Abstract
Environmentally sensitive poly(N-isopropylacrylamide) (PNIPAAm) nanofibrous scaffolds loaded with a hydrophilic drug were fabricated via an electrospinning process. First, thermally crosslinkable poly(NIPAAm-co-N-methylolacrylamide) (PNN) was synthesized by redox polymerization below the phase transition temperature of PNIPAAm. The phase transition temperature of the PNN copolymer could be altered from 34 to 40 °C by changing the ratio of N-methylolacrylamide (NMA) to NIPAAm. Subsequently, PNN/chitosan nanofibers were electrospun using ethanol/acetic acid/water as a cosolvent. The PNN/chitosan nanofibers were sensitive to both pH and temperature. The fibrous structure of the soaked PNN/chitosan nanofibers was successfully preserved by the crosslinking of NMA. Furthermore, the chitosan-based nanoparticles (NPs) were introduced into the PNN nanofibers (PNN/NPs) to achieve prolonged drug release. The nanoparticles were observed in the PNN nanofibers by transmission electron microscopy. All of the scaffolds examined had high tensile strengths (1.45 MPa or above) and exhibited no significant cytotoxicity toward human fetal skin fibroblasts. Finally, doxycycline hyclate was used as a model drug. The results illustrated that PNN/NPs nanofibrous scaffolds exhibited continuous drug release behavior for up to 1 week, depending on the pH and temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polymer nanofibrous scaffolds have received much attention in tissue engineering as scaffolding materials to restore, maintain, or improve the function of cell transplantation because of their imitative structure and their function as a natural extracellular matrix (ECM) (Piryaei et al. 2011; Liu et al. 2011). The high surface-to-volume ratio of nanofibrous scaffolds and the nanoscale diameter of the fibers provide favorable conditions for cell attachment and growth; cells have been shown to attach to and reorganize around the fibers. Lee et al. (2011) fabricated a nanofibrous scaffold composed of a synthetic biodegradable polymer, poly(epsilon-caprolactone) (PCL) to support the in vitro chondrogenesis of mesenchymal stem cells (MSCs). Li et al. (2005) reported that the PCL nanofibrous scaffolds are a practical carrier for MSC transplantation and a candidate scaffold for cell-based tissue engineering approaches to cartilage repair. Although a scaffold provides a template for tissue regeneration, biological factors such as cells, growth factors or genes are typically required to effectively regenerate challenging bone defects. Therefore, nanofibers loaded with different admixture molecules or drug carriers were studied (Kolambkar et al. 2011; Sahoo et al. 2010). Kolambkar et al. reported that osteoinductive growth factors such as rhBMP-2 demonstrated some clinical success for bone healing, but large doses were needed. Delivery systems that provide sustained release and improved local retention might contribute to efficacy at a lower protein dose, thereby minimizing complications and making the therapy more cost-effective.
Generally, a number of manufacturing processes have been explored to fabricate micro or nanoscale fibers, including drawing (Jayaraman et al. 2004), self-assembly (Hartgerink et al. 2002), template-directed synthesis (Hulteen et al. 1997), phase separation (Ma and Zhang 1999), and electrospinning (Jeong et al. 2011). Among these techniques, electrospinning has emerged as a simple yet versatile technique in manufacturing fibrous scaffolds from a variety of synthetic or natural polymers (Sill and von Recum 2008). The process begins with a polymer solution in a syringe, held at the end of the needle by its surface tension. A high-voltage charge is then induced on the needle by an external electric field. As the applied electric field is increased, the created charges directly oppose the surface tension. At a critical value, these forces cause the formation of a protruding conical shape known as the Taylor cone at the tip of the needle (Reneker and Yarin 2008). Beyond this critical value, the electric field causes the charges to overcome the surface tension, and a charged jet of solution is formed. As this jet travels in the air, it experiences instabilities and follows a spiral path while the solvent evaporates, leaving behind a charged polymer fiber that is deposited onto the collector. The fibrous material can be chosen in accordance with the application of the nanofibers.
Environmentally sensitive polymers have been widely investigated for use in biomedical applications such as drug delivery, sensors, and diagnostics (Caruso et al. 1998). poly(N-isopropylacrylamide) (PNIPAAm) is the best-documented thermo-responsive polymer; it has a phase transition temperature, also known as the lower critical solution temperature (LCST), at approximately 32 °C (Binkert et al. 1991). The transition from a hydrophilic to a hydrophobic structure occurs dramatically at the phase transition temperature (Schild 1992). In contrast, chitosan (CS) is a pH-responsive polymer because of the amino groups along its chain. It can be dissolved in acidic solutions thanks to the protonation of its amino group on the C-2 position of pyranose, and it then becomes a cationic polyelectrolyte with pH sensitivity.
However, the use of hydrophilic polymer nanofibers such as PNIPAAm and chitosan has been hindered by their relatively rapid dissolution in water. There have been a few studies of water-stable PNIPAAm-based nanofibers: PNIPAAm/polystyrene electrospun nanofibers exhibited reversible hydrophilicity and hydrophobicity upon a temperature transition from 20 to 50 °C. This temperature-responsive wettability was greatly enhanced by the surface roughness and surface ratio of the electrospun fibers (Sill and von Recum 2008). Poly(NIPAAm-co-methyl methacrylate) electrospun nanofibers demonstrated thermo-responsive and water-stable properties. Methyl methacrylate was expected to be sufficiently stable in water. When poly(NIPAAm-co-methyl methacrylate) nanofibers they were soaked in water for 4 days, weight losses were in the range of 1–10 % (Zhang and Yarin 2009).
The aim of this study was to develop water-stable PNIPAAm-based nanofibers as drug carriers with both thermal and pH sensitivities. First, the thermally crosslinkable copolymer poly(NIPAAm-co-NMA) (PNN) was synthesized by redox polymerization at room temperature below the LCST of the PNIPAAm. Subsequently, PNN-based nanofibers containing chitosan or chitosan-based nanoparticles (NPs) were fabricated via electrospinning. The morphologies of the PNN/CS and PNN/NPs nanofibers were observed by SEM. Additionally, doxycycline hyclate, an antibiotic used to treat a variety of infections, was used as a model drug and encapsulated into the nanofibers or NPs. The drug release profiles were then studied in several phosphate-buffered saline (PBS) solutions at different temperatures and pH values.
Experimental
Materials
Chitosan (degree of deacetylation 95 %, average molecular weight 2 × 105 Da) was obtained from Kio-Tek (Taipei, Taiwan). N-isopropylacrylamide (NIPAAm), ammonium persulfate (APS), sodium metabisulfite (SMBS) and acetic acid (HAc) were acquired from Acros Organic (Geel, Belgium). N-Methylol acrylamide (NMA, 48 wt%), phosphate-buffered saline (PBS), and doxycycline hyclate were obtained from Sigma-Aldrich (St. Louis, MO, USA). All the other chemicals were of analytical-grade and used without further purification. Cells used for the cytotoxicity test were WS1 human fetal skin fibroblasts (ATCC CRL-1502) obtained from the Bioresource Collection and Research Center (Hsinchu, Taiwan). The fibroblasts were cultured in Eagle’s minimum essential medium (MEM; M 3024, Sigma) supplemented with 10 % fetal bovine serum (FBS; Biological) and 1 % antibiotic antimycotic solution (A 5955, Sigma) in a 5 % CO2/95 % air incubator with saturated humidity at 37 °C.
Synthesis of poly(NIPAAm-co-NMA) (PNN)
To maintain the fibrous structure of the soaked nanofibers, a thermally crosslinkable monomer, NMA, was introduced into the copolymer, poly(NIPAAm-co-NMA) (PNN). First, 50 mmol of NIPAAm and NMA was dissolved in 36 mL of deionized water. The molar ratios of NMA to NIPAAm were 3:97 and 10:90; the solutions with these ratios were labeled PNN3 and PNN10, respectively. Second, APS and SMBS were dissolved separately in 2 mL deionized water. For PNN3 and PNN10-1, 0.6 mmol each of APS and SMBS was added; for PNN10-2, 0.25 mmol of each was added. The APS aqueous solution was mixed well with the monomer solution prior to adding the SMBS solution. Polymerization was carried out at room temperature (25 °C) for 24 h. The resulting solution was taken out for dialysis (3,500 Da molecular weight cut-off) at room temperature for 72 h in deionized water in order to remove the residual initiator and unreacted monomer. During this period, the fresh deionized water was replaced every 12 h. The final dialysis product was dried by lyophilization.
Fabrication of chitosan-based nanoparticles
Chitosan-based nanoparticles (NPs) were prepared by in situ polymerization of NIPAAm monomer in the presence of chitosan micelles, and details were shown in our previous study (Huang et al. 2013). Briefly, NIPAAm monomer (0.33 g) and MBA (0.0134 g) were dissolved in 45 mL chitosan micelle solution containing 0.50 g chitosan and 0.175 g acetic acid. After stirring at 400 rpm overnight, the solution was purged with nitrogen and heated to 70 °C. Potassium persulfate (0.082 g) was dissolved in 5 mL of DI water and pre-heated to 70 °C before it was poured into the solution to initiate the polymerization. After 2 h of reaction, the solution was cooled down to 40 °C, followed by the addition of glutaraldehyde to crosslink the CS shell. After 1 h of crosslinking reaction, the solution was taken out for dialysis (50 kDa molecular weight cut-off) at room temperature for 72 h in DI water in order to remove the residual initiator and un-reacted monomer.
Doxycycline hyclate (10 mg) was dissolved in 40 g dispersion solution of CS-PNIPAAm NPs at pH 4.5 and 25 °C. After incubation for 96 h, the dispersion solution was adjusted to pH 9.0. Drug-loaded NPs were separated from the solution by centrifugation at 12,000 rpm and 20 °C for 5 min. The drug-loaded CS-PNIPAAm NPs were dried by lyophilization and then weighed. Doxycycline hyclate concentration in the supernatant was analyzed by measuring the UV absorbance at the wavelength of 346 nm. The amount of the drug loaded in the NPs thus could be calculated by subtracting the amount of drug in the supernatant from the feed. The drug loading content (%) and encapsulation efficiency (%) were calculated by Eqs. (1) and (2).
Fabrication of nanofibrous scaffolds
Electrospinning
The electrospinning system used in this study consisted of a syringe pump (KD Scientific Model 100, USA), a needle (i.d. = 0.84 mm), a ground electrode (D = 21.5 cm, stainless steel plate), and a high voltage supply (chargemaster CH30P SIMCO, USA). The needle was connected to the high positive voltage supply of 14 kV. The working distance from needle tip to collecting plate was 15 cm. The solution feeding rate was controlled by a syringe pump at rate of 1.0 mL/h. The electrospinning process must keep as a stable cone-jet. The cone-jet tip shape is monitored by CCD camera (Xli 3 M CCD camera, USA) and macro video zoom lens (Optem MVZL, USA) for obtaining uniform fibers. All electrospinnings were carried out at room temperature.
PNN/CS scaffolds
The PNN10 series and PNN10-2/CS nanofibers were fabricated via electrospinning using ethanol/acetic acid/water as a cosolvent, in which the weight ratio of ethanol to acetic acid was 3:1. Specific amounts of PNN and chitosan were dissolved in ethanol and 1 wt% acetic acid, respectively (see Table 1). Subsequently, the two solutions were mixed for 30 min before electrospinning. The electrospinning process is described in detail in “Electrospinning” section. Finally, the thermal crosslinking was carried out in an oven (110 °C) for 5 h.
Drug loaded PNN/CS scaffolds
At the beginning, doxycycline hyclate (11 mg) was dissolved in 1 g of 1 wt% acetic acid aqueous solution, and then chitosan was dissolved in the drug solution. After mixing with PNN solution, the homogeneous solution was electrospun at the same condition as mentioned before. The drug loading content (%) and encapsulation efficiency (%) were calculated by Eqs. (1) and (2).
PNN/NPs scaffolds
The drug-loaded NPs were dispersed in 1 mL neutral deionized aqueous solution; the dispersion was soon added to a PNN10-2/ethanol solution and mixed for 5 min prior to electrospinning (see Table 2). The electrospinning process is described in detail in “Electrospinning” section, and then the electrospun scaffolds were thermally crosslinked at 110 °C for 5 h.
Characterization methods
PNN characterization
The number average molecular weight (Mn) and weight average molecular weight (Mw) of PNNs were characterized by GPC using THF as the mobile phase. The composition of the copolymer was determined from 1H NMR spectrum recorded for polymer solutions in CD3OD.
Phase transition temperature
The phase transition temperature was measured by differential scanning calorimetry DSC (TA Q20 System, USA). The PNN copolymer and chitosan were dissolved in 1 wt% acetic acid solution at the concentration of 2 wt% polymer. In addition, the crosslinked nanofibrous scaffolds were put into DSC cells and wetted by 50 times of water for 24 h. DSC thermograms were obtained at a heating rate of 10 °C/min from 10 to 70 °C. The transition temperature was defined as the onset temperature of transition zone.
Morphology
The scaffolds were mounted on the copper stubs with conductive adhesive tape and sputter-coated with platinum. The morphology and diameter of the nanofibers were observed by scanning electron microscopy (SEM; JOEL JSM-6700F, Japan). Furthermore, the PNN10-2/NPs nanofibers were electrospun on copper grids and observed by transmission electron microscopy (TEM; JOEL JEM-1230, Japan).
Gel fraction and swelling ratio
The weights of the crosslinked scaffolds were measured and noted as Wo. Afterwards, the scaffolds were immersed in water at 25 °C and pH 6.0 for 1 day. The weights of the swollen scaffolds were noted as Ws. Finally, the scaffolds were dried by lyophilization; the weights of the dried scaffolds were noted as Wd. The gel fraction and swelling ratio were calculated by Eqs. (3) and (4).
Mechanical properties
The mechanical properties of the scaffolds were determined using a tensile strength instrument (LRX, Lloyd, Hampshire, UK). The prepared scaffolds were cut into a specific dumbbell shape (6 cm long, 2 cm wide at the ends, and 1 cm wide in the middle), and the thicknesses were measured. The tensile analysis was performed at a stretching rate of 10 mm/min with a pre-load of 0.1 N. The tensile strength and tensile elongation were the maximum stress and maximum elongation before failing or breaking.
Cytotoxicity test
The method of the International Standards Organization (ISO) 10993-5 was followed to test the cytotoxicity of possible substances that could leach from the synthesized polymers. Briefly, the nanofibrous scaffolds (PNN10-2, PNN10-2/CS (88/12), and PNN10-2/NPs-13.6) were sterilized by 75 % ethanol solution for 24 h. After drying, the scaffolds were immersed separately in 40 mL of cell culture medium for 24 h at 37 °C, and then the scaffolds were removed from the medium. Human fetal skin fibroblasts were mixed with the medium containing the extracts from the scaffolds and seeded at a density of 15,000 cells/well in 24-well plates. Control group was treated with normal medium. The cells were incubated for 1–7 days at 37 °C with 5 % CO2. At the end of the exposure time, cell viability was measured (n = 4) using (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) (MTT) assay. Absorbance measured at 540 nm was recorded.
Drug release profiles
The drug-loaded nanofibrous scaffolds were first sectioned into 2 × 2 cm2 squares (0.0233 ± 0.0009 g). Each square sample was immersed in 30 mL of PBS medium. The drug release profile was determined under different conditions, including different temperatures (25 and 50 °C) and pH values (2 and 7). After a predetermined period, 1 mL of the PBS solution was drawn out from the system for analysis, and the solution was replenished with 1 mL fresh medium. The released amount of doxycycline hyclate was determined by UV analysis using a calibration curve. The results of drug-release experiment presented here indicate the means of at least triplicate measurements. The release percentage of the scaffolds was calculated by Eq. (5).
Results and discussion
The aim of this study was to develop an environmentally sensitive nanofibrous scaffold composed of PNN and chitosan as a drug carrier. The water-stable fibrous scaffold was formed by the thermal crosslinking of PNN.
Synthesis of poly(NIPAAm-co-NMA) (PNN)
The NIPAAm and NMA copolymer was synthesized by redox polymerization at room temperature. The composition of the copolymer was determined from the 1H NMR spectra recorded for polymer solutions in CD3OD. As indicated in Fig. 1, the chemical shift from 1.82 to 2.27 ppm (peak d) was attributed to the methylene protons (–CH2CH–) of NMA and NIPAAm. The chemical shifts at 1.15 ppm (a) and 3.95 ppm (b) were associated with NIPAAm (–CH(CH 3 )2) and NIPAAm (–CH(CH3)2), respectively. For NMA, the chemical shift at 4.69 ppm (c) was associated with NMA (–NHCH 2 –OH). Thus, we calculated the actual NMA content by using Eq. (6).
As shown in Table 3, the NMA molar content in each of the PNN copolymers was close to the monomer feeding ratio. In addition, with a decrease in APS and SMBS, the molecular weight (Mw) of PNN10-2 was higher than that of PNN10-1 (Table 3). A high molecular weight polymer, due to increased chain entanglements, could be easily electrospun into nanofibers.
The phase transition temperature of PNN in solution
The aqueous solutions containing 2 wt% uncrosslinked copolymer showed temperature-responsive behavior (Fig. 2). The phase transition temperature of the PNN solution increased due to the increase of NMA content (Fig. 2a). It was reported that the phase transition temperature can be controlled by copolymerizing NIPAAm with a hydrophilic monomer, which raises the phase transition temperature, or hydrophobic monomer, which lowers the phase transition temperature (Lee et al. 2008). Moreover, the interaction between the PNN copolymer and chitosan might affect the transition temperature. As evident in Fig. 2b, the transition temperature decreased with an increase in the chitosan content of the solution. It was suggested that the interaction between the chitosan and PNN, e.g., hydrogen bonding resulted in the decrease of the phase transition temperature.
Electrospinning
Although several polymers have been successfully electrospun by controlling the conditions such as the polymer solution concentration, solvent, pumping rate, tip-to-collector distance and supply voltage (Katti et al. 2004), natural polymers have solubility problems in many solvents; chitosan, for example, can only dissolve in acidic solution. Trifluoroacetic acid is normally chosen as a chitosan solvent for electrospinning (Sencadas et al. 2012). However, doxycycline hyclate is not soluble in trifluoroacetic acid. Song et al. (2008) electrospun gelatin from a water-based cosolvent composed of ethyl acetate and acetic acid in water. Hence an ethanol/acetic acid/water cosolvent was used in this study. The weight ratio of ethanol to 1 wt% acetic acid solution was 3:1 because the phase transition would occur at ratios below 2:1.
Morphology
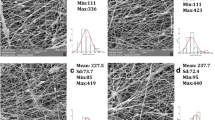
The morphology of the PNN nanofibers is shown in Fig. 3a, b. The nanofibers of the higher-Mw PNN10-2 had a uniform fibrous morphology with an average diameter of 295 ± 70 nm because the elongational strength was high enough with the higher Mw in the PNN10-2 solution. However, the lower-Mw PNN10-1 was materially insufficient to yield a fibrous structure and only sprayed droplets were obtained. Base on this result, a Mw of 33,600 g/mol was used for the further studies of the PNN/CS electrospinning.
The SEM images of the PNN and the PNN/CS nanofibers, fabricated from 2.44 wt% polymer solution under the feeding rate of 1.0 mL/h, voltage supply of 14 kV, and working distance of 15 cm: a pure PNN10-1 copolymer of Mw. 22,500, b pure PNN10-2 copolymer of Mw. 33,600, PNN10-2/CS ratio of c 95/5, d 88/12, and e 50/50
Scanning electron microscopy images of the as-spun PNN10-2/CS nanofibers with different weight ratios of PNN10-2 to chitosan are shown in Fig. 3c–e. The morphologies of the PNN10-2/CS (95/5) and (88/12) nanofibers were flat and noodle-like. However, as the PNN/CS ratio increased to 50/50, there were a few bead-like structures on the nanofibers. This result suggests that the solution with the higher chitosan content was too viscous to be electrospun because the high viscosity impeded the continuous flow of the polymer solution through the capillary tip.
In addition, for applications such as drug carriers, the drug-loaded scaffold should be stable in water. In this study, NMA segments were used for the thermal crosslinking function of the PNN copolymer, which led to the formation of a bis(methylene ether) and then of a methylene bridge by eliminating water and formaldehyde molecules, respectively. As illustrated in Fig. 4, the thermally crosslinked PNN10-2 and PNN10-2/CS (95/5) scaffolds retained their fibrous structures after being soaked in water, although the diameters increased (Fig. 4a, b). However, as the chitosan content increased, the fibrous structure of PNN10-2/CS (88/12) partially disappeared and was transformed into a film (Fig. 4c).
The drug-loaded NPs, which consisted of chitosan/PNIPAAm with a drug loading of 3.02 %, were electrospun with the PNN. The morphologies of the PNN10-2/NPs nanofibers with different amounts of NPs are shown in Fig. 5. At lower NP contents, a uniform fibrous structure was observed, and the average diameter was approximately 164 ± 31 nm, as shown in Fig. 5a. The PNN nanofiber containing NPs was observed by TEM (Fig. 5b). The size of the NPs was 81 nm in the picture, close to the 90 nm that was observed in our previous research (Huang et al. 2013). With an increasing content of NPs, irregular rock-like structures (543 ± 107 nm) were observed on the nanofibers (368 ± 76 nm), as shown in Fig. 5c. Because the NPs restricted the flow of the electrospun solution in the needle, continuous nanofibers could not be fabricated when the NPs content was increased to 25 wt%.
Gel fraction and swelling ratio
The gel fraction and swelling ratio of the scaffolds crosslinked in an oven (110 °C) for 5 h were summarized in Table 4. It was demonstrated that the gel fractions of all three types of scaffolds remained high (0.81) even though the incorporation of chitosan and the NPs had some influence on gel fraction. Moreover, the swelling ratio of PNN10-2 scaffold was 10.3. With the incorporation of chitosan, the swelling ratio increased to 13.3. It was suggested that the crosslinking of PNN was obstructed by chitosan chains, thus increasing the swelling ratio. Although NPs also decreased the crosslinking of PNN, however, swelling ratio did not increase because the distribution of NPs was not continuous in the scaffolds.
The phase transition temperature of the nanofibrous scaffolds
The thermal properties of the nanofibrous scaffolds were measured by DSC. As shown in Fig. 6a, the phase transition temperatures of the crosslinked PNN nanofibers shifted to a lower temperature because the hydrophilic hydroxyl groups of NMA were eliminated during the crosslinking reaction. Figure 6b shows the phase transition temperature of the PNN10-2/CS (95/5) nanofibrous scaffold in media of different pH values. The transition temperature at pH 2 was significantly lower than others. As described previously, the phase transition temperature of PNN10-1 was decreased by adding chitosan to the polymer solution. In addition, the crosslinked nanofibrous scaffold of PNN10-2/CS (95/5) at pH 2 had a lower phase transition temperature (27.5 °C) than the PNN10-1/CS solution (Fig. 2b). This result suggests that the interaction between chitosan and PNN would be stronger in the crosslinked nanofibrous scaffold than in the polymer solution. On the other hand, the shift of the transition temperature was negligible when the pH of the medium was higher than 6, which is the pKa of chitosan.
Mechanical properties
The prepared scaffolds were cut into a specific dumbbell shape with an average thickness of 0.14 0.05 mm. Table 5 shows that all of the crosslinked scaffolds had high tensile strengths (1.45 MPa or above) and the tensile elongations remained 6.11 % or above. The incorporation of NPs had no influence on the tensile strengths of the scaffolds though the tensile elongations decreased.
Cytotoxicity
The cytotoxicities of the nanofibrous scaffolds (PNN10-2, PNN10-2/CS (88/12), and PNN10-2/NPs-13.6) were determined by exposing human fetal skin fibroblasts to the mediums containing the extracts from these scaffolds. Figure 7 shows the viabilities of the fibroblasts cultured in the control (extract-free) and extracts-containing mediums for 1–7 days. The fibroblasts proliferated well with no obvious difference among the fibroblasts cultured in these different mediums. It is therefore speculated that PNN10-2, PNN10-2/CS (88/12), and PNN10-2/NPs scaffolds exhibited no significant cytotoxicity toward human fetal skin fibroblasts.
Cytotoxicity test of the nanofibrous scaffolds (PNN10-2, PNN10-2/CS (88/12), and PNN10-2/NPs-13.6). The viabilities of human fetal skin fibroblasts cultured in the control (extract-free) medium and also mediums containing the extracts from the scaffolds were determined. Studies consisted of at least four experiments with error bars representing the standard deviation
Release of doxycycline hyclate
PNN/CS scaffolds
As shown by the integrity of the soaked fibrous structure, PNN10-2/CS (95/5) scaffolds could be used as drug carriers. Doxycycline hyclate was added to the electrospun solution directly, so the encapsulation efficiency was 100 %. The drug loading content calculated by Eq. (1) was 9.9 %. The drug-loaded nanofibrous scaffolds were then immersed in several PBS solutions, and the drug release profiles under different conditions were recorded, as shown in Fig. 8. First, it was found that the initial release rate and the final release amount of doxycycline hyclate were higher in the acidic solutions of pH 2 than those in the neutral solutions of pH 7. After 8 h, the drug release percentage in the 25 °C pH 2 solutions reached 88 %; whereas in the 25 °C pH 7 solutions, it was only approximately 62 %. The difference was attributed to the pH-sensitive chitosan. It is known that chitosan has a pKa value of approximately 6 (Rinaudo et al. 1999); therefore, the chitosan chains, limited by the PNN networks, swelled to a larger extent in acidic solution because of the protonation of their amino groups. In contrast, the chitosan chains shrank significantly in the neutral solution. Therefore, the PNN10-2/CS scaffold in the pH 2 solution would have less diffusion resistance for the drug compared to that in the pH 7 solution. Furthermore, the drug release profile was also sensitive to temperature due to the presence of the thermo-sensitive PNN. As shown in Fig. 8, the drug would be expelled immediately along with water due to the shrinking of the PNN at 50 °C, which is above its phase transition temperature. This scenario would also lead to a large amount of released drug and even burst behavior at the initial stage of drug release. Additionally, it was noted that the final release percentage could not reach 100 %, suggesting that there were some interactions between the doxycycline hyclate and the PNN/CS nanofibers, as they all have polar functional groups such as amino, hydroxyl and amide groups. However, these results indicated that the diffusion resistance of the PNN/CS nanofibrous scaffold was too small to be applied in controlled release. Based on our previous studies (Huang et al. 2013), the chitosan-based NPs were used in our further studies.
PNN/NPs nanofibrous scaffolds
The encapsulation efficiency and drug loading content of the NPs were 58 and 3.02 %, respectively. It was assumed that the drug within the NPs was completely entrapped in the nanofibrous scaffolds after electrospinning. The drug loading contents of PNN10-2/NPs-8.20 and PNN10-2/NPs-13.6 were 0.25 and 0.41 %, respectively. Figure 9 shows the drug release profiles of PNN10-2/NPs-8.20 in several PBS solutions. First, the initial release rate and the final release amount of doxycycline hyclate were higher in the acidic solutions of pH 2 than in the neutral solutions. After 48 h, the drug release percentages in the pH 2 solutions at 37 and 25 °C reached 67 and 54 %, respectively, whereas in the pH 7 solutions, the release percentages were only approximately 44 and 45 % at the respective temperatures. The difference is attributed to the pH-sensitive chitosan shell of nanoparticles. Briefly, the crosslinked chitosan can swell to a larger extent in acidic solution. Therefore, the chitosan shell of NPs in the pH 2 solution would experience less diffusion resistance for the drug compared to that in the pH 7 solution.
In addition to pH-sensitivity, the drug release profile was also sensitive to temperature due to the presence of the thermo-sensitive PNN nanofibers. At a pH of 2, the amount of drug released was greater in the solution at 37 °C than in that at 25 °C. As mentioned before, the chitosan shell of nanoparticles swelled in acidic solution (pH 2), and the drug diffused into the nanofibers. It is generally believed that the drug would be expelled immediately along with water due to the shrinking of the PNN nanofibers at 37 °C, which was above its phase transition temperature. This behavior would lead to a greater amount of drug released. However, in the pH 7 solution, the amount of drug released was lower in the solution at 37 °C than at 25 °C. The chitosan nanoparticle shell shrank at the pH value above its pKa, and a limited amount of drug diffused out of the particles. Additionally, the crosslinked PNN nanofibers had a higher swelling ratio, leading to a low transfer resistance at the temperature below the phase transition temperature. The limited amount of drug was quickly released into the PBS solution at lower temperature. On the other hand, a gradual-release behavior was observed in the solutions at 37 °C: increasing from 3.5 % at 20 min to 45 % at 54 h.
Figure 10 shows the drug release profiles of PNN10-2/NPs-13.6. The drug release percentages in the pH 2 solutions at 37 and 25 °C reached 76 and 66 % at 168 h, respectively, whereas in the pH 7 solutions, they were only approximately 55 and 46 % at the respective temperatures. Generally, the trend of the profiles was similar to that observed with PNN10-2/NPs-8.20. First, the amount of drug released was higher in the acidic solutions of pH 2 than in the neutral solution (pH 7). Second, the amount of drug released was higher in the solution at 37 °C than in the solution at 25 °C at both pH 2 and 7 because the drug would be expelled along with water due to the shrinking of the PNN nanofibers.
Comparing PNN10-2/NPs-13.6 with PNN10-2/NPs-8.20 at 37 °C, the release amount of PNN10-2/NPs-13.6 was significantly higher than that of PNN10-2/NPs-8.20 at pH values of 2 and 7 due to the larger amount of drug loaded. At 25 °C, the release amount of PNN10-2/NPs-13.6 was higher than that of PNN10-2/NPs-8.20 in the pH 2 solution; in contrast, the release amount of PNN10-2/NPs-13.6 was lower than that of PNN10-2/NPs-8.20 in the pH 7 solution. These results suggest that the limited amount of drug released from the nanoparticles at pH 7 quickly diffused into the PBS solution due to the small diameter of the PNN10-2/NPs-8.20 fibers. This would also lead to a large amount of drug released from the PNN10-2/NPs-8.20 scaffold. In addition, a gradual-release behavior of PNN10-2/NPs-13.6 was observed for the drug in the pH 2 solution at 25 °C. Consequently, the addition of chitosan-based nanoparticles prolonged the drug release of nanofibrous scaffolds.
Conclusions
A copolymer of NIPAAm and NMA (PNN) was synthesized successfully via redox polymerization. The phase transition temperature of PNN was altered by changing the ratio between NIPAAm and NMA. Additionally, the interaction between chitosan and PNN decreased the phase transition temperature of PNN in acidic solution. Subsequently, the PNN10-2/CS nanofibers were fabricated from a cosolvent composed of aqueous ethanol and acetic acid. Moreover, the fibrous structure of the soaked PNN10-2/CS scaffold was successfully preserved by the crosslinking of NMA in the PNN through a thermal curing process. The crosslinked PNN10-2/CS nanofibers had transition temperatures at 27.5 and 35.8 °C in acidic and neutral solution, respectively. Moreover, the uniform fibrous composite scaffold of the PNN nanofibers containing chitosan-base nanoparticles was electrospun from the cosolvent. All of the scaffolds examined had high tensile strengths (1.45 MPa or above) and exhibited no significant cytotoxicity toward human fetal skin fibroblasts.
Doxycycline hyclate was used as a model drug to test the drug-release behavior. The amount of drug released was greater in the acidic solution of pH 2 than it was in the neutral solution of pH 7 in PNN10-2/CS because chitosan swelled in the acidic solution. In addition, the drug would be expelled immediately along with water due to the shrinking of the PNN nanofibers at 50 °C. In the PNN/NPs series, the trend of the release profiles was similar to that of PNN10-2/CS, but a gradual-release behavior in PNN10-2/NPs-13.6 was observed for the drug in the pH 2 solutions at 25 °C. The addition of chitosan-based nanoparticles improved the utility of the nanofibrous scaffolds. The fibrous scaffolds containing nanoparticles thus have the potential to be applied as controlled-release carriers in hydrophilic drug delivery.
References
Binkert T, Oberreich J, Meewes M, Nyffenegger R, Ricka J (1991) Coil-globule transition of poly(N-isopropylacrylamide): a study of segment mobility by fluorescence depolarization. Macromolecules 24(21):5806–5810
Caruso F, Caruso RA, Mohwald H (1998) Nanoengineering of inorganic and hybrid hollow spheres by colloidal templating. Science 282(5391):1111–1114
Hartgerink JD, Beniash E, Stupp SI (2002) Peptide-amphiphile nanofibers: a versatile scaffold for the preparation of self-assembling materials. Proc Natl Acad Sci USA 99(8):5133–5138
Huang C-H, Wang C-F, Don T-M, Chiu W-Y (2013) Preparation of pH- and thermo-sensitive chitosan-PNIPAAm core–shell nanoparticles and evaluation as drug carriers. Cellulose 20(4):1791–1805
Hulteen JC, Chen HX, Chambliss CK, Martin CR (1997) Template synthesis of carbon nanotubule and nanofiber arrays. Nanostruct Mater 9(1–8):133–136
Jayaraman K, Kotaki M, Zhang Y, Mo X, Ramakrishna S (2004) Recent advances in polymer nanofibers. J Nanosci Nanotechnol 4(1–2):52–65
Jeong SI, Krebs MD, Bonino CA, Samorezov JE, Khan SA, Alsberg E (2011) Electrospun chitosan-alginate nanofibers with in situ polyelectrolyte complexation for use as tissue engineering scaffolds. Tissue Eng Part A 17(1–2):59–70
Katti DS, Robinson KW, Ko FK, Laurencin CT (2004) Bioresorbable nanofiber-based systems for wound healing and drug delivery: optimization of fabrication parameters. J Biomed Mater Res B Appl Biomater 70(2):286–296
Kolambkar YM, Dupont KM, Boerckel JD, Huebsch N, Mooney DJ, Hutmacher DW, Guldberg RE (2011) An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects. Biomaterials 32(1):65–74
Lee C-F, Lin C-C, Chi W-Y (2008) Thermosensitive and control release behavior of poly (N-isopropylacrylamide-co-acrylic acid) latex particles. J Polym Sci Part A Polym Chem 46(17):5734–5741
Lee WY, Cheng WY, Yeh YC, Lai CH, Hwang SM, Hsiao CW, Huang CW, Chen MC, Sung HW (2011) Magnetically directed self-assembly of electrospun superparamagnetic fibrous bundles to form three-dimensional tissues with a highly ordered architecture. Tissue Eng Part C Methods 17(6):651–661
Li WJ, Tuli R, Okafor C, Derfoul A, Danielson KG, Hall DJ, Tuan RS (2005) A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials 26(6):599–609
Liu W, Yeh Y-C, Lipner J, Xie J, Sung H-W, Thomopoulos S, Xia Y (2011) Enhancing the stiffness of electrospun nanofiber scaffolds with a controlled surface coating and mineralization. Langmuir 27(15):9088–9093
Ma PX, Zhang R (1999) Synthetic nano-scale fibrous extracellular matrix. J Biomed Mater Res 46(1):60–72
Piryaei A, Valojerdi MR, Shahsavani M, Baharvand H (2011) Differentiation of bone marrow-derived mesenchymal stem cells into hepatocyte-like cells on nanofibers and their transplantation into a carbon tetrachloride-induced liver fibrosis model. Stem Cell Rev Rep 7(1):103–118
Reneker DH, Yarin AL (2008) Electrospinning jets and polymer nanofibers. Polymer 49(10):2387–2425
Rinaudo M, Pavlov G, Desbrieres J (1999) Influence of acetic acid concentration on the solubilization of chitosan. Polymer 40(25):7029–7032
Sahoo S, Ang LT, Goh JC-H, Toh S-L (2010) Growth factor delivery through electrospun nanofibers in scaffolds for tissue engineering applications. J Biomed Mater Res A 93A(4):1539–1550
Schild HG (1992) Poly(N-isopropylacrylamide): experiment, theory and application. Prog Polym Sci 17(2):163–249
Sencadas V, Correia DM, Ribeiro C, Moreira S, Botelho G, Gomez Ribelles JL, Lanceros-Mendez S (2012) Physical-chemical properties of cross-linked chitosan electrospun fiber mats. Polym Test 31(8):1062–1069
Sill TJ, von Recum HA (2008) Electrospinning: applications in drug delivery and tissue engineering. Biomaterials 29(13):1989–2006
Song JH, Kim HE, Kim HW (2008) Production of electrospun gelatin nanofiber by water-based co-solvent approach. J Mater Sci Mater Med 19(1):95–102
Zhang Y, Yarin AL (2009) Stimuli-responsive copolymers of n-isopropyl acrylamide with enhanced longevity in water for micro- and nanofluidics, drug delivery and non-woven applications. J Mater Chem 19(27):4732–4739
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, CH., Kuo, TY., Lee, CF. et al. Preparation of a thermo- and pH-sensitive nanofibrous scaffold with embedded chitosan-based nanoparticles and its evaluation as a drug carrier. Cellulose 21, 2497–2509 (2014). https://doi.org/10.1007/s10570-014-0290-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-014-0290-7