Abstract
A novel willow-based solid acid catalyst was successfully prepared through sulfonation of pencil refill. This solid acid catalyst bearing SO3H and COOH groups shows macroporous structure, which is more suitable for conversion of cellulose into glucose, resulting in 78.0 % conversion and 65.0 % glucose yield at 160 °C for 8 h reaction time. Its activity was comparable to other carbon sulfonated acid catalysts made from pure starch or glucose, respectively, due to its acidic property as well as macroporous structure. Additionally, the willow-derived catalyst could be repeatedly employed for at least three cycles while retaining around 89 % of its original activity, exhibiting excellent operational stability. These results clearly show that use of this willow-derived catalyst is an economic, ecofriendly, and promising approach for production of glucose from cellulose and may open wide applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
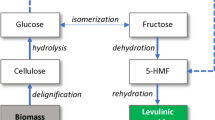
Cellulose is the most wildly distribution and richest biomass in the world (Rinaldi and Schüth 2009a). Cellulose is formed by connection of d-glucose molecules through β-1,4-glycosidic bonds into a long-chain polymer, making conversion difficult (Fan et al. 1987). Therefore, conversion of cellulose has been a key and difficult issue in utilization of this biomass (Chheda et al. 2007). To date, a great deal of scientific effort has been put into hydrolysis of cellulose using homogeneous acids including H2SO4, HCl, and HF (Saeman 1945; Torget et al. 2000) and sub- or supercritical systems (Sasaki et al. 2000; Zhao et al. 2009). However, these approaches suffer from various drawbacks, such as high cost or unavailability for large-scale applications. To solve these problems, different kinds of solid acid catalyst have been developed for conversion of cellulose (Suganuma et al. 2008; Onda et al. 2009, 2008; Pang et al. 2010; Fukuhara et al. 2011; Guo et al. 2012; Li et al. 2012; Zhang et al. 2012; Jiang et al. 2012; Vyver et al. 2010; Lai et al. 2011), showing certain activity and different selectivities for glucose (Table 1). Among these, production and application of carbon-based SO3H material have attracted much attention due to its high density of sulfonic acid groups (SO3H), stability, and reusability. Hara’s group has worked on synthesis of different amorphous carbon materials bearing SO3H, recently reporting hydrolysis of cellulose catalyzed by amorphous-carbon-based SO3H with 35 % yield of glucose (Nakajima and Hara 2012). Onda reported sulfonated active-carbon catalysts for hydrolysis of cellulose into glucose with 40.5 % glucose yield and selectivity above 90 % when applied at 423 K for 24 h (Onda et al. 2008). Zhang’s group employed sulfonated carbon with mesoporous structure for hydrolysis of cellulose with 75 % yield of glucose, being the highest recorded yield for a solid acid catalyst (Pang et al. 2010). Other carbon-derived solid acid catalysts have been prepared from various types of pure carbohydrates, including d-glucose, sucrose, cellulose, and starch (Suganuma et al. 2008; Fukuhara et al. 2011; Guo et al. 2012; Zhang et al. 2012; Jiang et al. 2012) by sulfonation. Polymers can also be used as starting materials (Li et al. 2012). However, sulfonation of incompletely carbonized resin, amorphous glassy carbon, activated carbon, or natural graphite could not result in the same high performance in esterification, hydration, or hydrolysis (Okamura et al. 2006). According to the reports by Suganuma et al. (2008) and Wu et al. (2010), sulfonated carbon materials exhibiting good cellulose hydrolysis efficiency will possess not only SO3H groups, but also OH groups as well. This could help to solve the mass transfer resistance between solid acids and insoluble cellulose in water. Therefore, it is highly desirable to prepare novel solid acid catalysts from new materials and to investigate their catalytic performance in degradation of lignocellulosic materials to produce various biomass products.
Appropriate natural biomass would be an attractive starting material for a novel catalyst, being much better than polysaccharides (Huber et al. 2006; Rinaldi and Schüth 2009b). In addition, carbon-based catalysts prepared from wood powder or bamboo were reported to be much less stable than those obtained from pure carbohydrates (Lou et al. 2008; Zong et al. 2007; Hara 2010). Such lower efficiency might be related to the effect of the different preparation conditions on the catalytic and textural properties. More recently, Zong’s group used waste biomass bagasse as feedstock to prepare sulfonated carbon materials, which were shown to be highly active for production of biodiesel (Lou et al. 2012). In addition, they described the relationships between the synthesis conditions and the characteristic properties of the resulting catalysts in detail. Zhang et al. prepared ionic liquid-functionalized biochar sulfonic acids using bamboo as a starting material, claiming biomimetic catalytic activity for hydrolysis of cellulose and bamboo under microwave irradiation (Miller 1959).
Based on the above-cited references, we prepared a novel carbon-based solid acid catalyst from twig of willow, containing amounts of SO3H, COOH, and OH functional groups. This carbon catalyst was active in hydrolysis of cellulose with 78.0 % conversion and 65.0 % yield of glucose at 160 °C for 8 h reaction time. This high efficiency results from the acidic property and macroporous textile structure of the carbon catalyst. This is the first report of use of sulfonated carbon material from nature with macroporous structure with potential for conversion of cellulose into glucose.
Experiments
Microcrystalline cellulose was obtained from J&K Chemical Ltd. (Beijing, China). X-ray diffraction analysis was carried out on a Rigaku (Japan) Dmax 2000 X-ray diffractometer using Cu Kα radiation (λ = 0.154178 nm) to analyze the structure of cellulose; the degree of cellulose crystallinity was about 60 %. The degree of polymerization was 300 as measured by viscosity. All other reagents were of analytical-reagent (AR) grade and used without further purification. 3,5-Dinitrosalicylic acid (DNS) reagent was prepared according to Miller (1959).
Twigs of willow were selected from the willow tree in our university, milled into powder, and oven-dried at 378 K for 24 h before carbonization.
Preparation of catalyst
Willow-derived catalysts were prepared according to modified published methods (Lou et al. 2012). Then, this black solid (7 g) was boiled in 150 cm3 fuming sulfuric acid (15 wt% SO3) at 353 K under N2. After heating for 10 h and then cooling to room temperature, the suspension was filtered. The black precipitate was washed repeatedly with hot distilled water (>353 K, 1,000 cm3) until impurities such as sulfate ions were no longer detected in the wash water. As only a small amount of the excess fuming sulfuric acid is consumed in the reaction, the sulfuric acid recovered after filtration of the powder can be reused for repeated sulfonation of the carbon material. The densities of the functional groups in the carbon material are mainly dominated by the degree of sulfonation.
Physical measurements
Structural information for the prepared carbon material was obtained by scanning electron microscopy (SEM, S-570) and Fourier-transform infrared spectroscopy (FTIR, Nicolet Magna 6,700 IR spectrometer). The SO3H content was determined by acid–base titration. XPS spectra of willow-derived samples were obtained using an AXISHS (Shimadzu/Kratos) photoelectron spectrometer with nonmonochromated Mg Ka radiation (1,253.6 eV) from a source operated at 15 KV and 10 mA.
Catalytic procedure
For hydrolysis of cellulose, a mixture of cellulose (50 mg) and catalyst (50 mg) in distilled water (5 mL) was heated at 160 °C in a Teflon-lined steel autoclave under air for 8 h with agitation (300 rpm). The reaction mixture was centrifuged to separate unreacted cellulose and catalyst. The clear solution was used for analysis. The conversion of cellulose was calculated according to Tian et al. (2010). The concentration of glucose (g/mL) was measured in the aqueous phase by high-performance liquid chromatography (HPLC) conducted on a system equipped with a refractive index detector (Shimadzu LC-10A, HPX-87H column).
Total reducing sugars (TRS) analysis (Tian et al. 2010)
A mixture containing 2 mL of DNS regent and 1 mL of reaction sample was heated for 2 min in a boiling water bath, then cooled to room temperature by flowing water, and mixed with deionized water to 25 mL. The color intensity of the mixture was measured using a UV757CRT model spectrophotometer at 540 nm. The concentration of total reducing sugars was calculated based on a standard curve obtained with glucose.
Results and discussion
Characterization of the catalysts
The FTIR spectra of the original carbon (a) and the prepared carbon material (b) are shown in Fig. S1. The vibration bands at 1,032 and 1,167 cm−1 are attributed to stretching vibration of SO −3 and O=S=O, respectively, indicating that the resulting material possesses SO3H groups. The absorption bands at 1,695 cm−1 correspond to COO− stretching vibrations (Suganuma et al. 2008). The peak at around 3,420 cm−1 is typically assigned to stretching modes of oxygen-containing OH (Ishimaru et al. 2007). Therefore, the FTIR spectrum clearly demonstrates that sulfonation of natural carbon introduces SO3H, OH, and COOH groups into the willow-derived catalyst.
A SEM image of the carbon material obtained by sulfonation at 180 °C for 10 h is shown in Fig. 1. It can be seen that the obtained carbon material mainly consisted of uniform macropores with diameter of 10–80 µm. In addition, this sulfonated carbon material exhibits cylindrical channels, being suitable for hydrolysis of cellulose. The EDAX measurement results demonstrated that the as-prepared material contained S, C, and O. The sulfur content in the material was about 1.18 mmol/g. The total SO3H+ and COOH content was determined by titration to be 3.08 mmol/g.
The XPS spectrum of the samples exhibited a single S 2p peak at 168 eV (Fig. 2a), which was attributed to the sulfur species in the SO3H group (Okamura et al. 2006). Fitting of the O 1s peak for the carbon-SO3H catalyst (Fig. 2b) revealed a peak for C=O-type oxygen (C=O and COOR; 530.9–531.9 eV) and another for C–O-type oxygen (C–OH, C–O–C, and CO–O–R; 532.6–533.9 eV) (Darmstadt et al. 2000, 2002). A peak for polyaromatic carbons (C–C, 285.7 eV) is shown in Fig. 2c (Proctor and Sherwood 1982; Xie and Sherwood 1990). The atomic C/S ratio was 25:1.
Catalyst activity
The activity of the carbon-based solid acid catalysts was studied through cellulose hydrolysis (Table 1), with the main product being glucose. It can be seen that the conversion of cellulose depends on the acidic property of the catalysts (Table 1). It can be seen that the carbon-SO3H catalysts with different acidic capacity resulted in different catalytic efficiency. The higher the acidic strength, the higher the conversion and glucose yield obtained. The highest yields of TRS and glucose reached 71.8 and 65.0 %, respectively, under 160 °C for 8 h. These results suggest that the carbon-SO3H material provided acidic sites for dehydration of cellulose to some water-soluble carbohydrates then to glucose.
The reaction time and temperature are key parameters determining the products of cellulose hydrolysis (Fig. 3). It can be seen that, at experimental temperatures of 150, 160, and 170 °C, increasing the reaction time could increase the conversion of cellulose. The reaction time and temperature had different influences on the yields of TRS and glucose. The time for the maximum yield of TRS and glucose depended on the reaction temperature. At the lower temperature of 150 °C, enhancement of the reaction time could increase the TRS yield, which reached 34.54 % at 150 °C for 12 h; the yield of glucose reached a maximum of 15.2 % within 10 h. On increasing the temperature to 160 °C, the yields of TRS and glucose reached maximum values of 71.8 and 65 %, respectively. Enhancement of the reaction time resulted in a decrease of the yield of TRS and glucose, showing the occurrence of by-production. On further increase of the temperature to 170 °C, the yields of both TRS and glucose did not increase significantly. This experiment proves that shorter reaction time can promote the efficiency of hydrolysis reactions.
Figure 4 displays the effect of catalyst dosage on the TRS or glucose yield from hydrolysis of cellulose. In a little amount of catalyst about 20 mg, at reaction temperature of 160 °C, conversion of 13.8 %, TRS yield of 11.2 %, and glucose yield of 4.2 % were obtained for 8 h reaction time, which can probably be attributed to cellulose hydrolysis catalyzed by H+ resulting from dissociation of water added to the mixture. Addition of the carbon-SO3H catalyst could improve the efficiency of hydrolysis. It could be seen that, on addition of 50 mg of carbon catalyst, the conversion of cellulose and the TRS yield were remarkably increased to 58.1 and 51.2 %, respectively, for the same reaction conditions. Further increases of the catalyst content resulted in a decrease in the selectivity for glucose, but an increase in the conversion of cellulose. This is probably due to accelerated degradation of reducing sugars caused by excess active sites in the system.
Water is a necessary reactant in hydrolysis of cellulose, but excess amounts of water will affect the dissolution and hydrolysis of cellulose. It can be seen from Fig. 5 that the TRS conversion and glucose yield increased with increasing amount of water from 3 to 5 mL, reaching a maximum (78.0 % conversion, 71.8 % yield of TRS , and 65.0 % yield of glucose, respectively) at 5 mL of water. Further addition of water into the reaction system caused the efficiency of hydrolysis to decrease sharply, which might be attributed to decrease of acidic sites.
Recycling of the carbon catalyst was tried for three times to study its activity and stability (Fig. 6) at 160 °C for 8 h. After the first reaction run, we separated the unreacted cellulose and catalyst by high-speed centrifugation and then dried the solid mixture. After drying, we refilled a certain amount of fresh cellulose into the sample for the next reaction to maintain the amount of cellulose as 50 mg. The results showed that the catalyst was still active in each recycle run, although the conversion, and TRS and glucose yields gradually decreased to 68.9, 64.6, and 50.1 %, respectively. The total leaching amount of sulfur was 2.06 %, showing the stability of the SO3H groups on the catalyst. The decrease in the efficiency of this carbon-derived acid catalyst with the number of recycles is probably the result of catalyst mass loss in the centrifugation and washing step.
Conclusions
Macroporous carbon material bearing SO3H, COOH, and OH groups was prepared using natural willow biomass as feedstock. This carbon-derived material displayed very high catalytic efficiency in hydrolysis of cellulose to glucose due to its acidic property and macroporous structure. The maximum conversion (78.0 %) and yield of glucose (65.0 %) were obtained by reaction for 8 h at 160 °C in distilled water solution. After three uses, the catalyst retained high activity. This carbon material is a kind of efficient and environmentally friendly catalyst.
References
Chheda JN, Huber GW, Dumesic JA (2007) Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals. Angew Chem Int Ed 46:7164–7183
Darmstadt H, Pantea D, Summchen L, Roland U, Kaliaguine S, Roy C (2000) Surface and bulk chemistry of charcoal obtained by vacuum pyrolysis of bark: influence of feedstock moisture content. J Anal Appl Pyrolysis 53:1–17
Darmstadt H, Roy C, Kaliaguine S, Choi S, Ryoo R (2002) Surface chemistry of ordered mesoporous carbons. Carbon 40:2673–2683
Fan LT, Gharpuray MM, Lee YH (1987) Cellulose hydrolysis biotechnology monographs, vol 3. Springer, Berlin, pp 57–68
Fukuhara K, Nakajima K, Kitano MI, Kato H, Hayashi S, Hara M (2011) Structure and catalysis of cellulose-derived amorphous carbon bearing SO3H groups. ChemSusChem 4:778–784
Guo HX, Qi XH, Li LY, Richard L, Smith J (2012) Hydrolysis of cellulose over functionalized glucose-derived carbon catalyst in ionic liquid. Bioresour Technol 116:355–359
Hara M (2010) Biomass conversion by a solid acid catalyst. Energy Environ Sci 3:601–607
Huber GW, Iborra S, Corma A (2006) Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering. Chem Rev 106:4044–4098
Ishimaru K, Hata T, Bronsveld P, Meier D, Imamura Y (2007) Spectroscopic analysis of carbonization behavior of wood, cellulose and lignin. J Mater Sci 42:122–129
Jiang YJ, Li XT, Wang XC, Meng LQ, Wang HS, Peng GM, Wang XY, Mu XD (2012) Effective saccharification of lignocellulosic biomass over hydrolysis residue derived solid acid under microwave irradiation. Green Chem 14:2162–2167
Lai DM, Deng L, Li J, Liao B, Guo QX, Fu Y (2011) Hydrolysis of cellulose into glucose by magnetic solid acid. ChemSusChem 4:55–58
Li XT, Jiang YJ, Shuai L, Wang LL, Meng LQ, Mu XD (2012) Sulfonated copolymers with SO3H and COOH groups for the hydrolysis of polysaccharides. J Mater Chem 22:1283–1289
Lou WY, Zong MH, Duan ZQ (2008) Efficient production of biodiesel from high free fatty acid-containing waste oils using various carbohydrate-derived solid acid catalysts. Bioresour Technol 99:8752–8758
Lou WY, Guo Q, Chen WJ, Zong MH, Wu H, Smith TJ (2012) A highly active bagasse-derived solid acid catalyst with properties suitable for production of biodiesel. ChemSusChem 5:1533–1541
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Nakajima K, Hara M (2012) Amorphous carbon with SO3H groups as a solid Brønsted acid catalyst. ACS Catal 2:1296–1304
Okamura M, Takagaki A, Toda M, Kondo JN, Domen K, Tatsumi T, Hara M, Hayashi S (2006) Acid-catalyzed reactions on flexible polycyclic aromatic carbon in amorphous carbon. Chem Mater 18:3039–3045
Onda A, Ochi T, Yanagisawa K (2008) Selective hydrolysis of cellulose into glucose over solid acid catalysts. Green Chem 10:1033–1037
Onda A, Ochi T, Yanagisawa K (2009) Hydrolysis of cellulose selectively into glucose over sulfonated activated-carbon catalyst under hydrothermal conditions. Top Catal 52:801–807
Pang JF, Wang AQ, Zheng MY, Zhang T (2010) Hydrolysis of cellulose into glucose over carbons sulfonated at elevated temperatures. Chem Commun 46:6935–6937
Proctor A, Sherwood PMA (1982) X-ray photoelectron spectroscopic studies of carbon fibre surfaces. III—Industrially treated fibres and the effect of heat and exposure to oxygen. Surf Interface Anal 4:212
Rinaldi R, Schüth F (2009a) Acid hydrolysis of cellulose as the entry point into biorefinery schemes. ChemSusChem 2:1096–1107
Rinaldi R, Schüth F (2009b) Design of solid catalysts for the conversion of biomass. Energy Environ Sci 2:610–626
Saeman JF (1945) Kinetics of wood saccharification-hydrolysis of cellulose and decomposition of sugars in dilute acid at high temperature. Ind Eng Chem 37:43–52
Sasaki M, Zhen F, Fukushima Y, Adschiri T, Arai K (2000) Dissolution and hydrolysis of cellulose in subcritical and supercritical water. Ind Eng Chem Res 39:2883–2890
Suganuma S, Nakajima K, Kitano M, Yamaguchi D, Kato H, Hayashi S, Hara M (2008) Hydrolysis of cellulose by amorphous carbon bearing SO3H, COOH, and OH groups. J Am Chem Soc 130:12787–12793
Tian J, Wang JH, Zhao S, Jiang CY, Zhang X, Wang XH (2010) Hydrolysis of cellulose by the heteropoly acid H3PW12O40. Cellulose 17:587–594
Torget RW, Kim JS, Lee YY (2000) Fundamental aspects of dilute acid hydrolysis/fractionation kinetics of hardwood carbohydrates, 1. Cellulose hydrolysis. Ind Eng Chem Res 39:2817–2825
Vyver SV, Peng L, Geboers J, Schepers H, Clippel F, Gommes CJ, Goderis B, Jacobs PA, Sels BF (2010) Sulfonated silica/carbon nanocomposites as novel catalysts for hydrolysis of cellulose to glucose. Green Chem 12:1560–1563
Wu YY, Fu ZH, Yin DL, Xu Q, Liu FL, Lu CL, Mao LQ (2010) Microwave-assisted hydrolysis of crystalline cellulose catalyzed by biomass char sulfonic acids. Green Chem 12:696–700
Xie YM, Sherwood PMA (1990) X-ray photoelectron-spectroscopic studies of carbon fiber surfaces. 11. Differences in the surface chemistry and bulk structure of different carbon fibers based on poly(acrylonitrile) and pitch and comparison with various graphite samples. Chem Mater 2:293
Zhang C, Fu ZH, Liu YC, Dai BH, Zou YH, Gong XL, Wang YL, Deng XL, Wu HT, Xu Q, Steven KR, Yin D (2012) Ionic liquid-functionalized biochar sulfonic acid as a biomimetic catalyst for hydrolysis of cellulose and bamboo under microwave irradiation. Green Chem 14:1928–1934
Zhao Y, Lu WJ, Wang HT (2009) Supercritical hydrolysis of cellulose for oligosaccharide production in combined technology. Chem Eng J 150:411–417
Zong MH, Duan ZQ, Lou WY, Smith TJ, Wu H (2007) Preparation of a sugar catalyst and its use for highly efficient production of biodiesel. Green Chem 9:434–437
Acknowledgments
This work was supported by the National Natural Science Foundation of China (51078066, 51102042), the Fundamental Research Funds for the Central Universities (10JCXK011), and the major projects of Jilin Provincial Science and Technology Department (201105001, 20140204085GX).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sun, Z., Tao, M., Zhao, Q. et al. A highly active willow-derived sulfonated carbon material with macroporous structure for production of glucose. Cellulose 22, 675–682 (2015). https://doi.org/10.1007/s10570-014-0540-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-014-0540-8