Polym 12(2):226–232. doi:10.1007/s12221-011-0226-x
Schramm C, Binder W, Tessadri R (2004) Durable press finishing of cotton fabric with 1,2,3,4-butanetetracarboxylic acid and TEOS/GPTMS. J Sol-Gel Sci Technol 29:155–165
Schramm C, Rinderer B, Tessadri R (2014) Non-formaldehyde, crease resistant agent for cotton fabrics based on an organic–inorganic hybrid material. Carbohydr Polym 105:81–89. doi:10.1016/j.carbpol.2014.01.063
Shishoo R (2005) Textiles in sport. CRC-Press, New York
Stalder AF, Kulik G, Sage D, Barbieri L, Hoffmann P (2006) A snake-based approach to accurate determination of both contact points and contact angles. Colloids Surf A 286(1–3):92–103. doi:10.1016/j.colsurfa.2006.03.008
Tao X (2001) Smart fibres, fabrics and clothing. Woodhead Publishing Limited, Cambridge
Wei Q (2009) Surface modification of textiles. Woodhead Publishing Limited, Cambridge.
Welch CM (1988) Tetracarboxylic acids as formaldehyde-free durable press finishing agents. Part I: catalyst, additive and durability studies. Text Res J 58(8):480–486
Welch CM (1990) Durable press finishing without formaldehyde. Text Chem Color 22(5):13–16
Welch CM (1992) Formaldehyde-free durable press finishing. Rev Prog Color 22:32–41
Welch CM (1994) Formaldehyde-free DP finishing with polycarboxylic acids. Am Dyest Rep 83(9):19–36
Wenzel RN (1936) Resistance of solid surfaces to wetting by water. Ind Eng Chem 28(8):988–994. doi:10.1021/ie50320a024
Williams DBG, Lawton M (2005) Aluminium triflate: a remarkable Lewis acid catalyst for the ring opening of epoxides by alcohols. Org Biomol Chem 3(18):3269–3272. doi:10.1039/B508924G
Wright JD, Sommerdijk NAJM (2001) Sol–gel materials chemistry and applications. Gordon and Breach Science Publisher, The Netherlands
Yamamoto K (1982) Crease-resistant treatment of cotton fabrics with non-formaldehyde crosslinking agents. Text Res J 52(6):357–362
Acknowledgments
The authors would like to thank the Testing Institute of the HTL Dornbirn (Austria) for making available textile-physical devices.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schramm, C., Rinderer, B. Non-formaldehyde, crease-resistant modification of cellulosic material by means of an organotrialkoxysilane and metal alkoxides. Cellulose 22, 2811–2824 (2015). https://doi.org/10.1007/s10570-015-0664-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-015-0664-5