Abstract
Raw cotton knitted fabrics of Greek, Indonesian, and Indian origin were investigated through surface characterization before and after processing. Surface modifications of all the knitted cotton fabrics were objectively evaluated through zeta potential measurements within a range of the electrolyte solution pHs, and swelling over time. Streaming potential coefficients of different cotton knitted fabrics were applied in order to establish a correlation between the properties of the raw and treated cotton knitted fabrics of different origins. The rate of swelling was calculated from the streaming potential coefficients of the raw knitted fabrics before and after different treatments. Swelling rate for different origins and different treatments of cotton knitted fabrics and the correlation coefficients were obtained using linear regression. Additionally, a data cluster analysis was performed in order to group different origins of cotton, while all the treatments were sorted according to zeta potential and the rate of swelling coefficients. The obtained results showed differences among the clusters, depending on the origin of the cotton knitted fabrics and treatments they were exposed to.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chemically, cotton is constituted of 88–96.5 % cellulose as well as non-cellulosic pectin, waxes, proteins, minerals and other organic compounds that are mainly localised within the cuticle of the primary wall. The properties of cotton strongly depend on the growing conditions of the cotton plant (influence of soil, weather, and harvested time) (Karamkar 1999).
Waxy materials and pectins are responsible for the hydrophobic properties of raw cotton, where the pectin acts as cement, including waxes (Tzanov et al. 2001; Calafell and Garriga 2004).
Various treatments should be performed for the removal of natural non-cellulosic materials from cotton as a preparation for all the subsequent finishing processes such as dyeing, printing, and finishing. The selection of methodology for their removal should be based on proper efficiency and minimum damage to the fibre.
Cotton is sensitive to acidic treatment, therefore it is necessary to decrease the risk of hydrolysis and prevent degradation by selecting a weak acid. Demineralisation using hydrochloric acid is the simplest solution for preventing damage.
Textile processing depends on the quality of the supplied raw cotton fibres, which also strongly influences the quality of the final product. Low-quality cotton requires repetition of different process steps, thus increasing production costs. All the preparatory procedures, each in its own way, can impact structural changes and surface modifications of cotton.
A number of references (Ribitsch et al. 1996; Stana-Kleinschek and Ribitsch 1998; Pothan et al. 2002; Bismarck et al. 2002; Bellmann et al. 2005) show that the surface properties of the cellulose materials can be successfully characterised by electrokinetic methods. Electrokinetic phenomena can be observed by the contact of a solid surface with a polar liquid medium, due to the existence of an electrical double layer at the solid–liquid interface. The potential on the surface of a solid in contact with a polar medium is governed by the dissociations of surface groups, the preferential adsorption of cations or anions and the adsorption of polyelectrolytes, the isomorphic substitution of cations and anions and, lastly, the accumulation or depletion of electrons (Lyklema 1995).
In most cases, zeta potential, which is calculated from the electrokinetic effect of the streaming potential, is recorded at different pH of an aqueous solution or with time. Recently, different techniques for textile fabric characterization with some focus on the zeta potential have been reviewed (Ripoll et al. 2010; Luxbacher 2012). Ripoll et al. (2012) even explored the streaming potential measurement for the direct analysis of nanoparticle adsorption on textile fabrics.
Measuring streaming potential can also be applied to the monitoring the swelling process of cotton fabrics. Swelling process actually represents the process of water adsorption and can be described by pseudo first-order kinetics (Kanamaru 1960). It depends on the origin of the cotton and the pre-treatment applied prior to the finishing or dyeing processes. Based on the streaming potential coefficients for raw and treated cotton, it is thus possible to group together cotton of different origins.
The aim of this work was to study the influence of processing on the zeta potential and the swelling of cotton knitted fabrics. In regard to this, the zeta potential of raw and treated cotton knitted fabrics was determined and as an objective parameter for their qualifications, in view of different origin of cotton knitted fabrics, using multivariate statistical analysis. This analysis can be applied for the individual groups, according to the origin of the cotton in question or certain treatment previously applied.
Experimental
Materials and procedures
Cotton knitted fabrics of different origin were used, i.e. Greek, Indonesian and Indian (provided by Beti Pletiva d.d., Slovenia). The characteristics of the raw cotton knitted fabrics of different origin are presented in Table 1.
Raw cotton knitted fabrics were subjected to various pre-treatment processes (removal of impurities) and finishing (dyeing). The pre-treatment processes included acid treatment and de-waxing being performed on raw cotton knitted fabrics under laboratory conditions. The samples were separately treated with hydrochloric acid (1.5 %) at 50 °C for 15 min in a Linitest apparatus (Original Hanau, Germany). Soxhlet extraction was used in order to remove impurities, labelled as the de-waxing of knitted fabrics, with dichloromethane, following DIN 54 728, part 1.
All the knitted fabrics were bleached with hydrogen peroxide under industrial conditions. The auxiliaries Biavin 109 (a concentrated gliding and grease-preventing agent) and CHT-Entschäumer MI (defoaming agent) were supplied by CHT Tübingen (Germany), whilst the auxiliaries Imerol JSF (wetting and washing agents), Sirix SB (stabilizing agent) and Bactosol ARL liquid c (powerful enzyme for the bio-degradation of hydrogen peroxide) were supplied by Clariant, Switzerland. A bleaching process for raw knitted fabrics was performed at 115 °C for 15 min in a bath (BR 1:5) containing Imerol JSF (1 %), Sirix SB (1.2 %), 32 % NaOH (1.36 %), 35 % H2O2 (5 %), Biavin 109 (0.5 g/l), and Entschäumer MI (0.2 g/l). After draining, treatment with Bactosol ARL (0.5 g/l) and acetic acid (0.3 g/l) at 50 °C followed for 15 min.
Dyeing of the bleached knitted fabrics with reactive dyes, as specified in Table 2, was applied under industrial conditions. The dyeing auxiliaries, Meropan DPE (sequestering agent) and Cotoblanc NSR (soaping agent) were supplied by CHT Tübingen (Germany).
The bleached knitted fabrics were treated at 20 °C for 40 min in a dyeing bath containing Meropan DPE (1.0 g/l), Biavin 109 (0.5 g/l), dyestuffs (0.02–1.5 %), sodium chloride (50 g/l) and sodium carbonate (5.0 g/l). After processing, the bath was heated to 60 °C for 10 min and 32 % NaOH (0.88 g/l) was added to the bath, while the process was continued for another 60 min. The bath was drained and rinsing and neutralisation with HCl (0.5 g/l) followed afterwards. Soaping was performed using Cotoblanc NSR (0.3 g/l) and Biavin 109 (0.5 g/l) at 100 °C for 10 min. Rinsing followed in three steps.
Methods
The streaming potential method was used to measure the electrokinetic properties of different cotton knitted fabrics during all the process stages by applying an electrokinetic analyzer (SurPASS, Anton Paar GmbH, Austria). A fabric sheet of 3 cm × 3 cm was mounted on the cylindrical cell, thereby creating a permeable plug. Movement of the liquid adjacent to the fabric surface resulted in a net-flow of the counter ions, which compensate the net charge on the surface. The flow of ions balanced by a back-current gives rise to the streaming potential, which was detected using Ag/AgCl electrodes.
The reproducibility of electrokinetic measurements depends on the sample packing and the electrolyte composition. In order to ensure comparability, care was taken to prepare fabric samples of same packing density. A 0.001 mol/l KCl solution was used to measure the time-dependences of the streaming potential coefficients for the fabrics. A background electrolyte of 0.001 mol/l KCl solution was also used during automatic titrations, while the initial pH was adjusted to pH 10 with 0.1 M NaOH. KCl was a typical 1:1 electrolyte which was expected not to interfere specifically with most solid material surfaces. At lower ionic strength we have to expect an effect of the interfacial conductance on the measured streaming potential (Werner et al. 1998). However, for complex material surfaces such as porous and swelling cotton fibres, we expected a significant contribution of material conductance (Yaroshchuk and Ribitsch 2002). On the other hand, the streaming potential decays almost double-exponentially with increasing ionic strength. Therefore, we selected ionic strength of 0.001 mol/l as the optimum measuring condition.
The evaluation of zeta potential is based on the Smoluchowski equation (Lyklema 1995).
where U is the streaming potential, Δp the pressure difference between both sides of the fabric plug, ε and ε 0 the dielectric constant and the vacuum permittivity, η the viscosity, and κ B the conductivity of the measuring fluid. The surface conductivity of the fibrous samples was not taken into account.
Cellulose fibres swell in water, causing a decrease in the zeta potential over time that can be described by the pseudo first order kinetics expressed as follows (Kanamaru 1960):
where ζ t is a measured ζ-potential value at time t, ζ∞ the ζ-potential value that the function ζ = f(t) approaches asymptotically, and k the rate constant.
Electrokinetic measurement under constant measuring conditions resulted in streaming potential data, and quotient dU/dp could be assigned to SP (streaming potential coefficient). The modification of Eq. (2) is as follows
The integration of Eq. (3) leads to:
with SP0 the starting streaming potential value measured immediately after the fibres got in contact with the electrolyte solution.
The streaming potential of the cotton knitted fabrics was measured in the presence of 0.001 mol/l KCl solution at its initial pH. The rate of swelling for different origin and different treatments of cotton and the correlation coefficient were obtained from Eq. (4) employing linear regression.
Cluster analysis
Data clustering was performed for clustering different origin of cotton and various treatments according to streaming potential measurements. Cluster analysis is an exploratory data analysis tool for solving classification problems in environmental, chemical, food and textile engineering. Its objective is to sort cases (origin of cotton) into groups, or clusters, so that the degree of association is strong between members of the same cluster and weak between members of different clusters. The similarities–dissimilarities were quantified for our purpose through Euclidian distance measurements (Zupan 2009).
During hierarchical clustering clusters were formed sequentially by starting with the more similar pairs of objects and forming higher clusters step by step. The normalised Euclidean distance and Ward methods were used to obtain dendrograms linkage distances reported as Dlink/Dmax × 100, which represented the quotient between the linkage distances for a particular case divided by the maximal distance, multiplied by 100, as a way of standardising the linkage distance represented on the y-axis. Cluster analysis was performed by applying the Statistica 10.0 software package.
Results and discussions
The composition and amount of impurities depend on the origin of cotton. Therefore, it is important to choose a proper pre-treatment process to remove impurities from cotton and ensure proper quality of the final product. These processes cause changes in the surface charge and the zeta potential, which are determined from streaming potential measurements.
A randomly-arranged plug of cotton fabrics with an undefined network of capillaries represents a system with enhanced complexities compared to the assumptions of the electric double layer model. Although this experimental approach of zeta potential analysis for fibrous and porous materials leads to reasonable and surprisingly reproducible results, the zeta potential remains underestimated at higher ionic strengths. The ionic conductance inside the porous fabric plug is increased, compared to the electric conductivity of the bulk aqueous solution, by the interfacial conductivity at the outer and inner surfaces of the cotton fibres. Whilst the compression of the fabric plug inside the measuring cell affects the distances the between individual fibres of the knitted fabric, and thus the outer surface conductance, the extension of the fibres due to swelling is driven by the electrolyte pH. Fibres with an open structure accommodate water and a streaming potential signal is generated and superimposed to the outer fibre streaming potential. The proximity of the adjacent fibre walls induces a significant interfacial conductivity effect, which suppresses the contribution of the inner fibre streaming potential to the zeta potential. However, the conductive water volume inside the swollen fibres offers a second path for the back-current which opposes and thus equilibrates the streaming current in the streaming potential mode of measurement. The streaming potential is therefore reduced as compared to a plug of non-porous or non-swelling fibres of the same surface charge densities. While the effect of surface swelling of materials can be compensated by the alternative measurement of the streaming current (Werner et al. 2001), bulk material swelling, which is dominating for cotton fibres and fabrics, affects both the streaming potential and the streaming current (Zimmermann et al. 2010). On the other hand, the sample packing in the measuring cell does not differentiate between different mesh size of the fabrics.
For the discussion of the charging behaviour of different cotton fabrics and its changes due to the modification processes of de-waxing, acid treatment, bleaching and dyestuff application, we refer to the streaming potential coefficient dU/dp = SP. The streaming potential coefficient is representative for the charge at the solid–liquid interface while the apparent zeta potential may lead to wrong interpretation of the results due to underestimation of the conductivity inside the porous fabric plug. The degree of underestimation depends on the ionic strength of the electrolyte solution and thus on the pH (at low pH the ionic strength inevitably increases). The requirement of a reproducible packing of the fibre plug holds for both the measurement of the streaming potential and the analysis of the apparent zeta potential.
It is well-known that most textile fibres are negatively-charged within a neutral aqueous solution. Surface charge is a result of reactive group dissociation or preferential adsorption of different species in a bulk. Cotton fibres are negatively charged due to the presence of hydroxyl groups in the cellulose polymer. Impurities cover cotton surface and their hydrophobic nature disables wet processing.
The streaming potential coefficients SP of the Greek, Indian, and Indonesian raw knitted cotton fabrics are shown in Fig. 1, which presents a specific titration curve of raw cotton knitted samples contaminated with hydrophobic impurities. Titration with HCl results in a decrease in the negative SP below pH 4.5. All the raw samples have similar curve shapes, whilst the magnitude of streaming potential differs. Indian raw cotton fabric is less negative in comparison with the Indonesian and Greek raw cotton fabrics. It implies different impurities content reflecting a more the prominent hydrophobic nature of Indian raw cotton knitted fabric.
In order to see the changes in the surface charges that occurred after each individual pre-treatment process prior to dyeing, the streaming potential coefficients of the raw and all treated cotton knitted fabrics were measured depending on the pH-value of the electrolyte solution.
Processing raw cotton with acid and solvent affected surface modifications of the cotton fabrics treated, which was finally characterised by zeta potential. As a representative example, Fig. 2 shows the streaming potential coefficients for raw, de-waxed, acid-treated, bleached and dyed cotton fabrics of Indonesian origin.
The curves of SP variations of pH indicated that the processing of raw knitted fabrics resulted in diverse effects. The SP after acid treatment was less negative than for raw cotton. A possible reason could be the residual hydrophobic impurities superimposed with non-soluble calcium or magnesium pectate that could stick to the surface of the cotton fabric. Extraction with dichloromethane resulted in the efficient removal of hydrophobic impurities from the Indonesian knitted fabric. The electrokinetic behaviour of de-waxed and bleached knitted fabrics was similar. It was clear that bleaching had an impact on the removal of the primary wall and the possible formations of new groups (–CO, –CHO, –COOH) that had an effect on the more negative surface charge. Dyeing the knitted cotton fabric had an impact on further increase of negative SP, especially when pH was higher than 5. This effect suggested permanent interactions of dyestuff molecules with available cellulose groups as covalent bonds.
The isoelectric point (IEP, i.e., pH where SP = 0 mV/mbar and thus ζ = 0 mV) remained at pH 2.6, and was almost unaffected by different treatment processes undertaken. This observation indicated the dominance of the inner structures of cotton fibres, which were less affected by impurities and the surface treatment.
Dissociation of reactive groups in an aqueous solution generated a negative value of zeta potential. The sign and magnitude of the charge of fibres surface depended very much on the pH. Highly polar cotton fibres possess hydroxyl and carboxyl groups, able to form hydrogen bonds with water molecules. The distinctions among various knitted fabrics were more sensitive within the alkaline range for both streaming potential coefficients and apparent zeta potentials. The magnitude of the charge decreased with increasing pH, meanwhile a characteristic titration curve from pH 9 to pH 7 was mostly stable.
Furthermore, negative maximum at around pH 4.3 was indicative for the conditions of the cotton samples. Such maximum is often observed for the pH dependence of the zeta potential of porous and strongly swelling natural fibres like cellulose and lignocellulose fibres (Bismarck et al. 2002; Buschle-Diller et al. 2005; Paul et al. 2008). Several phenomena contribute to the observed trend in the pH dependence of SP, i.e., an increase of the negative SP, which passed through a (negative) maximum before decreasing again in the pH range from pH 10 to pH 2. First, the swelling of the cellulose fibres got suppressed with decreasing electrolyte pH. This suppression in the swelling led to a shift of the shear plane of the electrochemical double towards the fibre surface and thus to an increase in zeta potential (Reischl et al. 2006). A reduced swelling also eliminated the effect of interfacial conductance, which affected the streaming potential signal. Second, the ionic strength was increased due to the addition of acid to the aqueous solution. Therefore, the effect of interfacial conductance on the streaming potential was even further reduced.
Due to these phenomena, which are, in our opinion responsible for the observed maximum in the negative SP, this maximum was less pronounced when choosing a higher ionic strength. However, the sensitivity of the individual measurement and thus the significance of observed difference between differently treated cotton fabrics, was considerably reduced.
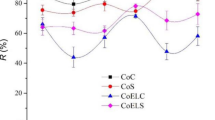
Figure 3 displays the change in the streaming potential coefficients at pH 4.3 for various treated cotton fabrics relative to the SP of the corresponding raw cotton samples.
Greek and Indonesian raw cotton knitted fabrics exhibited similar electrokinetic behaviour for variations of pH, so we assumed that all the performed laboratory and industrial processes resulted in similar relationships. The results presented in Fig. 3 confirmed that the impact of processing on the zeta potentials of Greek and Indonesian cotton was predictable and similar.
The zeta potential of Indian raw cotton knitted fabric was less negative than the Greek and Indonesian (Fig. 1). It indicated a higher content of hydrophobic impurities in the Indian raw cotton, which could require repeated re-processing of Indian cotton in comparison to the Greek and Indonesian ones.
Swelling processes
Strong sorption power and high sorption capacity provide cotton fabrics with a pronounced hydrophilic character. The accessibility in water-swollen state is of even greater importance than in the dry or conditioned state for many industrially-performed processes on cellulose fabrics.
As for the pH dependence of the charging behaviour of the cotton fabrics in aqueous solutions, the rate of swelling was monitored by making use of the streaming potential coefficients rather than the apparent zeta potentials. As a representative example, Fig. 4 shows the change of SP with time after exposure to a 0.001 mol/l KCl solution for Indian raw cotton fabric and its derivative samples, which were obtained after several treatment processes. The measurement time was limited to 10 h whilst the equilibrium condition was expected to be achieved after 40–50 h. The initial and final streaming potential coefficients SP0 (at t = 0 min) and SP∞ (at t = 4,000 min) were used to estimate the rates of swelling according to the pseudo-first order kinetics proposed by Kanamaru (Eqs. 2 and 3). Whilst the streaming potential coefficients for the purified and oxidised cotton fabrics obtained after de-waxing, acid treatment, and bleaching, respectively, underwent the expected temporal changes, the raw cotton fabrics showed a continuous but almost linear decrease of SP over time. We assumed a superposition of the processes of swelling and of the removal of surface impurities (the measurements of streaming potential analysis and rinsing of the material surface occurred simultaneously) with the latter process dominating the change in SP. We therefore excluded the raw cotton fabrics from the statistical analysis of the swelling kinetics.
a Changes in streaming potential coefficients over time upon the exposures of differently-treated Indian cotton fabric to aqueous 0.001 mol/l KCl solution. b Evaluation of the swelling rates according to Kanamaru (Eq. 3)
The first pre-treatment process was the extraction in dichloromethane where cotton fabrics were de-waxed. Despite of only a partly removed content of pectin, the hydrophilicity increased as indicated by the more pronounced maximum in the SP at pH 4.3 and a decrease in the negative SP at higher pH. A correlation between hydrophilicity, which is commonly described by the water contact angle, and zeta potential was already reported by Ribitsch et al. (2001) for a series of cellulose and regenerated cellulose fibres. The residual pectin impurities could hinder water uptake which was witnessed by low swelling rate. Demineralisation removed hydrophobic impurities completely thus enabling a faster water uptake indicated by a higher swelling rate.
Bleaching with hydrogen peroxide affected the creation of carboxylic groups and a more negative SP curve. After bleaching, cotton samples assumed most hydrophilic character and highest water uptake capacity.
The temporal change in the streaming potential coefficient for the dyed cotton fabric also indicated more complex kinetics, either of a higher order or, more likely, of a combination of two processes: swelling and dissolution of the dyestuff. The initial fast rate was assigned to the desorption of excess dyestuff whilst the slower process resembled the swelling characteristics of the treated but non-dyed cotton fabrics.
Based on the streaming potential measurements, the rates of the swelling process using Eq. (3) (modified the Kanamaru equation) were determined for different origin of cotton and different treatments. Equation (4) predicts a linear relation when plotting the term \( \ln \left( {\frac{{SP - SP_{\infty } }}{{SP_{0} - SP_{\infty } }}} \right) \) versus time. Figure 4b shows this dependence for treated cotton knitted fabrics of Indian origin. In Fig. 4b zeta potentials in Eq. (3) are replaced by the corresponding streaming potential coefficients SP. The rates k of the swelling process were determined based on linear regression analysis. Values of k (h−1) with corresponding correlation coefficients (R2) for different sorts of cotton and different treatments are shown in Table 3.
According to the obtained results, the rates of the swelling process can be associated with the pre-treatment processes and the differences in the origin of cotton. The reason for the latter assignment could be the non-uniform quality of cotton due to the presence of short fibres, less maturity and higher content of impurities that could influence the process of water uptake.
When considering both, the observation that different origin of the cottons determined the impacts of particular treatments, and the indications from kinetic analysis that the raw and dyed cotton deviated from the pseudo first order kinetics, chemometric analyses (CA) were performed for the acid-treated, de-waxed and bleached cottons. Figure 5 presents a dendrogram of the origins of the cottons and different modification patterns as a result from the CA of the measured streaming potential coefficients. Using the CA of cotton, variations in the origin and treatments can be clearly distinguished.
The dendrogram shows that all the treatments and origins of the cottons analysed may be generally grouped into two main clusters. Cluster I can be formed from the bleached Greek, Indian, and Indonesian cotton knitted fabrics. Cluster II can be formed regarding the acid and de-waxing treatments of cotton knitted fabrics of different origin. Due to the differentiations of the treatments, it was impossible to provide a grouping of three different origins of cotton, as in Cluster I.
Additional cluster analysis was performed to identify the origin of cotton on the basis of similarities/dissimilarities in their treatment processes. Using cluster analysis, cotton sorts of different origin were clearly distinguishable in two clusters: cluster I with very small Euclidean distances and separate groups, and cluster II with much higher Euclidean distance values. The results of these analyses are shown in Fig. 6.
It was clear that cotton of different origin could be grouped on the basis of streaming potential coefficients. These measurements could help in the selection of a pre-treatment procedure before dyeing. Specific distributions of treatments indicated that each individual treatment had a different impact on cotton structure and properties, which could impact dyeing properties and colouration of the fabrics made from it. Raw and dyed cotton from Greece and Indonesia belonged to the same group whilst the Indian cotton was singled-out. Cotton from the same group, Greek and Indonesian, could be treated employing identical procedures, while the cotton from India would require a modified procedure. Streaming potential coefficients showed that other treatments (de-waxing, acid treatment and bleaching) resulted in different distributions and groupings, indicating that each treatment had a specific impact on cotton structure. De-waxing and acid treatment were separately performed on raw knitted cotton fabric under laboratory conditions. These laboratory processes were optional as preparatory treatments for dyeing cotton. Streaming potential coefficients singled out a de-waxed Greek cotton and acid-treated Indonesian cotton. The real preparatory process for dyeing is bleaching, where streaming potential coefficients singled out Greek and Indian cotton, whilst the Indonesian cotton was separated.
Conclusions
The solid surface zeta potential is a suitable parameter for the surface characterization of raw cellulosic materials. However, different chemistries of non-cellulosic impurities and complex structure of cotton fibres make it difficult to interpret zeta potential results. Instead, streaming potential coefficient is an appropriate indicator for surface charge of cellulose-based materials. Furthermore swelling of cotton fabrics observed by streaming potential measurements is convenient to determine the rate of water uptake through pseudo first-order kinetics. Streaming potential coefficients of different cotton knitted fabrics were used in order to establish a correlation between the properties of raw and treated cotton knitted fabrics of different origin. It was proved that the properties of the cotton of different origin can be grouped on the bases of streaming potential measurements. This information can help in the selection of appropriate procedures for treating cotton before dyeing processes. Streaming potential coefficients provided the grouping and qualifications of Greek and Indonesian cottons as substrates where it was possible to achieve the same shade, whilst the same shade for Indian cotton could only be reached by a modified recipe.
References
Bellmann C, Caspari A, Albrecht V, Loan Doan TT, Mäder E, Luxbacher T, Kohl R (2005) Electrokinetic properties of natural fibres. Colloids Surf A 267:19–23
Bismarck A, Aranberri-Akargorta I, Springer J, Lampke T, Wielage B, Stamboulis A, Shenderovich I, Limbach HH (2002) Surface characterization of flax, hemp and cellulose fibers; surface properties and the water uptake behaviour. Polym Compos 23:872–894
Buschle-Diller G, Inglesby MK, Wu Y (2005) Physicochemical properties of chemically and enzymatically modified cellulosic surfaces. Colloids Surf A 260:63–70
Calafell M, Garriga P (2004) Effect of some process parameters in the enzymatic scouring of cotton using an pectinase. Enzyme Microb Technol 34:326–331
Kanamaru K (1960) Wasseraufnahme in ihrer Beziehung zur zeitlichen Erniedrigung des & #x03B6; Potentials yon Fasern in Wasser. Kolloid Z 168:115–121
Karamkar SR (1999) Textile science and technology 12: chemical technology in the pretreatment processes of textiles. Elsevier, Amsterdam
Luxbacher T (2012) Electrokinetic properties of natural fibers. In: Kozlowski R (ed) Handbook of natural fibres, vol 2. Woodhead Publishing, Cambridge
Lyklema J (1995) Fundamentals of interface and colloid science. Vol. II Solid–liquid interfaces, vol II. Academic press, San Diego
Paul AP, Piasta D, Spange S, Pothan LA, Thomas S, Bellmann C (2008) Solvatochromic and electrokinetic studies of banana fibrils prepared from steam-exploded banana fiber. Biomacromolecules 9:1802–1810
Pothan LA, Bellman C, Kailas L, Thomas S (2002) Influence of chemical treatments on the electrokinetic properties of cellulose fibres. J Adhes Sci Technol 16:157–178
Reischl M, Stana-Kleinschek K, Ribitsch V (2006) Electrokinetic investigations of oriented cellulose polymers. Macromol Symp 244:31–47
Ribitsch V, Stana-Kleinschek K, Jeler S (1996) The influence of classical and enzymatic treatment on the surface charge of cellulose fibres. Colloid Polym Sci 274:388–394
Ribitsch V, Stana-Kleinschek K, Kreze T, Strnad S (2001) The significance of surface charge and structure on the accessibility of cellulose fibres. Macromol Mater Eng 286:648–654
Ripoll L, Bordes C, Etheve S, Elaissari A, Fessi H (2010) Cosmeto-textile from formulation to characterization: an overview. e-Polymers 40:1–34
Ripoll L, Bordes C, Marote P, Etheve S, Elaissari A, Fessi H (2012) Electrokinetic properties of bare or nanoparticle-functionalized textile fabrics. Colloids Surf A 397:24–32
Stana-Kleinschek K, Ribitsch V (1998) Electrokinetic properties of processed cellulose fibers. Colloids Surf A 140:127–138
Tzanov T, Calafell M, Gübitz GM, Cavaco-Paulo A (2001) Bio-preparation of cotton fabrics. Enzyme Microb Technol 29:357–362
Werner C, Körber H, Zimmermann R, Dukhin S, Jacobasch HJ (1998) Extended electrokinetic characterization of flat solid surfaces. J Colloid Interface Sci 208:329–346
Werner C, Zimmermann R, Kratzmüller T (2001) Streaming potential and streaming current measurements at planar solid/liquid interfaces for simultaneous determination of zeta potential and surface conductivity. Colloids Surf A 192:205–213
Yaroshchuk A, Ribitsch V (2002) Role of channel wall conductance in the determination of zeta-potential from electrokinetic measurements. Langmuir 18:2036–2038
Zimmermann R, Kuckling D, Kaufmann M, Werner C, Duval JFL (2010) Electrokinetics of a poly(N-isopropylacrylamid-co-carboxyacrlyamid) soft thin film: evidence of diffuse segment distribution in the swollen state. Langmuir 26:18169–18181
Zupan J (2009) Kemometrija in obdelava eksperimentalnih podatkov. Kemijski inštitut Ljubljana, Inštitut nove revije, Ljubljana
Acknowledgments
Acknowledgment goes to Ministry of Higher Education, Science and Technology of Slovenian Republic, Directorate of Technology, and to Ministry of Science, Education and Sports of Croatian Republic for financial support of EUREKA project APTEX E!4178, entitled “Improving the application and durability of surface functionalisation on textile fabrics” and EUREKA project FLAMEBLEND E!5785, entitled “Improvement in the flame retardant properties of cotton and wool blends”. We also acknowledge our reviewers who helped us to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luxbacher, T., Čurlin, M., Petrinić, I. et al. Assessing the quality of raw cotton knitted fabrics by their streaming potential coefficients. Cellulose 21, 3829–3839 (2014). https://doi.org/10.1007/s10570-014-0388-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-014-0388-y