Abstract
Porous celluloses (PCs) were successfully prepared by a simple process of freezing the cellulose solution in ionic liquid, followed by solvent exchange and drying at normal temperature instead of the supercritical drying. PCs were composed of cellulose sheets of low crystallinity, as evidenced by SEM, XRD, TGA and FTIR, oriented into unidirectional structures when the cellulose concentration was low (1 %). When the cellulose concentration was high (4 %) the structure was twisted and randomly oriented. PCs had low apparent densities of 44–88 mg/cm3 and oil adsorption capacities ranging from 9.70 to 22.40 g/g (oil/PC) due to the ultralight porous structures. Oxidation of sodium periodate introduced dialdehyde groups into the porous structure. After the same reaction time, the aldehyde content of PC was much higher than the untreated cellulose counterpart. The resultant dialdehyde modified-PC had better urea adsorption than modified-viscose fiber. The high reactivity of PCs was related to the low crystallinity and porous structure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulose, a linear β-1,4-glycosidically linked polyglucan and the most abundant natural polymer, has many attractive properties such as low cost, renewability, biodegradability, non-toxicity, and biocompatibility (Mahmoudian et al. 2012). However, cellulose is insoluble in common solvents due to its hydrogen-bonded supramolecular structure and high crystallinity (Zhao et al. 2009), which hinder its industrial application (Vitz et al. 2009).
Many solvent systems have been developed for preparing cellulose materials and derivatives, including N,N-dimethylacetamide (DMAc)/lithium chloride (LiCl), dimethyl sulfoxide (DMSO)/paraformaldehyde, N-methyl-morpholine-N-oxide (NMMO)/H2O, and NaOH/urea. However, the practical utilization of these solvent systems is very restrictive due to limited dissolving capability, toxicity, high cost, solvent recovery, uncontrollable side reactions, and instability during processing or modification of cellulose (Cao et al. 2009). In 2002, it was found that 1-butyl-3-methylimidazolium chloride ([BMIM]Cl), an ionic liquid (IL), could dissolve up to 10 % cellulose at around 100 °C (Swatloski et al. 2002). Imidazolium-based ILs are non-volatile, non-flammable, chemically and thermally stable, easy to recycle, and are gradually accepted as cellulose solvents (Zhang et al. 2014). Currently, ILs are widely used for dissolution, chemical modification, and processing. In a sugarcane bagasse system, 1-allyl-3-methylimidazolium-chloride ([AMIM]Cl) was used to prepare cellulose acetate butyrate and cellulose acetate propionate (Huang et al. 2011), while [BMIM]Cl was reacted with maleic anhydride without the use of a catalyst (Chen et al. 2013). [BMIM]Cl was initially used at 130 °C for 6 h to dissolve ball-milled bamboo meal for the production of bamboo derivatives by subsequent reaction with lauroyl chloride (Wen et al. 2011). Regenerated cellulose/montmorillonite nanocomposite films were successfully prepared in [BMIM]Cl using a solution casting method (Mahmoudian et al. 2012). Lignocellulosic polymers were dissolved in [BMIM]Cl and coagulated from solution by adding aqueous ethanol. The thus obtained gel was further washed with ethanol and liquid carbon dioxide and then dried by releasing carbon dioxide from the porous structure at a supercritical temperature to obtain an aerogel (Aaltonen and Jauhiainen 2009). Ultralight and porous cellulose material was prepared by cellulose dissolution in 1-ethyl-3-methylimidazolium acetate ([EMIM]Ac) and [BMIM]Cl, followed by regeneration and drying under supercritical CO2 conditions (Sescousse et al. 2011).
In this work, porous cellulose (PC) materials were fabricated from hot cellulose solution in [BMIM]Cl using a simple cooling, solvent exchange ([BMIM]Cl-water) and drying (CSD) method rather than supercritical drying. Presumably, the porous structure and low crystallinity of PC would make chemical modification of PC simple and effective. The oxidation of sodium periodate introduced dialdehyde groups into the porous structure of cellulose, and the aldehyde contents were used to estimate the reactivity of the PCs. PCs functionalized with dialdehyde groups could be used to remove urea from aqueous solution.
Experimental
Materials
Viscose fiber (VF) from cotton pulp was provided by CHTC, HELON, Co., Ltd, China. [BMIM]Cl was provided by the Centre for Green Chemistry and Catalysis, LICP, CAS. Soybean oil was provided by COFCO Northsea Oils & Grains (Tianjin) Co., Ltd, China. All other reagents were commercially available and of analytical grade.
Preparation of porous celluloses (PCs)
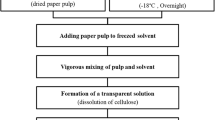
VFs were added into ILs at different cellulose concentrations (1, 2, 3, and 4 wt%) and then heated at 130 °C for 2 h to completely dissolve the VF. The solutions were stored in beakers and sealed in plastic (PE) bags. The solutions were frozen at 0 °C for 72 h. The frozen mixtures were immersed in water to obtain PCs, and then washed several times to remove ILs—until testing with AgNO3 solution showed no Cl− was present. PCs were freeze dried under vacuum. PC1, PC2, PC3 and PC4 represent the PCs fabricated from cellulose solutions with different concentrations (1, 2, 3, and 4 wt%, respectively).
Synthesis of dialdehyde cellulose
Sodium periodate solution (0.3 mol/L) was adjusted to pH 3.0 with sulfuric acid (0.1 mol/L). PC4 was added to the solution without stirring in the modification of PC, while VF was added under vigorous mechanical stirring in the modification of VF. The molar ratio between sodium periodate and cellulose was 0.3. The molar mass of cellulose (i.e. anhydroglucose unit) is 162. The reaction was kept at 37 °C for 2, 4, 6, 8 or 10 h, and the products were washed with distilled water. Anhydrous ethanol was then used to remove the water and the resulting products were dried at 50 °C for 8 h.
Characterization
Fracture surfaces of PCs were viewed using a Hitachi S-4800 scanning electron microscope. Nitrogen adsorption–desorption measurements were performed with an Autosorb-1 specific surface area analyzer (Quantachrome Instruments, USA). X-ray diffraction (XRD) patterns for PCs and VF were recorded in the reflection mode over the angular range of 2–80° (2θ) at ambient temperature using a Bruker D8-S4 Pioneer operated at a CuKα wavelength of 1.542 Å. PCs and VF were measured at 2 cm−1 resolution with a Bio-Rad FTS 3000 IR spectrum scanner. The sample powders were evenly dispersed in KBr and pressed into transparent sheets for testing.
Thermogravimetric analysis of PCs was measured with a STA 409 PC thermal analyzer (NETZSCH, Germany). The sample weights were about 15 mg and they were heated from room temperature to 500 °C at a heating rate of 15 °C/min in a nitrogen atmosphere.
Apparent density (also called bulk density) of PCs was defined as the mass of material divided by the total volume occupied.
Oil adsorption capacity
Dried PC cubes (weight = w0) were immersed in soybean oil for 0.5 h at room temperature with constant stirring. The mixture was filtered under gravity. When there was no more oil dripping from the filter paper, the PC was weighed (w). The oil adsorption capacity was calculated as follows:
Determination of aldehyde content
The aldehyde content of cellulose was determined according to the method of Veelaert et al. (1997), with some modification. A quantity of 0.2 g dialdehyde cellulose (including PC and VF modification) was added to hydroxylamine hydrochloride (25 mL of a 0.25 M) solution. The pH was adjusted to 5.0 with NaOH (0.1 M) solution. The conversion of aldehydes into oximes continued at 50 °C for 2 h. The aldehyde content was determined using Eq. (2) by recording the consumption V sample (mL) of NaOH (0.1 M), performing the reaction at a constant pH of 5.0. A quantity of 0.2 g PC (or VF) was used as a control to record the consumption V control (mL) of NaOH.
162 represents the molecular weight of the glucose unit.
Urea adsorption
The modified-PC4s and VFs with different aldehyde contents were weighed (about 20 mg) into 20 mL glass bottles containing 10 mL urea (100 mg/L) solution. Urea solutions were adsorbed in a water bath shaker (100 rpm) at 30 °C for 12 h to reach equilibrium. According to the method for diacetyl monoxime-antipyrine (GB/T 18204.29-2000, China), the residual urea concentrations were analyzed at 460 nm by UV–Vis spectrometry to determine the urea capacity of dialdehyde- modified PC4s and VFs.
Results and discussion
Characterization of PCs
Figure 1 shows the morphology of PCs from cellulose solutions with different concentrations. SEM images of PC1 (Fig. 1a, c) exhibited a unidirectional porous structure, in which the adjacent PC sheets connected and divided the long pore into several parts. The image of PC4 (Fig. 1b) revealed that its porous structure was composed of randomly oriented cellulose sheets, and the PC sheets were twisted and broken. During formation of PCs, the cellulose solutions were cooled so the ILs began to solidify and the cellulose separated out. As the solid ILs grew, they easily penetrated the cellulose material and unidirectional cellulose sheets formed at low cellulose concentrations. This structure was very similar to the porous chitosan–gelatin/graphene oxide monoliths prepared using the freeze-drying method of Zhang et al. (2011). At high cellulose concentrations (4 wt%) the more cellulose sheets separated from the solidification of IL, and restrained each other, so the twisted cellulose sheets formed and randomly constituted the porous structure.
When the surfaces of cellulose sheets in PC4 were magnified, many holes of about ten nanometers were observed (Fig. 1d). The large BET surface area of PC4 (33.16 m2/g) could be related to these mesopores. PC1 had similar holes on the surface of the cellulose walls (not shown here). As is well known, strong interactions can be formed between cellulose and BMIMCl; because of this, BMIMCl is a good solvent for cellulose. When BMIMCl was cooled and solidified, tiny particles of IL remained wrapped up with cellulose molecules due to the strong interaction. The nanometer sized holes formed on the walls of PCs as ILs were removed by water in the solvent exchange.
The X-ray diffraction patterns of VF and PCs are shown in Fig. 2. VF displayed the characteristic peaks of cellulose I with a sharp high peak (200) centered at 2θ values of about 22.5°, two overlapped weaker peaks at about 14.8° and 16.2°, and a weak peak at about 34.1° (Duchemin and Staiger 2009). There were only small differences among the patterns of PCs from the different cellulose concentrations. PCs did not show the sharp peaks characteristic of the VF pattern, but, at least for the PC1 and PC2 patterns, there were small increases in intensity near 12 and 20° 2-θ as could be expected for very small crystals of cellulose II (French and Santiago Cintrón 2013; French 2014), probably mixed with substantial fractions of amorphous cellulose. Swelling and dissolution of VF in IL inevitably disrupted VF’s crystallinity, and after freezing of IL and subsequent removal of water, a much-less organized material formed in PC. Any time that cellulose in the native cellulose I form is dissolved, the subsequently precipitated form tends to be cellulose II, but some precipitates have very little crystallinity depending on the exact procedures. Cellulose II has been previously found for cellulose precipitate from ionic liquids, including [BMIM]Cl (Zhang et al. 2005; Azubuike et al. 2012; Han et al. 2013).
The FTIR spectra of VF and PC4 are shown in Fig. 3. The absorption peak at 3,383 cm−1 was ascribed to the –OH groups of cellulose in VF, which shifted to higher wavenumbers (3,402 cm−1) in PC4. A similar effect appeared at about 1,060 cm−1 and was attributed to –C–O bond stretching of –C–O–C groups in the anhydroglucose ring of cellulose (Yu et al. 2009). This redshift may be related to the weakening of hydrogen-bond interactions among cellulose molecules of PC4 (Chang et al. 2010). It was also noticed that the relative intensities of –C–O–C groups and –OH groups changed little, and no characteristic peak for C = O groups was observed at 1,740 cm−1 in the FTIR spectra of PC4. This was related to the no degradation of cellulose when PCs were made from VF. Similarly, a study by Heinze et al. (2005) exhibited no obvious degradation of cellulose dissolved in [BMIM]Cl.
Thermogravimetric (TG) and derivative thermogravimetric (DTG) curves of VF and PC4 are exhibited in Fig. 4. Decomposition takes place at the maximum rate of mass loss in TG, i.e., the peak temperature in DTG. The degradation of VF and PC4 occurred at 369 and 341 °C, respectively. The weakened interaction among cellulose molecules and the low crystallinity obviously decreased the thermal stability of PC.
Apparent densities and adsorption of soybean oil
Table 1 shows the bulk density of PCs and the adsorption values for soybean oil. PCs were ultralight with apparent densities ranging from 44 to 88 mg/cm3. The dependence of apparent densities on cellulose concentration in BMIMCl is also exhibited in Table 1. During the cooling process the apparent densities of PCs significantly increased as the concentration increased from 1 to 4 wt%. At the higher cellulose concentrations (>4 wt%) cellulose solution exhibited the high viscosities, and the dissolution with long times could result in the degradation of cellulose.
As is also shown in Table 1, the oil adsorption capacity of PC decreased rapidly with increasing cellulose concentration. The oil adsorption capacity of PC1 reached 22.40 g/g (oil/PC) while PC4 was 9.70 g/g. Despite the hydrophilic nature of cellulose, PCs exhibited good capacity for oil adsorption; this could be attributed to the porous structures which trapped and retained oil. When cellulose concentrations increased, the apparent density increased, and the macro pore volume of the PCs decreased. It resulted in the lower oil adsorption capacity. PC1 exhibited effective adsorption of soybean oil. In comparison, the adsorption capacity for vegetable oil by graphene gel was about 17 g/g (oil/gel) (Cong et al. 2012), and silica or rectorite gels absorbed oil nearly 15 g/g (Rao et al. 2007; Zheng et al. 2013).
The dialdehyde modification
As shown in Fig. 5, dependence of the aldehyde content of VF and PC4 on reaction time is linear. Linear equations with high correlation coefficients (R = 0.998) were expressed as y = 19.79 + 7.249x and y = 1.826 + 7.391x for the modification of PC4 and VF, respectively, where x and y represent the reaction time (h) and aldehyde content (%), respectively. Periodate oxidation mainly breaks the bond between the C2 and C3 in the anhydroglucose units forming double aldehyde groups that take the place of the C–OH groups at C2 and C3 (Yu et al. 2010). The aldehyde content of PC4 was much higher than that of VF after the same reaction time. The high reactivity of PC could be related to the low crystallinity and porous structure, which were propitious for the available IO4 − molecules in the vicinity of anhydroglucose units.
Dialdehyde starch has been used in medicine to remove urea from blood or from the gastrointestinal tract of uremic patients because aldehyde groups can form a Schiff base with the amino groups of urea via covalent bonding. The effect of aldehyde content on urea adsorption of modified-VF and PC4 is shown in Fig. 6. With increasing aldehyde content the adsorption capacity for urea increased until the aldehyde content was 62.78 % for modified-VF and 75.33 % for modified-PC4. The redundant aldehyde groups could not adsorb urea due to steric hindrance; therefore, the porous structure of modified-PC4 can adsorb more urea than modified-VF. Modified-PC4 can adsorb 7.01 mg urea/g with 75.33 % aldehyde groups, while the modified-VF can adsorb 3.09 mg urea/g with 62.78 % aldehyde groups.
Conclusions
The porous cellulose was successfully prepared with IL in the serial process of dissolving, freezing, solvent exchange (ILs-water), and drying, and as such is distinctly different from supercritical drying. The obtained ultralight PCs exhibited good oil adsorption that reached 22.40 g/g (oil/PC). The weakened interaction between cellulose molecules and the low crystalline structures obviously decreased the thermal stability of PC. The porous structures and low crystallinity of PC made chemical modification simple and effective. During oxidation of sodium periodate the relationship between the aldehyde content (y) and the reaction time (x) was linear. The linear equations were expressed as y = 19.79 + 7.249x and y = 1.826 + 7.391x for the modification of PC4 and VF; and the porous modified-PC4 adsorbed more urea than modified-VF.
Porous cellulose alters the modification of cellulose from its typical homogenous pattern to heterogeneous. Ultralight, porous and functionalized PCs have a wide range of potential applications as bio-based materials for thermal insulators, bio-medical, delivery systems, scaffolds, sorbents, catalyst supports, etc.
References
Aaltonen O, Jauhiainen O (2009) The preparation of lignocellulosic aerogels from ionic liquid solutions. Carbohyd Polym 75:125–129
Azubuike CP, Rodríguez H, Okhamafe AO, Rogers RD (2012) Physicochemical properties of maize cob cellulose powders reconstituted from ionic liquid solution. Cellulose 19:425–433
Cao Y, Wu J, Zhang J, Li H, Zhang Y, He J (2009) Room temperature ionic liquids (RTILs): a new and versatile platform for cellulose processing and derivatization. Chem Eng J 147:13–21
Chang PR, Jian R, Zheng P, Yu J, Ma X (2010) Preparation and properties of glycerol plasticized-starch (GPS)/cellulose nanoparticle (CN) composites. Carbohyd Polym 79:301–305
Chen M, Chen C, Liu C, Sun R (2013) Homogeneous modification of sugarcane bagasse with maleic anhydride in 1-butyl-3-methylimidazolium chloride without any catalysts. Ind Crop Prod 46:380–385
Cong H, Ren X, Wang P, Yu S (2012) Macroscopic multifunctional graphene-based hydrogels and aerogels by a metal ion induced self-assembly process. ACS Nano 6:2693–2703
Duchemin B, Staiger MP (2009) Treatment of Harakeke fiber for biocomposites. J Appl Polym Sci 112:2710–2715
French AD (2014) Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 21:885–896
French AD, Santiago Cintrón M (2013) Cellulose polymorphy, crystallite size, and the segal crystallinity index. Cellulose 20:583–588
Han J, Zhou C, French AD, Han G, Wu Q (2013) Characterization of cellulose II nanoparticles regenerated from 1-butyl-3-methylimidazolium chloride. Carbohyd Polym 94:773–781
Heinze T, Schwikal K, Barthel S (2005) Ionic liquids as reaction medium in cellulose functionalization. Macromol Biosci 5:520–525
Huang K, Wang B, Cao Y, Li H, Wang J, Lin W, Mu C, Liao D (2011) Homogeneous preparation of cellulose acetate propionate (CAP) and cellulose acetate butyrate (CAB) from sugarcane bagasse cellulose in ionic liquid. J Agr Food Chem 59:5376–5381
Mahmoudian S, Wahit MU, Ismail AF, Yussuf AA (2012) Preparation of regenerated cellulose/montmorillonite nanocomposite films via ionic liquids. Carbohyd Polym 88:1251–1257
Rao AV, Hegde ND, Hirashima H (2007) Absorption and desorption of organic liquids in elastic superhydrophobic silica aerogels. J Colloid Interf Sci 305:124–132
Sescousse R, Gavillon R, Budtova T (2011) Aerocellulose from cellulose-ionic liquid solutions: preparation, properties and comparison with cellulose-NaOH and cellulose-NMMO routes. Carbohyd Polym 83:1766–1774
Swatloski RP, Spear SK, Holbrey JD, Rogers RD (2002) Dissolution of cellulose with ionic liquids. J Am Chem Soc 124:4974–4975
Veelaert S, de Wit D, Gotlieb KF, Verhé R (1997) Chemical and physical transitions of periodate oxidized potato starch in water. Carbohyd Polym 33:153–162
Vitz J, Erdmenger T, Haensch C, Schubert US (2009) Extended dissolution studies of cellulose in imidazolium based ionic liquids. Green Chem 11:417–424
Wen J, Sun Y, Meng L, Yuan TQ, Xu F, Sun RC (2011) Homogeneous lauroylation of ball-milled bamboo in ionic liquid for bio-based composites production Part I: modification and characterization. Ind Crop Prod 34:1491–1501
Yu J, Yang J, Liu B, Ma X (2009) Preparation and characterization of glycerol plasticized-pea starch/ZnO-carboxymethylcellulose sodium nanocomposites. Bioresourc Technol 100:2832–2841
Yu G, Chang P, Ma X (2010) The preparation and properties of dialdehyde starch and thermoplastic dialdehyde starch. Carbohyd Polym 79:296–300
Zhang H, Wu J, Zhang J, He J (2005) 1-Allyl-3-methylimidazolium chloride room temperature ionic liquid: a new and powerful nonderivatizing solvent for cellulose. Macromolecules 38:8272–8277
Zhang N, Qiu H, Si Y, Wang W, Gao J (2011) Fabrication of highly porous biodegradable monoliths strengthened by graphene oxide and their adsorption of metal ions. Carbon 49:827–837
Zhang QL, Shi F, Wang P, Lin DQ, Yao SJ (2014) Preparation of cellulose adsorbents with ionic liquid and pore expansion for chromatographic applications. J Appl Polym Sci. doi:10.1002/APP.40060
Zhao H, Jones CL, Baker GA, Xia S, Olubajo O, Person VN (2009) Regenerating cellulose from ionic liquids for an accelerated enzymatic hydrolysis. J Biotechnol 139:47–54
Zheng P, Chang PR, Ma X (2013) Preparation and characterization of rectorite gels. Ind Eng Chem Res 52:5066–5071
Acknowledgments
This research was supported by the Science and Technology Project of Jiangxi Provincial Office of Education (KJLD12082 and Innovation Platform “project 311″) and Nature Science Foundation of Jiangxi Province (20132BAB 206006) and the National Nature Science Foundation of China (51162011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, X., Chang, P.R., Zheng, P. et al. Porous cellulose facilitated by ionic liquid [BMIM]Cl: fabrication, characterization, and modification. Cellulose 22, 709–715 (2015). https://doi.org/10.1007/s10570-014-0467-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-014-0467-0