Abstract
A series of N-(aryl) and their quaternary N-(aryl) chitosan derivatives were synthesized and evaluated for their antifungal activity against crop-threatening fungus Botrytis cinerea. Schiff bases were firstly synthesized by the reaction of chitosan with cinnamaldehyde, cuminaldehyde and 4-dimethylaminobenzaldehyde followed by reduction with sodium borohydride to form N-(aryl) chitosans. Quaternary N-(aryl) chitosans were then obtained by reaction of N-(aryl) chitosan compounds with ethyl iodide. The chemical structures were characterized by 1H-NMR, FT-IR and UV spectroscopic techniques. The antifungal activity was evaluated in vitro against B. cinerea by mycelial growth inhibition method and in vivo by application of compounds to tomato plants prior to inoculation with fungal spores. In an in vitro experiment, all quaternized chitosans were more active than N-(aryl) chitosan derivatives and N,N,N-(diethylcinnamyl) chitosan (QC1) was the most potent (EC50 = 1,147 mg/L) against mecelia however, N,N,N-(diethyl-p-dimethylaminobenzyl) chitosan (QC3) was the most potent (EC50 = 334 mg/L) against spores. In an in vivo study, no disease incidence (0.0 %) was observed with QC1 and QC3 at 1,000 mg/L. Spray liquid chitosan enhanced total phenolics and guaiacol peroxidase in inoculated leaves.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gray-mould disease caused by Botrytis cinerea is probably one of the most common and widely distributed diseases of economically important fruits, vegetables and even field crops throughout the world during the growing season and postharvest storage. It is also a major obstacle to long-distance transport and storage (Sommer et al. 1992). This fungal pathogen infects leaves, stems, flowers and fruit, either by direct penetration or through wounds caused by cultivation practices (McKeen 1974; O’Neill et al. 1997). Fungicides are effective in reducing the damage to plants caused by fungal pathogens, but repeated fungicide applications are harmful to the environment (Carson 1962; Houeto et al. 1995). Furthermore, chemical control of B. cinerea has only been partially successful due to the continued appearance and establishment of resistant Botrytis spp. strains. The development of new alternatives, which would eliminate the use of harmful pesticides, is a goal of considerable interest within the framework of a sustainable, economically profitable agriculture (Tripathi and Dubey 2004). Consequently, the possibility of stimulating internal plant defenses has become an interesting option for enhancing natural disease resistance.
Among the elicitors known to date, chitosan, a polysaccharide including both β-1,4-anhydroglucosamine and N-acetyl-β-1,4-anhydroglucosamine units, has the best prospects as a biocontrol agent (El Ghaouth et al. 1992, 1994). Chitosan is particularly attractive for biological applications due to its non mammalian toxicity, biodegradability, physiological, and antibacterial properties (Badawy and Rabea 2011; No and Meyers 1997; Rabea et al. 2003). The antimicrobial activity of chitosan has been shown against a wide variety of microorganisms including fungi and bacteria (Guo et al. 2013, 2014; No et al. 2002; Rabea et al. 2003; Xia et al. 2011). However, its antimicrobial activity was prominent only in acidic medium because of its poor solubility in neutral and basic media where chitosan start to lose its cationic nature (Liu et al. 2001; Zhang et al. 2012).
To overcome this obstacle, several chitosan derivatives with high solubility in water have been prepared such as N-, O-(carboxymethyl) chitosan (Muzzarelli and Tanfani 1982), N-(sulphate) chitosan (Holme and Perlin 1997), O-(butyryl) chitosan (Grant et al. 1988), N-(methylenephosphonic) chitosan (Heras et al. 2001; Ramos et al. 2003) and quaternary ammonium chitosan compounds (Badawy 2010; Badawy and Rabea 2012; Jia et al. 2001; Muzzarelli and Tanfani 1985). Quaternary ammonium chitosans, a kind of chitosan derivatives with permanent cationic charges on the polysaccharide backbone, have attracted much attention to be used as bacteriostatic and fungistatic agents (Rúnarsson et al. 2007) and drug delivery carriers (Mourya and Inamdar 2009). That is because chitosan modified with quaternary ammonium salts can become water-soluble and exhibit better antimicrobial activity than unmodified chitosan (Li et al. 2011).
The application of a chitosan solution may sensitize the plants to respond more rapidly to pathogen attack by stimulating chitinase and glucanase production (Benhamou 1996; El Hadrami et al. 2010). Thus, there is a growing interest in the use of chitosan as a means to providing more protection against plant pathogens since it has been shown to have a wide range of antifungal activity (El Ghaouth et al. 1992, 1994).
In this article, a series of N-(aryl) and their quaternary N-(aryl) chitosan derivatives were synthesized to enhance the antimicrobial activity of chitosan molecule against gray mold caused by B. cinerea in tomato plant. The chemical structure and physical properties of the chitosan derivatives were characterized by Fourier transform infrared (FT-IR) spectroscopy, UV spectroscopy, 1H-NMR spectroscopy, and solubility testing. The antifungal activity was investigated in vitro by mycelia growth inhibition method and in vivo by application of compounds to tomato plants prior to inoculation with the fungal spores. Furthermore, the elicitation of defence markers including total soluble phenolic compounds and guaiacol peroxidase activity by chitosan treatments in leaves was investigated. The ultimate purpose of our research is to provide an alternative treatment for preventing gray mould damage to tomato plant.
Materials and methods
Materials and tested microorganism
Low molecular weight of acid-soluble chitosan was purchased from Sigma-Aldrich Co. (USA). The degree of deacetylation (DDA) of this product was determined to be 0.91 by 1H-NMR spectroscopy. Cinnamaldehyde, cuminaldehyde and 4-dimethylaminobenzaldehyde were purchased from Fluka (Deisenhofen, Germany). Ethyl iodide, deuterium oxide, deuterated acetic acid, sodium borohydride (NaBH4), gallic acid, polyvinylpyrrolidone (PVP), pyrocatechol, sodium–potassium tartarate, Folin–Ciocalteu phenol reagent, guaiacol, and Bovine Serum Albumin (BSA) were purchased from Sigma-Aldrich Chemical Co. (USA) and used without further purification. Potato Dextrose Agar (PDA) was purchased from Oxoid Ltd. (Basingstoke, Hampshire, UK).
A culture of Botrytis cinerea (Pers.) (Family: moniliaceae; Class: Deuteromycetes), was provided by Microbiology Laboratory, Department of Plant Pathology, Faculty of Agriculture, Alexandria University, Alexandria, Egypt. Tomato plants (Solanum lycopersicum L. var. lycopersicum) were cultivated in black sheet (10 cm in diameter and 20 cm in depth) that filled with 600 g soil (1:1, clay: sand). The fertilization of plants was carried out every 2 weeks with NPK (20–20–20) by drench the soil. The irrigation was performed every 2 days.
Synthesis of N-(aryl) chitosans and their quaternary derivatives
The N-(aryl) chitosan derivatives were firstly obtained by a reductive amination reaction according to previously reported procedure (Scheme 1) described by Sajomsang et al. (2008). 18 mmol chitosan (3.0 g calculated as glucosamine unit) was dissolved in 1 % (v/v) aqueous acetic acid. One equivalent (calculated as glucosamine unit) of an aromatic aldehyde (cinnamaldehyde, cuminaldehyde and 4-dimethylaminobenzaldehyde) was dissolved in methanol and added dropwise to the chitosan solution at room temperature. After 1 h of stirring at room temperature, the pH of the solution was adjusted to 4.5 by NaOH solution (1 M). To this solution, sodium borohydride (NaBH4) (1.5 equivalents to the aldehyde) was added, and the solution was stirred for 1.5 h. The precipitate of the N-(aryl) chitosan derivative was obtained by adjusting the pH to 10. The precipitate was washed with distilled water to neutralize and the unreacted aldehyde was soxhlet-extracted with 1:1 (v/v) ethanol/diethyl ether. The solid residue was then oven-dried overnight at 60 °C giving the N-(aryl) chitosan derivatives.
Synthesis of N-(aryl) and quaternary N-(aryl) chitosan derivatives. DA is a degree of acetylation and Ac is an acetyl group. C1: N-(cinnamyl) chitosan; C2: N-(cuminyl) chitosan; C3: N-(p-dimethylaminobenzyl) chitosan; QCh: N,N,N-(triethyl) chitosan; QC1: N,N,N-(diethyl cuminyl) chitosan; QC2: N,N,N-(diethyl cinnamyl) chitosan and QC3: N,N,N-(diethyl-p-dimethylaminobenzyl) chitosan
For the synthesis of quaternary N-(aryl) chitosan derivatives, the procedure described by (Saxena et al. 2006) was used with some modification as follows: 2.5 % (w/v) of chitosan or N-(aryl) chitosan derivative was dispersed in 42 % methanol/water and the mixture was kept under constant stirring for 1 h at 30 °C. Then 5 % (v/v) ethyl iodide (C2H5I) was added dropwise under constant stirring and the reaction mixture was refluxed at 70 °C overnight. 5 % NaCl (w/v) was added and the reaction was extra stirred for 2 h at 70 °C. Quaternized chitosan derivatives [N,N,N-(diethylaryl) chitosan] was then precipitated and washed several times by acetone and then dried overnight under vacuum at 50 °C (Scheme 1).
Spectroscopic analysis
1H-NMR measurements were performed on a JEOL A-500 NMR Spectrometer (Faculty of Science, Alexandria University, Alexandria, Egypt) under a static magnetic field of 500 MHz at 25 °C. Chitosan compounds were dissolved in 1 % CD3COOD/D2O solution and were introduced into 5 mm Φ NMR tubes, and finally the tubes were kept at room temperature to dissolve the polymer. IR spectra were recorded with KBr discs in the range of 4,000–400 cm−1 with resolution of 4.0 cm−1 on Perkin Elmer FT-IR Spectrophotometer (Faculty of Pharmacy, Alexandria University, Alexandria, Egypt). UV–Vis absorption spectra of the chitosan compounds were recorded on Alpha-1502 UV–Visible Spectrophotometer (LAXCO, Inc., Bothell, WA, USA) in 0.5 % (v/v) aqueous acetic acid solution at the range of 200–340 nm. A quartz cell path length of 1 cm was employed.
1H-NMR and IR spectral data
Spectral data of chitosan
1 H-NMR: δ 2.09–2.12 (br s, NHCOCH 3 ), 3.15–3.30 (br m, H-2 of GlcN residue), 3.57–4.10 (br m, H-3,4,5,6 of GlcN unit and H-2,3,4,5,6 of GlcNAc unit), 4.88–5.00 (m, H-1 of GlcN and GlcNAc units). IR (KBr): ν 3,400–3,460 cm−1 (br, OH/NH), 2,855–2,930 cm−1 (m, C–H of CH2 and CH3 groups), 1,656 cm−1 (>C=O stretching, amide I) and 1,564 cm−1 (NH2 stretching, amide II), 1,380–1,420 cm−1 (C–H stretching of methyl groups in GlcNAc units), 944–1,150 cm−1 assigned to the saccharide structure.
Spectral data of N-(cinnamyl) chitosan (C1)
1 H-NMR: δ 2.03–2.14 (br s, NHAc), 3.04–3.28 (br s, H-2 of GlcN residue), 3.40–3.51 (CH 2 –NH), 3.62–4.20 (br m, H-2 of GlcNAc and H-3,4,5,6 of GlcN unit), 4.51–4.63 (br s, H-1 of GlcNAc residue), 4.79–4.95 (br s, H-1 of GlcN residue), 6.82–6.95 (br, CH=CH), 7.55–7.64 (br, CH=CH), 7.80–7.85 (br m, 3H of the phenyl ring) and 8.20–8.27 (br m, 2H of the phenyl ring). IR (KBr): ν 3,400–3,450 cm−1 (br, OH/NH), 2,800–2,950 cm−1 (m, C–H of CH2 and CH3 groups), 1,654 cm−1 (>C=O stretching, amide I) and 1,580 cm−1 (NH2 stretching, amide II), 1,370–1,410 cm−1 (C–H stretching of methyl groups in GlcNAc units) and 900–1,150 cm−1 assigned to the saccharide structure.
Spectral data of N-(cuminyl) chitosan (C2)
1 H-NMR: δ 1.32–1.36 (m, (CH3)2 on the phenyl ring), 2.07–2.10 (s, NHAc), 3.05–3.15 (br s, H-2 of GlcN residue), 3.40–3.51 (CH 2 –NH), 3.60–4.10 (br m, H-2 of GlcNAc and H-3,4,5,6 of GlcN unit), 4.51–4.63 (br s, H-1 of GlcNAc residue), 4.79–4.95 (br s, H-1 of GlcN residue) and 7.43 (s, 4H of the phenyl ring). IR (KBr): ν 3,400–3,500 cm−1 (br, OH/NH), 2,800–2,950 cm−1 (m, C–H of CH2 and CH3 groups), 1,628 cm−1 (>C=O stretching, amide I), 1,550 cm−1 (NH2 stretching, amide II), 1,380–1,430 cm−1 (C–H stretching of methyl groups in GlcNAc units) and 1,000–1,250 cm−1 assigned to the saccharide structure.
Spectral data for N-(p-dimethylaminobenzyl) chitosan (C3)
1 H-NMR: δ 2.03–2.15 (br s, NHCOCH 3 ), 3.12–3.25 (br s, H-2 of GlcN residue), 3.25–3.40 (m, (CH3)2), 3.61–4.15 (br m, H-2 of GlcNAc and H-3,4,5,6 of GlcN unit and NHCH 2), 4.74–4.87 (m, H-1 of GlcN and GlcNAc units), 7.48–7.70 (br m, Ph). IR (KBr): ν 3,400–3,440 cm−1 (br, OH/NH), 2,800–2,900 cm−1 (m, C–H of CH2 and CH3 groups), 1,654 cm−1 (>C=O stretching, amide I), 1,550 cm−1 (NH2 stretching, amide II), 1,370–1,450 cm−1 (C–H stretching of methyl groups in GlcNAc units) and 1,000–1,190 cm−1 assigned to the saccharide structure.
Spectral data of N,N,N-(triethyl) chitosan (QCh)
1 H-NMR: δ 1.36–1.40 (br t, CH3), 2.0.9–2.12 (s, NHCOCH3), 3.15–3.20 (br m, H-2 of GlcN residue), 3.30–3.40 (br s, tertiary N-CH2), 3.76–3.94 (br m, H-3,4,5,6 of GlcN unit and H-2,3,4,5,6 of GlcNAc unit), 4.75 (s, H-1 of GlcN unit) and 4.85–4.94 (m, H-1 of GlcNAc unit). IR (KBr): ν 3,400–3,450 cm−1 (br, OH/NH), 2,860–2,950 cm−1 (m, C–H of CH2 and CH3 groups), 1,633 cm−1 (>C=O stretching, amide I), 1,510 cm−1 (NH2 stretching, amide II), 1,480 cm−1 (tertiary N-(C2H3)3), 1,380 cm−1 (C–H stretching of methyl groups in GlcNAc units) and 1,000–1,150 cm−1 assigned to the saccharide structure.
Spectral data of N,N,N-(diethylcinnamyl) chitosan (QC1)
1H-NMR: δ 1.29 (CH3), 2.03–2.05 (s, NHAc), 3.05–3.15 (br s, H-2 of GlcN residue), 3.49 (CH 2 –NH), 3.60–4.05 (br m, H-2 of GlcNAc and H-3,4,5,6 of GlcN unit), 4.58 (br s, H-1 of GlcNAc residue), 4.71 (br s, H-1 of GlcN residue), 6.20 (br, PhCH=CH), 6.80 (br, PhCH=CH),7.38 (br, 3H of the phenyl ring) and 7.49 (br s, 2H of the phenyl ring). IR (KBr): ν 3,390–3,400 cm−1 (br, OH/NH), 2,900 cm−1 (m, C–H of CH2 and CH3 groups), 1,650 cm−1 (>C=O stretching, amide I), 1,590 cm−1 (NH2 stretching, amide II), 1,475 cm−1 (ethyl group of quaternary –N +–(C2H5)2), 1,385 cm−1 (C–H stretching of methyl groups in GlcNAc units) and 1,100–1,120 cm−1 assigned to the saccharide structure.
Spectral data of N,N,N-(diethylcuminyl) chitosan (QC2)
1 H-NMR: δ 1.23–1.25 (CH3), 1.32–1.36 (m, (CH3)2 on the phenyl ring), 2.07–2.10 (s, NHAc), 3.05–3.15 (br s, H-2 of GlcN residue), 3.40–3.51 (CH 2 –NH), 3.60–4.10 (br m, H-2 of GlcNAc and H-3,4,5,6 of GlcN unit), 4.51–4.63 (br s, H-1 of GlcNAc residue), 4.79–4.95 (br s, H-1 of GlcN residue) and 7.43 (s, 4H of the phenyl ring). IR (KBr): ν 3,390–3,450 cm−1 (br, OH/NH), 2,800–2,950 cm−1 (m, C–H of CH2 and CH3 groups), 1,650 cm−1 (>C=O stretching, amide I), 1,570 cm−1 (NH2 stretching, amide II), 1,457 cm−1 (ethyl group of quaternary –N +–(C2H5)2), 1,380 cm−1 (C–H stretching of methyl groups in GlcNAc units) and 1,000–1,200 cm−1 assigned to the saccharide structure.
Spectral data of N,N,N-(diethyl-p-dimethylaminobenzyl) chitosan (QC3)
1 H-NMR: δ 1.29 (CH3), δ 2.03–2.15 (br s, NHCOCH 3 ), 3.12–3.25 (br s, H-2 of GlcN residue), 3.25–3.40 (m, (CH3)2), 3.61–4.15 (br m, H-2 of GlcNAc and H-3,4,5,6 of GlcN unit and NHCH 2), 4.74–4.87 (m, H-1 of GlcN and GlcNAc units), 7.48–7.70 (br m, Ph). IR (KBr): ν 3,330–3,360 (br, OH/NH), 2,900–2,950 (m, C–H of CH2 and CH3 groups), 1,653 cm−1 (>C=O stretching, amide I), 1,570 cm−1 (NH2 stretching, amide II), 1,458 cm−1 (ethyl group of quaternary -N +-(C2H5)2), 1,378 cm−1 (C–H stretching of methyl groups in GlcNAc units) and 1,000–1,200 cm−1 assigned to the saccharide structure.
Solubility test
The solubility of chitosan and its derivatives was examined in water and aqueous acetic acid solution (0.1, 0.5 and 1 %, v/v). A sample was soaked in each solvent at the concentration of 10 mg/mL and the solubility was checked after standing for 24 h at room temperature (Sugimoto et al. 1998).
The antimicrobial assay of chitosan compounds against B. cinerea
Pathogens and inoculums preparation for bioassays
Botrytis cinerea was obtained by growing the fungus in PDA medium at 36 °C. The spores were harvested from 2-weeks-old PDA culture grown under fluorescent lights in 9-cm diameter Petri dishes at 25 °C. An amount of 5 mL of sterile water was added to a Petri plate culture. The spores were gently dislodged from the surface with a sterile glass rod and the suspension was filtered through three layers of cheesecloth to remove mycelia fragments. The suspension was diluted with sterile water to an absorbance of 0.25 at 425 nm as determined by a Unico 1,200-Spectrophotometer. This suspension contained about 1.0 × 106 conidia/mL (Mlikota Gabler and Smilanick 2001) and was then diluted with sterile distilled water to obtain 1.0 × 105 conidia/mL.
The in vitro assay against mycelial growth
The antifungal activity on the mycelial growth of B. cinerea was tested using mycelia radial growth technique according to El Ghaouth et al. (1992). Chitosan and N-(aryl) chitosan compounds were dissolved in 0.5 % (v/v) aqueous acetic acid and the pH was adjusted to 5.5–6.0 with 1 M NaOH, whereas quaternary N-(aryl) chitosan derivatives dissolved in water. Different concentrations of chitosan compounds ranged from 500 to 4,000 mg/L were, respectively, added to sterilized PDA medium immediately before pouring into the Petri dishes. Each concentration was tested in triplicate. Parallel controls were maintained with water and aqueous acetic acid (0.5 %, v/v) mixed with PDA medium. The discs of mycelial culture (0.5 cm diameter) of fungi, taken from 8-day-old culture on PDA plates, were transferred aseptically to the centre of the Petri dishes. The plates were incubated in the dark at 26 °C. The colony growth diameter was measured when the fungal growth in the control had completely covered the Petri dishes. Inhibition percentage of mycelial growth was calculated as follows:
where DC and DT are average diameters of fungal colonies of control and treatment, respectively. The concentration inhibited 50 % of the fungal mycelial growth (EC50) and its corresponding 95 % confidence limits were estimated by probit analysis (Finney 1971).
The in vitro assay against spore germination
Aliquots of 50 μL of spore suspension were placed in eppendorf tubes containing 500 μL of potato dextrose broth (PDB) medium with chitosan concentrations ranged from 250 to 1,000 mg/L. The tests were performed at concentrations ranged from 250 to 1,000 mg/L. The tubes were incubated at 25 °C during 16 h. The samples were placed on both chambers of a hemocytometer by carefully touching the edges of cover slip with the pipette tip and allowed capillary action to fill the counting chambers and observed under the microscope for spore germination. Spore counting was done using a Neubauer haemocytometer and light microscopy at 40x. All experiments were conducted in four replicates. A spore was considered germinated when the length of the germ tube equaled or exceeded the length of the spore (Griffin 1994).
The in vivo assay of chitosan in tomato leaves
The in vivo activity of chitosan compounds against B. cinerea on tomato plants were evaluated as described by (Meziane et al. 2005). The conidial suspension was diluted in a solution of 0.01 M glucose and 6.7 mM KH2PO4 to a final concentration of 1.0 × 105 conidia/mL. Tomato plants were grown in plastic pots under greenhouse conditions (24 °C with a 16 h photoperiod and high humidity). Chitosan compounds at concentrations of 250, 500 and 1,000 mg/L were applied with spraying to run off. The control plants were sprayed with water and 0.5 % (v/v) aqueous acetic acid. After 1 day drying, tertiary leaves of tomato plants were artificially inoculated with B. cinerea isolate by the detached leaf method as described by (Audenaert et al. 2002). Each tomato leaf was inoculated with ten droplets of 10 μL of conidial suspension (1.0 × 105 conidia/mL). Treated leaves were put in 200 mm × 130 mm × 50 mm plastic boxes with sterile water to maintain a high relative humidity (~95 %). Disease incidence (%) was evaluated 2 days later by recording numbers of spreading and non-spreading lesions as follows:
Biochemical studies
Preparation of enzyme and protein extracts
One gram of leaf tissue was ground in 2 mL of enzyme extraction buffer with glass beads by mortar and pestle chilled on ice. The extraction buffer consisted of 0.5 % (w/v) PVP, 3 mM disodium EDTA, and 0.1 M potassium phosphate buffer, pH 7.5. The homogenate was centrifuged at 13,000 rpm for 10 min at 4 °C and was used as the crude enzyme extract (Horii et al. 2007). The supernatant was used in protein determination and as the crude enzyme extract. Biochemical constituents were determined, after 48 h of inoculation. The final supernatant was subjected to protein and enzymatic assays. All of the experiments were done in triplicate. Protein concentrations were determined by (Lowry et al. 1951) method. A protein standard curve was generated using BSA.
Total soluble phenolics assay
Total phenolic content in leave tissues was determined according to (McCue et al. 2000). Briefly, 100 mg of leaf tissue was taken after 2 days of inoculation then was placed in 2.5 mL of 95 % ethanol and frozen for 48–72 h. Samples were homogenized with a Tissue Tearor and centrifuged at 10,000 rpm for 10 min. One milliliter of the resulting supernatant was combined with 1 mL 95 % ethanol, 5 mL distilled water and 0.5 mL of 50 % Folin–Ciocalteu phenol reagent. After a 5 min incubation period at room temperature, 1 mL of 5 % (w/v) sodium carbonate was added followed by brief vortexing to mix. The reaction mixture was incubated for 1 h in a dark-cupboard. After briefly vortexing, the absorbance at 725 nm was determined by spectrophotometer (Unico 1,200-Spectrophotometer, USA). A standard curve was established using a gallic acid in 95 % ethanol. Total phenolics content was standardized against gallic acid and absorbance values were converted to μg of phenolics per gram of fresh weight tissue. Each value reported is the average of three replicate assays of three separate samples for each replicate.
Guaiacol peroxidase assay
Guaiacol peroxidase was determined according to the method described by (McCue et al. 2000). Guaiacol peroxidase polymerizes mono-phenols into di- and polyphenolics for use in lignification during plant growth. The formation of tetraguaiacol was followed at k = 470 nm. The enzyme reaction mixture consisted of 0.1 M pH 6.8 potassium phosphate buffer, 50 mM guaiacol, and 0.2 mM hydrogen peroxide. To 1 mL of the above reaction mixture was added 50 µL of the crude enzyme extract. At a wavelength of 470 nm, the increase in absorbance (i.e. production) of tetraguaiacol was assayed over a period of 5 min. The rate of change in absorbance obtained was then used to quantify the enzyme activity in the mixture using the extinction co-efficient of tetraguaiacol (the oxidized product). The millimolar extinction coefficient of tetraguaiacol at 470 nm is 26.6 mM−1 cm−1. Guaiacol peroxidase activity was reported as nmol tetraguaiacol/min/mg protein.
Statistical analysis
Statistical analysis was performed using the SPSS 17.0 software program (Statistical Package for Social Sciences, USA). The log dose–response curves allowed determination of the EC50 values for the fungal bioassay according to the probit analysis (Finney 1971). The 95 % confidence limits for the range of EC50 values were determined by the least-square regression analysis of the relative growth rate (% control) against the logarithm of the compound concentration. The data of the in vivo experiments were analyzed by one-way analysis of variance (ANOVA). Mean separations were performed by Student–Newman–Keuls (SNK) test and differences at p < 0.05 were considered as significant.
Results and discussion
Chemical characterization of chitosan derivatives
The N-(aryl) chitosan derivatives were synthesized by reductive amination of chitosan with three aromatic aldehydes (Scheme 1). Reductive amination reaction is a versatile and specific method for creating a covalent bond between aldehyde and the amino group. In the present work, chitosan was reacted with cinnamaldehyde, cuminaldehyde and 4-dimethylaminobenzaldehyde to produce the corresponding Schiff base intermediate which was then reduced with sodium borohydride to produce N-(cinnamyl) chitosan (C1) N-(cuminyl) chitosan (C2) and N-(p-dimethylaminobenzyl) chitosan (C3), respectively. The formation of quaternary ammonium salt into the chitosan and N-(aryl) chitosan derivatives backbone was carried out by nucleophilic substitution of chitosan amine protons with ethyl groups of ethyl iodide leading to water-soluble chitosan compounds [N,N,N-(triethyl) chitosan (QCh), N,N,N-(diethylcinnamyl) chitosan (QC1), N,N,N-(diethylcuminyl) chitosan (QC2) and N,N,N-(diethyl-p-dimethylaminobenzyl) chitosan (QC3)] as shown in Scheme 1. Finally, the counterion I− was exchanged in the presence of sodium chloride in a methanol/water medium with Cl− to obtain N,N,N-(diethylaryl) chitosans chloride having higher solubility than the iodide counterpart.
The chemical structure of the compounds, DDA, degree of substitution (DS) and degree of qutarnization (DQ) were estimated by 1H-NMR spectra in according to the method of Hirai et al. (1991) and Sashiwa and Shigemasa (1999) and the data are presented in Table 1. DDA was calculated to be 0.91 in chitosan from the integral ratio between the δ 3.20 ppm (x) that attributed to H-2 of GlcN unit vs 3.40–4.40 ppm (6 − x) which attributed to H-3,4,5,6 of GlcN unit and H-2,3,4,5,6 of GlcNAc unit however, the DDA of C1, C2 and C3 derivatives were 0.71, 0.64 and 0.65, respectively. It is important to notice that the aromatic proton signals in the 1H-NMR spectra of the N-(aryl) chitosan derivatives were selected to calculate the DS because it did not overlap with the proton resonances of GlcN (Fig. 1). It was based on the ratio between the areas of the protons in the phenyl ring and the protons of the pyranose unit (Rabea et al. Rabea, Badawy, Steurbaut and Stevens 2009). In this paper, the DQ was calculated between the integral area of one hydrogen from the methyl protons in –[N +(C2H5)2R] at the range of δ 1.30–1.50 ppm and the integral area of one hydrogen from the protons of H-1,2,3,4,5 and 6 of GlcN unit in the range of δ 3.15–4.20 ppm in 1H-NMR spectra of the quaternary ammonium chitosan compounds as shown in Eq. 1 (Sajomsang et al. 2010).
where DQ is the degree of quaternization, A is the peak area of methyl protons in –[N +(C2H5)2R] at the range of δ 1.30–1.50 ppm, B is the peak area of H-2 of GlcNAc and H-3,4,5,6 of GlcN. It was found that QCh, QC1, QC2 and QC3 were prepared with DQ of 0.21, 0.23, 0.16 and 0.13, respectively (Table 1).
Further evidence for confirmation the chemical structures of the synthesized compounds was also obtained from the FT-IR spectroscopy. The spectral data of the quaternized derivatives were similar to those of their corresponding N-substituted aryl precursors except for the presence of absorption band around 1,457–1,480 cm−1 due to C H symmetric bending of the methyl groups on the quaternary ammonium moieties (Fig. 2). The characteristic absorption bands at 3,427, 2,920, and 2,855 cm−1 represent the presence of OH group, CH2 and CH3 groups, respectively. The amino group has a characteristic absorption band in the region of 3,250–3,500 cm−1, which must have been masked by the absorption band due to the OH group (Shanmugasundaram et al. 2001). The >C=O stretching (amide I) peak at 1,656 cm−1 representing the structure of GlcNAc, as well as the NH2 stretching (amide II) peak at 1,564 cm−1 representing the GlcN functional group, appeared in the spectrum of chitosan powder. A region of 1,375–1,414 cm−1 representing the structure of methyl groups in GlcNAc units. In addition, the absorption band in the region of 890–1,150 cm−1 assigned to the saccharide structure. Absorption peak of the amino group at 1,560–1,575 cm−1 considerably decreased due to the reaction of amino group with aldehydes and ethyl iodide, and a new peak by the methyl groups appeared at about 1,450–1,480 cm−1 (Badawy 2010; Jia et al. 2001). These results demonstrate that the quaternized chitosan derivatives were obtained.
The solubility of chitosan derivatives was evaluated in water and aqueous acetic acid solutions (0.1, 0.5 and 1 %, v/v) and the data are shown in Table 2. Chitosan and N-(aryl) chitosan compounds could not dissolve in water. C1, C2 and C3 were showed swelling in diluted acetic acid (0.1 %), whereas they formed gel in 0.5 % and were soluble in 1 % aqueous acetic acid. However, the quaternary chitosan derivatives QCh, QC1, QC2 and QC4 were soluble in water and diluted acetic acid (0.1, 0.5 and 1 %). It can be noticed that with a quaternization process, the derivatives turn water soluble, owing to the presence of positively charged groups imparting polycation character to the chain.
UV–Visible spectral analysis
UV–Visible spectra of chitosan, N-(aryl) chitosan derivatives and their quaternized derivatives are displayed in Figs. 3 and 4, respectively. As seen in Fig. 3, chitosan itself is transparent in the UV and visible region, and so its conformation is hard to characterize by spectroscopy methods. However, we can overcome this natural handicap by borrowing chromophore from extrinsic molecule. The UV spectra of the N-(aryl) chitosan derivatives solution showed a broad absorption bands between 200 and 280 nm due to the presence of aromatic ring and the maximum absorption peaks (λmax) were found at 250, 210 and 212 for C1, C2 and C3, respectively (Fig. 3). However, the maximum absorption peaks for quaternary chitosan derivatives were found at 227, 252, 225 and 227 nm for QCh, QC1, QC2 and QC3, respectively (Fig. 4). While the chitosan solution had no absorption peaks from 205 to 340 nm (Fig. 3). It can be notice that the quaternization of chitosan derivatives showed shifting in λmax to a higher value. The chitosan derivative was expected to be sensitive to the light at ≤280 nm in the emissions from the sun light and possibly used as UV protection materials.
The in vitro antifungal activity of chitosan derivatives
The effect on mycelia growth
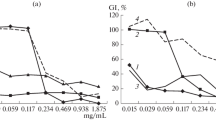
The in vitro antifungal activity of chitosan and its derivatives was analyzed by mycelia radial growth inhibition technique against B. cinerea and the data are presented in Table 3. It was found that all chitosan derivatives yield high antifungal activity (about twofold) in comparison with the unmodified chitosan molecule. Compared with the antifungal activity of chitosan (EC50 = 2,960 mg/L), the N-(aryl) chitosan derivatives (C1, C2 and C3) have good antifungal activity, and the EC50 values are 1,520, 1,673 and 1,786 mg/L, respectively. In comparison to N-(cinnamyl) chitosan, both of C2 and C3 were not significantly different in fungal growth inhibition. It can be notice that the antifungal activity was enhanced with conversion of the derivatives to quaternary ammonium as water soluble compounds. This is clear in the case of QCh with EC50 1,428 mg/L compared to 2,960 mg/L for chitosan. In addition, QC1 and QC3 exerted prominent antifungal activity with EC50 1,147 and 1,325 mg/L against B. cinerea, respectively.
The effect on spore germination
The effect of chitosan derivatives on spore germination of B. cinerea was examined and the data are presented in Table 4 as the concentration causing 50 % inhibition in spore germination (EC50) with its 95 % confidence limits. As can be seen that the spore germination was significantly affected and all the derivatives showed promising inhibition (The EC50 ranged from 334 to 606 mg/L) compared to the unmodified chitosan (EC50 = 859 mg/L). As shown in the antifungal activity against mycelia growth, the conversion of the N-(aryl) chitosan derivatives to quaternary ammonium aryl form led to enhance the activity. QC2 and QC3 showed to be the most active compounds against spores (EC50 = 396 and 334 mg/L, respectively).
The in vivo antifungal activity of chitosan derivatives in tomato leaves
Table 5 shows the in vivo antifungal activities of chitosan compounds on tomato leaves against B. cinerea as disease incidence (%). The chitosan compounds inhibited fungal decay when used at concentrations of 250, 500 and 1,000 mg/L after 48 h of artificial inoculation. The number of B. cinerea spreading lesions was significantly reduced on leaves from the plants treated with chitosans after inoculation compared to the control. A significant effect on the B. cinerea infection was observed at the highest concentration (1,000 mg/L). The results also indicate that the conversion of the N-(aryl) chitosan derivatives to quaternary ammonium form as water-soluble compounds led to enhance the activity and QC1 and QC3 were the most active compounds. No disease incidence (0.0 %) was observed with QC1 and QC3 at 1,000 mg/L. However, the unmodified chitosan was the lowest active one which showed 90.0, 46.67 and 30.0 % disease incidence at 250, 500 and 1,000 mg/L, respectively.
Influence of chitosan treatments on total phenolic contents and guaiacol peroxidase activity in tomato plant leaves
The results showed that total phenolics content in leaf extracts of tomato plants treated with chitosan compounds after 48 h of artificial inoculation by B. cinerea was observed to correlate with concentration as shown in Fig. 5. The lowest value of phenolics content was found in the untreated leaves (11.86 μg of phenolics/g fresh weight tissue in control acid). The tested chitosan compounds at 250, 500 and 1,000 mg/L significantly induced the phenolics content. The phenolics content in all treatments was significantly induced to the highest value with the highest concentration (1,000 mg/L). Quaternary ammoniums of N-(aryl) chitosan derivatives were more effective than N-(aryl) chitosan derivatives and the treatment with QC2 and QC3 induced high phenolics content (21.69 and 23.28 μg of phenolics/g fresh weight tissue at 1,000 mg/L, respectively).
Effect of various concentrations of chitosan compounds on total soluble phenolic content in tomato plant leaves after 48 h of treatment and inoculation with B. cinerea. Ch: chitosan; C1: N-(cinnamyl) chitosan; C2: N-(cuminyl) chitosan; C3: N-(p-dimethylaminobenzyl) chitosan; QCh: N,N,N-(triethyl) chitosan; QC1: N,N,N-(diethylcinnamyl) chitosan; QC2: N,N,N-(diethylcuminyl) chitosan and QC3: N,N,N-(diethyl-p-dimethylaminobenzyl) chitosan
Guaiacol peroxidase activity in leaf extracts of tomato plants treated with 250, 500 and 1,000 mg/L of chitosan compound after 48 h of artificial inoculation by B. cinerea was observed to correlate with concentration as shown in Fig. 6. Lower levels of guaiacol peroxidase activity were observed in the untreated leaves plants (714.97 and 645.96 nmol tetraguaiacol/min/mg protein in controls water and aqueous acetic acid, respectively). Chitosan compounds significantly promoted guaiacol peroxidase activity and high effect was observed at high concentration (1,000 mg/L). Quaternary ammonium of N-(aryl) chitosan derivatives was also more effective than N-(aryl) chitosan derivatives and QCh induced a high guaiacol peroxidase activity (1,123, 1,410 and 1,668 nmol tetraguaiacol/min/mg protein at 250, 500 and 1,000 mg/L, respectively).
Effect of various concentrations of chitosan compounds on Guaiacol peroxidase activity in tomato plant leaves after 48 h of treatment and inoculation with B. cinerea. Ch: chitosan; C1: N-(cinnamyl) chitosan; C2: N-(cuminyl) chitosan; C3: N-(p-dimethylaminobenzyl) chitosan; QCh: N,N,N-(triethyl) chitosan; QC1: N,N,N-(diethylcinnamyl) chitosan; QC2: N,N,N-(diethylcuminyl) chitosan and QC3: N,N,N-(dieth)
The use of natural compounds to control plant pathogens may lead to reduction in using of synthetic harmful pesticides. Among natural elicitor compounds, chitosan offers a great potential as antimicrobial agent. Unmodified chitosan molecule has a moderate antimicrobial activity against plant pathogens. According to this fact, several research groups have started to modify chitosan to produce high antimicrobial active compounds. For example, we have prepared in our laboratory some of hydrophobic chitosan derivatives through the reductive amination reaction with various aldehydes. We noted that the N-alkylation or -arylation of chitosan with aliphatic or aromatic aldehydes, respectively, effectively enhanced the antifungal activity of chitosan against B. cinerea, Fusarium oxysporum and Pythium debaryanum (Badawy 2008; Rabea et al. 2006, 2009). However, N,N,N-(dimethylalkyl) chitosans as water soluble derivatives that recently prepared in our laboratory enhanced the antibacterial activity against Agrobacterium tumefaciens and Erwinia carotovora and N,N,N-(dimethylpentyl) chitosan was the most active with MIC 750 and 1,225 mg/L, respectively. However, both of N,N,N-(dimethylpentyl) chitosan and N,N,N-(dimethyloctyl) chitosan were significantly the highest in fungal mycelial growth inhibition of B. cinerea, F. oxysporum and P. debaryanum (Badawy 2010). These results are in agreement with the present study that indicates that all quaternary ammonium chitosan have better antimicrobial activity than N-(aryl) chitosan compounds.
The activity of chitosan has been explained as being based on the electrostatic interaction of the charged amino groups of chitosan with the negatively charged cell wall surface of the targeted microorganisms, which can led to the disruption of the cell wall and therefore to the death of the microbial cell (Roller and Covill 1999). Another possibility for antifungal activity of chitosan accounts for its chains to cross the cell membrane inhibiting the cell growing from inside (Guo et al. 2007). In the present work, chitosan compounds having aryl substituents as resulting in a hydrophopic molecule, the mechanism on B. cinerea may be occurs by the interaction with cell surface as the result of forming an impermeable layer around the cell, thus blocking the transport of essential solutes into the cell. However, the modification of chitosan molecule by introducing permanently charged quaternary groups could improve the antifungal activity (Eaton et al. 2008; Sajomsang et al. 2012). Similarly, Jia et al. (2001) and (Sajomsang et al. 2009) reported that quaternary ammonium derivatives of chitosan had better antibacterial activities against Escherichia coli (Gram-negative) and Staphylococcus aureus (Gram-positive) bacteria than chitosan, which was attributed to the high charge density of quaternized chitosan. We propose that the antifungal activity of the quaternized chitosan derivatives could also be caused by the cation groups in these quaternized derivatives. As shown in Scheme 1, the cationic charge of quaternized chitosan is higher than that of chitosan and N-substituted chitosan and thus has the best antifungal activity. Regarding to the chemical substituent on the chitosan molecule, the presence of the N-(cinnamyl) showed the higher activity (EC50 = 1,530 mg/L) than N-(cuminyl) chitosan and N-(p-dimethylaminobenzyl) against B. cinerea. Previously, we have reported that the MIC values of the N-(cinnamyl) chitosan derivatives against A. tumefaciens and E. carotovora ranged from 1,025 to 2,900 mg/L (Badawy and Rabea 2013). We also reported that the antifungal activity of these derivatives against Alternaria alternata, B. cinerea, Botryodiplodia theobromae, F. oxysporum, F. solani, P. debaryanum and Phytophthora infestans and the presence of the N-(o-methoxycinnamyl) substituent on chitosan backbone showed the highest antifungal activity among the tested compounds against the airborne fungi A. alternata, B. cinerea, Bd. theobromae and Ph. infestans (EC50 = 672, 796, 980 and 636 mg/L, respectively). However, N-(p-N-dimethylaminocinnamyl) chitosan was the most active against the soil born fungi F. oxysporum, F. solani and P. debaryanum (EC50 = 411, 566 and 404 mg/L, respectively).
Chitosan has the ability to inhibit the development of gray mould caused by B. cinerea in tomato plants in the present study. The result is in agreement with El Ghaouth et al. (1992)who reported that chitosan reduces decay incidence, mainly caused by B. cinerea in strawberry. Liu et al. (2007) reported that chitosan was effective in controlling gray mould and blue mould in tomato fruit stored at 25 and 2 °C, indicating that storage temperature had little influence on the control effects of chitosan on postharvest diseases of tomato fruit. Badawy and Rabea (2009) added that different molecular weights of chitosan significantly reduced fungal decay of B. cinerea in tomato fruit and all compounds (0.5 × 104, 3.7 × 104, 5.7 × 104 and 2.9 × 105 g/mol) at 2,000 and 4,000 mg/L showed complete control of the fungus in wound-inoculated fruit. Chitosan of 5.7 × 104 g/mol also completely inhibited the development of gray mould caused by B. cinerea on potato plants at 500 and 1,000 mg/L after 48 h of the treatment (Rabea and Badawy 2012). Reglinski et al. (2005) added that the germination of B. cinerea was completely inhibited in malt extract broth containing chitosan at concentrations greater than 125 mg/L. As a foliar treatment, chitosan has been reported to enhance disease resistance against many fungal diseases when applied as either a pre or postharvest treatments. It protected detached Chardonnay leaves against B. cinerea and reduced lesion diameter compared with untreated controls (Reglinski et al. 2005).
Besides its antifungal activity, chitosan also has a potential for inducing defense-related enzymes and phenolic contents in plants (Bautista-Baños et al. 2006; Benhamou 1996). Our study indicated that the total soluble phenolic compounds and guaiacol peroxidase in chitosan-treated tomato plants were increased significantly with increase of the concentrations. This result is in agreement with Benhamou and Thériault (1992) and Liu et al. (2007), who reported that the production of phenolic compounds was induced in tomato plants and fruit after treatment with chitosan.
Antioxidant activity is an important biochemical parameter due to its ability to show whether the phenolics present are antioxidant in nature or are being used for lignification purposes (Randhir et al. 2002). Antioxidants have the ability to intercept and neutralize the effects of destructive oxygen free radicals produced during normal cellular metabolism. In the current study, it was found that the activity of guaiacol peroxidase was highest after 2nd day, correlating with the data showing the highest levels of the total phenolics (Figs. 5, 6). This may be attributable to the higher demand for oxygen during the growth, requiring higher levels of antioxidant phenolics to protect the cells from potential oxidative damage. Guaiacol peroxidase in an enzyme that catalyses the transformation of the phenolics produced through the phenylpropanoid pathway to lignin and lignans (Morales and Barcelo 1997). An increase in guaiacol peroxidase activity during the progression of plant growth indicates the need for phenolics to be used in lignification and structural development during their growth. Results suggest that chitosan controlled grey mold B. cinerea infection and increased the resistance in plants by stimulating the accumulation of phenolics and then lignin. Thus, chitosan compounds have a potential for protecting of the tomato plant and then enhancement of crop yields.
Conclusion
There are growing environmental problems caused by fungicides, especially chemical products. It is imperative under this situation to explore new chemical fungicides, which not only can control the pathogenic diseases of crop but which are also biodegradable and environmentally friendly. The synthesis and characterization of aryl chitosans and their quaternary derivatives were carried out and their antifungal activities were evaluated in vitro and in vivo against B. cinerea. The results have shown that the antifungal activity of chitosan can be improved by substitution with aryl moieties and increasing their water solubility by quternization with ethyl iodide on the polymer chain. The fungal inhibition for both synthesized series N-(aryl) chitosans and quaternary ammonium of N-(aryl) chitosans was increased and both of N,N,N-(diethylcinnamyl) chitosan (QC1) and N,N,N-(diethyl-p-dimethylaminobenzyl) chitosan (QC3) were the most substituted derivatives exhibiting an inhibition value about three times higher than those obtained with chitosan. The synthesis and characterization of chitosan derivatives having biologically active chains are ongoing in our laboratory aiming to obtain derivatives with higher antifungal activities. This study suggests that the use of chitosan could be a promising handling as a natural antimicrobial agent to partially substitute for the harmful synthetic microbicides in tomato plants.
References
Audenaert K, De Meyer G, Hofte M (2002) Abscisic acid determines basal susceptibility of tomato to Botrytis cinerea and suppresses salicylic acid dependent signaling mechanisms. Plant Physiol 128:491–501
Badawy MEI (2008) Chemical modification of chitosan: synthesis and biological activity of new heterocyclic chitosan derivatives. Polym Int 57:254–261
Badawy MEI (2010) Structure and antimicrobial activity relationship of quaternary N-alkyl chitosan derivatives against some plant pathogens. J Appl Polym Sci 117:960–969
Badawy MEI, Rabea EI (2009) Potential of the biopolymer chitosan with different molecular weights to control postharvest gray mold of tomato fruit. Postharvest Biol Technol 51:110–117
Badawy MEI, Rabea EI (2011) A biopolymer chitosan and its derivatives as promising antimicrobial agents against plant pathogens and their applications in crop protection. Int J Carbohydr Chem ID 460381. doi:10.1155/2011/460381
Badawy MEI, Rabea EI (2012) Characterization and antimicrobial activity of water-soluble N-(4-carboxybutyroyl) chitosans against some plant pathogenic bacteria and fungi. Carbohydr Polym 87:250–256
Badawy MEI, Rabea EI (2013) Synthesis and structure-activity relationship of N-(cinnamyl) chitosan analogs as antimicrobial agents. Int J Biol Macromol 57:185–192
Bautista-Baños AN, Hernández-Lauzardo MG, Velázquez-del Valle M, Hernández-López E, Ait Barka E, Bosquez M, Wilson CL (2006) Chitosan as a potential natural compound to control pre and postharvest diseases of horticultural commodities. Crop Prot 25:108–118
Benhamou N (1996) Elicitor-induced plant defence pathways. Trends Plant Sci 1:233–240
Benhamou N, Thériault G (1992) Treatment with chitosan enhances resistance of tomato plants to the crown and root pathogen Fusarium oxysporum f. sp. radicis-lycopersici. Physiol Mol Plant Pathol 41:33–52
Carson RL (1962) Silent spring. Riverside Press, Cambridge
Eaton P, Fernandes JC, Pereira E, Pintado ME, Malcata FX (2008) Atomic force microscopy study of the antibacterial effects of chitosans on Escherichia coli and Staphylococcus aureus. Ultramicroscopy 108:1128–1134
El Ghaouth A, Arul J, Grenier J, Asselin A (1992) Antifungal activity of chitosan on two post-harvest pathogens of strawberry fruits. Phytopathology 82:398–402
El Ghaouth A, Arul J, Grenier J, Benhamou N, Asselin A, Belanger R (1994) Effect of chitosan on cucumber plants: suppression of Pythium aphanidermatum and induction of defense reactions. Phytopathology 84:313–320
El Hadrami A, Lorne R, Adam LR, El Hadrami I, Daayf F (2010) Chitosan in plant protection. Mar Drugs 8:968–987
Finney DJ (1971) Probit analysis, 3rd edn. Cambridge University Press, Cambridge
Grant S, Blair H, Mckay G (1988) Water-soluble derivatives of chitosan. Polym Comm 29:342–344
Griffin DH (1994) Fungal physiology, 2nd edn. Wiley, New York
Guo ZY, Xing R, Liu S, Zhong Z, Ji X, Wang L, Li P (2007) The influence of the cationic of quaternized chitosan on antifungal activity. Int J Food Microbiol 118:214–217
Guo Z, Ren J, Dong F, Wang G, Li P (2013) Comparative study of the influence of active groups of chitosan derivatives on antifungal activity. J Appl Polym Sci 127:2553–2556
Guo Z, Li Q, Wang G, Dong F, Zhou H, Zhang J (2014) Synthesis, characterization, and antifungal activity of novel inulin derivatives with chlorinated benzene. Carbohydr Polym 99:469–473
Heras A, Rodriguez NM, Ramos VM, Agullo E (2001) N-methylene phosphonic chitosan: a novel soluble derivative. Carbohydr Polym 44:1–8
Hirai A, Odani H, Nakajima A (1991) Determination of degree of deacetylation of chitosan by 1H-NMR spectroscopy. Polym Bull 26:87–94
Holme KR, Perlin AS (1997) Chitosan N-sulfate. A water-soluble polyelectrolyte. Carbohydr Res 302:7–12
Horii A, McCue P, Shetty K (2007) Seed vigour studies in corn, soybean and tomato in response to fish protein hydrolysates and consequences on phenolic-linked responses. Biores Technol 98:2170–2177
Houeto P, Bindoula G, Hoffman JR (1995) Ethylenebisdithiocarbamates and ethylenethiourea: possible human health hazards. Environ Health Perspect 103:568–573
Jia Z, Shen D, Xu W (2001) Synthesis and antibacterial activities of quaternary ammonium salt of chitosan. Carbohydr Res 333:1–6
Li P, Poon YF, Li WF, Zhu HY, Yeap SH, Cao Y, Qi XB, Zhou CC, Lamrani M, Beuerman RW, Kang E, Mu YG, Li CM, Chang MW, Leong SSJ, Chan-Park MB (2011) A polycationic antimicrobial and biocmpatible hydrogel with microbe membrane suctioning ability. Nat Mater 10:149–156
Liu XF, Guan YL, Yang DZ, Li Z, Yao KD (2001) Antibacterial action of chitosan and carboxymethylated chitosan. J Appl Polym Sci 79:1324–1335
Liu J, Tian SP, Meng XH, Xu Y (2007) Effects of chitosan on control of postharvest diseases and physiological responses of tomato fruit. Postharvest Biol Technol 44:300–306
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
McCue P, Zheng Z, Pinkham JL, Shetty K (2000) A model for pea seedling vigour following low pH and salicylic acid treatments. Proc Biochem 35:603–613
McKeen WE (1974) Mode of penetration of epidermal cell walls of Vicia faba by Botrytis cinerea. Phytopathology 64:461–467
Meziane H, van der Sluis I, Van Loon LC, Höfte M, Bakker PEHM (2005) Determinants of Pseudomonas putida WCS358 involved in inducing systemic resistance in plants. Mol Plant Pathol 6:177–185
Mlikota Gabler F, Smilanick JL (2001) Postharvest control of table grape gray mold on detached berries with carbonate and bicarbonate salts and disinfectants. Am J Enol Vitic 52:12–20
Morales M, Barcelo AR (1997) A basic peroxidase isoenzyme from vacuoles and cell walls of Vitis vinifera. Phytochemistry 45:229–232
Mourya VK, Inamdar NN (2009) Trimethyl chitosan and its applications in drug delivery. J Mater Sci Mat Med 20:1057–1079
Muzzarelli RAA, Tanfani F (1982) N-(o-carboxybenzyl) chitosan, N-carboxymethyl chitosan and chitosan dithiocarbamate. New chelating derivatives of chitosan. Pure Appl Chem 54:2141–2150
Muzzarelli RAA, Tanfani F (1985) The N-permethylation of chitosan and the preparation of N-trimethyl chitosan iodide. Carbohydr Polym 5:297–307
No HK, Meyers SP (1997) Preparation of chitin and chitosan. In: Muzzarelli RAA, Peter MG (eds) Chitin handbook. European Chitin Society, Grottammare, pp 475–489
No HK, Park NY, Lee SH, Hwang HJ, Meyers SP (2002) Antibacterial activities of chitosans and chitosan oligomers with different molecular weights on spoilage bacteria isolated from Tofu. J Food Sci 67:1511–1514
O’Neill TM, Shtienberg D, Elad Y (1997) Effect of some host and microclimate factors on infection of tomato stems by Botrytis cinerea. Plant Dis 81:36–40
Rabea EI, Badawy MEI (2012) Inhibitory effects on microbial growth of Botrytis cinerea and Erwinia carotovora on potato using of a biopolymer chitosan at different molecular weights. Arc Phytopathol Plant Prot 45:1939–1949
Rabea EI, Badawy MET, Stevens CV, Smagghe G, Steurbaut W (2003) Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules 4:1457–1465
Rabea EI, Badawy MEI, Rogge TM, Stevens CV, Steurbaut W, Höfte M, Smagghe G (2006) Enhancement of fungicidal and insecticidal activity by reductive alkylation of chitosan. Pest Manag Sci 62:90–97
Rabea EI, Badawy MEI, Steurbaut W, Stevens CV (2009) In vitro assessment of N-(benzyl) chitosan derivatives against some plant pathogenic bacteria and fungi. Eur Polym J 45:237–245
Ramos VM, Rodriguez NM, Diaz MF, Rodriguez MS, Heras A, Agullo E (2003) N-methylene phosphonic chitosan. Effect of preparation methods on its properties. Carbohydr Polym 52:39–46
Randhir R, Shetty P, Shetty K (2002) L-DOPA and total phenolic stimulation in dark germinated fava bean in response to peptide and phytochemical elicitors. Process Biochem 37:1247–1256
Reglinski T, Elmer PAG, Taylor JT, Parry FJ, Marsden R, Wood PN (2005) Suppression of Botrytis bunch rot in Chardonnay grapevines by induction of host resistance and fungal antagonism. Australas Plant Pathol 34:481–488
Roller S, Covill N (1999) The antifungal properties of chitosan in laboratory media and apple juice. Int J Food Microbiol 47:67–77
Rúnarsson OV, Holappa J, Nevalainen T, Hjalmarsdottir M, Jarvinen T, Loftsson T, Einarsson JM, Jonsdottir S, Valdimarsdottir M, Masson M (2007) Antibacterial activity of methylated chitosan and chitooligomer derivatives: synthesis and structure activity relationships. Euro Polym J 43:2660–2671
Sajomsang W, Tantayanon S, Tangpasuthadol V, Daly WH (2008) Synthesis of methylated chitosan containing aromatic moieties: chemoselectivity and effect on molecular weight. Carbohydr Polym 72:740–750
Sajomsang W, Gonil P, Saesoo S (2009) Synthesis and antibacterial activity of methylated N-(4-N, N-dimethylaminocinnamyl) chitosan chloride. Eur Polym J 45:2319–2328
Sajomsang W, Ruktanonchai UR, Gonil P, Warin C (2010) Quaternization of N-(3-pyridylmethyl) chitosan derivatives: effects of the degree of quaternization, molecular weight and ratio of N-methylpyridinium and N, N, N-trimethyl ammonium moieties on bactericidal activity. Carbohydr Polym 82:1143–1150
Sajomsang W, Gonil P, Saesoo S, Ovatlarnporn C (2012) Antifungal property of quaternized chitosan and its derivatives. Int J Biol Macromol 50:263–269
Sashiwa H, Shigemasa Y (1999) Chemical modification of chitin and chitosan 2: preparation and water soluble property of N-acylated or N-alkylated partially de-acetylated chitins. Carbohydr Polym 39:127–138
Saxena A, Kumar A, Shahi VK (2006) Preparation and characterization of N-methylene phosphonic and quaternized chitosan composite membranes for electrolyte separations. J Colloid Interface Sci 303:484–493
Shanmugasundaram N, Ravichandran P, Neelakanta RP, Nalini R, Subrata P, Panduranga RK (2001) Collagen-chitosan polymeric scaffolds for the in vitro culture of human epidermoid carcinoma cells. Biomaterials 2:1943–1951
Sommer NF, Fortlage RJ, Edwards DC (1992) Postharvest diseases of selected commodities. In Kader AA (ed) Postharvest technology of horticultural crops, University of California Davis Division of Agriculture and Natural Resources, Publication 3311; pp 117–160
Sugimoto M, Morimoto M, Sashiwa H, Saimoto H, Shigemasa Y (1998) Preparation and characterization of water-soluble chitin and chitosan derivatives. Carbohydr Polym 36:49–59
Tripathi P, Dubey NK (2004) Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest Biol Technol 32:235–245
Xia W, Liu P, Zhang J, Chen J (2011) Biological activities of chitosan and chitooligosaccharides. Food Hydr 25:170–179
Zhang X, Gen XD, Jiang HJ, Li JR, Huang JY (2012) Synthesis and characteristics of chitin and chitosan with the [(2-Hydroxy-3-trimethylammonium)propyl] functionality, and evaluation of their antioxidant activity in vitro. Carbohydr Polym 89:486–891
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Badawy, M.E.I., Rabea, E.I. Synthesis and antifungal property of N-(aryl) and quaternary N-(aryl) chitosan derivatives against Botrytis cinerea . Cellulose 21, 3121–3137 (2014). https://doi.org/10.1007/s10570-014-0333-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-014-0333-0