Abstract
Morphological changes to the different components of lignocellulosic biomass were observed as they occurred during steam pretreatment by placing a pressure reaction cell in a neutron beam and collecting time-resolved neutron scattering data. Changes to cellulose morphology occurred mainly in the heating phase, whereas changes in lignin morphology occurred mainly in the holding and cooling phases. During the heating stage, water is irreversibly expelled from cellulose microfibrils as the elementary fibrils coalesce. During the holding phase lignin aggregates begin to appear and they increase in size most noticeably during the cooling phase. This experiment demonstrates the unique information that in situ small angle neutron scattering studies of pretreatment can provide. This approach could be useful in optimizing the heating, holding and cooling stages of pretreatments to allow the exact size and nature of lignin aggregates to be controlled in order to enhance enzyme accessibility to cellulose and therefore the efficiency of biomass conversion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
We have been developing neutron scattering technologies to characterize the morphology of lignocellulosic biomass and how it evolves in response to industrial thermochemical pretreatments (Langan et al. 2012). Neutron scattering is sensitive, but differently to both hydrogen (H) and its isotope deuterium (D). This sensitivity to H and the ability to replace it by D to alter scattering levels provides distinct advantages for using small angle neutron scattering (SANS) techniques to highlight different components of complex biological systems over length scales from 1–1,000 nm. In particular, SANS can be used to study the morphology of the different components within biomass; lignin, hemicellulose, and cellulose (Pingali et al. 2010; Cheng et al. 2011; Li et al. 2011). This can be achieved by soaking samples in D2O so that the scattering level of the water accessible matrix of lignin and hemicellulose polymers is raised compared to the water impenetrable elementary crystalline cellulose fibrils. Further, the ability of neutrons to penetrate through robust sample environments raises the possibility for studying pretreatment in situ, thereby providing a parametric picture of the morphological transitions taking place.
In previous studies we applied SANS in combination with molecular dynamics (MD) simulations to study morphological changes in biomass during steam explosion pretreatment (SEP) (Langan et al. 2014). Wood chips from poplar trees were chosen for study due to their potential as an abundant, low cost, feed stock (Sannigrahi et al. 2010). We found that these chips are composed mostly of cellulose in the form of elementary fibrils, with well-ordered cores and hydrated surfaces that are more disordered, which pack together tightly in microfibrillar bundles in some regions (a schematic is shown in Fig. 1). Within the bundles, and between the elementary fibers, a thin layer of water is trapped. These bundles are surrounded by a disordered hydrated matrix of covalently linked and entangled hemicellulose and lignin polymers. After the wood chips were steam pretreated in a pressure reaction vessel for about half an hour at 180 °C it was observed that the core water molecules originally trapped within the microfibrils had been released into the surrounding hydrated matrix, causing the fibrils to coalesce into bundles with more confined, and therefore ordered, surface chains. We also found that the lignin-hemicellulose matrix had gone through a phase separation, with the more hydrophobic lignin aggregating into crumpled globules and with some loss of hemicellulose through autohydrolysis.
These experimental results were interpreted using MD simulations of two separate systems, one that modeled a hydrated microfibril and the other a hydrated matrix of lignin and hemicellulose. In simulations of the microfibril, enthalpic interactions between cellulose and water keep some (core) water trapped within the microfibril, but as the temperature is increased during SEP the entropic penalty dominates allowing and the core water molecules to be released to bulk solvent. In simulations of the matrix, lignin transitions to conformations that contain fewer kinks, thus leading to fewer lignin hemicellulose entanglements, and allowing the observed phase separation. The balance between the entropy and enthalpy of water is one of the key drivers of the morphological changes to both cellulose microfibrillar bundles and the matrix of lignin and hemicellulose within which they are encrusted.
A limitation of this earlier work is that the wood chips were examined outside of the pressure reaction vessel before and after SEP; there is no experimental information on what happens within the vessel during pretreatment. Therefore our interpretation of the morphological changes, informed by the MD simulations, as the emergence of lignin globules and the aggregation of cellulose fibers during the heating stage of the reaction has not been experimentally verified; it is possible that the morphological changes occur during the subsequent holding or cooling stage of the reaction. In order to address this knowledge gap we carried out an experiment to monitor morphological changes as they occur during pretreatment by placing a pressure reaction cell in a neutron beam and collecting time-resolved neutron scattering data.
Materials and methods
Biomass samples
Quaking aspen (Populus tremuloides) samples were obtained by Benchmark International in High Level, Alberta, Canada. Trees were destructively sampled to collect approximately 20–80 mm thick disks, or cross sections, from individual trees at 0.3 m from the point of germination. All chips were taken from the outer portion of the section to minimize variability within a single chip. Chips had the following dimensions: 50.8 × 38.1 × 12.7 mm (2 × 1.5 × 0.5) in length, width, and thickness, respectively. The fiber axis ran along the length of the chip.
Bleaching
Lignin was removed to leave holocellulose by treating aspen chips in 1 % sodium chlorite solution at 70 °C for 2 days, at a pH below 5 by adding acetic acid.
Small angle neutron scattering (SANS) of biomass
SANS measurements on biomass were carried out on GP-SANS (CG-2) and Bio-SANS (CG-3) instruments at the high flux isotope reactor facility of Oak Ridge National Laboratory. The samples consisted of thin (~1 mm thick) circular wafers of 10 mm diameter tangential section cut from aspen chips. For the in situ measurements, the sample was soaked in D2O for several hours and then, while still wet, placed in a stainless steel pressure reaction cell with 12.7 mm thick quartz windows (Melnichenko et al. 1999). A wavelength, λ, of 4.75 Å, a neutron spread Δλ/λ of 0.14, and a sample-to-detector distance of 3.336 m were used to cover a Q range of 0.0125–0.375 Å−1. The center of the area detector was offset to allow access to larger values of Q. The instrument resolution was defined using a circular aperture of 10 mm diameter. The detector images were normalized to incident beam monitor counts, and corrected for detector dark current, pixel sensitivity and scattering from backgrounds. The images were then transformed into polar reciprocal space coordinates Q and ϕ, where ϕ is the azimuthal angle around the beam direction (ϕ has values of 0° and 180° in the equatorial direction and 90° and 270° in the meridional direction).
Results
In situ SANS images were collected every 15 min as the temperature was ramped from 25 to 140 °C, held for 4 h at 140 °C, and then quenched to 25 °C (see Fig. 2). Data was collected in intervals of 15 min to optimize two aspects: signal-to-noise ratio of the data and maintain a close resemblance to the protocols employed by the biofuel refineries.
The polar reciprocal space transformed data (Q vs. ϕ) were then separated into an isotropic background component and an anisotropic equatorial component using the following approach. At each value of Q the intensity distribution in ϕ was fitted, using least-squares refinement, by two Gaussian profiles
where the peak intensity IA, peak position ϕ0 and orientation fluctuation Δϕ were refined together with an isotropic background, B(Q) (which varies with Q but is constant with ϕ) as described by Nishiyama et al. (2013). The resulting fitted points for IA(Q) in the equatorial direction (ϕ0 = 0o) and B(Q) over the course of the pretreatment are shown in Fig. 3. Further data over a more extensive Q range were collected from samples before and after SEP that had been bleached to remove lignin. The samples were sealed in quartz “Banjo” cuvettes (0.5 and 14 mm inner thickness and diameter) with D2O. A wavelength, λ, of 4.75 Å, a neutron spread Δλ/λ of 0.14, and three sample-to-detector distances of 1.089, 6.789 and 19.289 m were used to cover a Q range of 0.003–1 Å−1. The center of the area detector was offset to allow access to larger values of Q. These data were collected to help interpret scattering features from cellulose and lignin in the in situ studies.
Neutron scattering collected as the temperature in the reaction vessel was (left column) increased from 40 to 140 °C (middle column) held at 140 °C and (right column) reduced from 140 to 40 °C. Top row The anisotropic component of the neutron scattering intensity in the equatorial directions, IA(Q) Bottom row the isotropic component of the neutron scattering intensity, B(Q). The intensity plots change color from blue to red as the temperature varies from 40 to 140 °C in the left and right columns. In the middle column the curves change from blue to red as the holding time increases. (Color figure online)
Discussion
During the heating phase of SEP a peak in the anisotropic equatorial scattering (at ~0.17 Å−1) begins to decrease in intensity after the temperature has reached ~80 °C. This diffraction peak has been reported before and has been shown to be due to side–side-packing of elementary cellulose fibrils in microfibrillar bundles; when soaked in 40 % D2O to match out scattering from cellulose the diffraction peak disappears, confirming its origin in side-by-side packing of cellulose elementary fibrils into aligned bundles (Langan et al. 2014). We interpret this result as a gradual reduction in the population of microfibrillar bundles that contain core water layers between elementary fibrils and a coagulation of those fibrils as the temperature is increased above ~80 °C. There is little change in the isotropic background scattering which remains featureless.
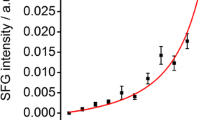
During the holding phase the anisotropic equatorial scattering shows little further change except for a small shift in peak position to lower Q. However a scattering feature appears and increases in intensity in the isotropic scattering background which can be interpreted as a Guinier region. This feature, which has been reported before (Pingali et al. 2010), is considerably diminished in SANS from the wood chip that had been pretreated and then bleached and we therefore interpret it as being due to lignin (R g is in the range of 20–30 nm). As the temperature is ramped down there is little change in the anisotropic equatorial scattering. However, there is a continued increase in the strength of the Guinier region in the isotropic scattering, which we interpret as the continued formation of lignin aggregates, and the Guinier shoulder moves to slightly lower Q, indicating that their sizes are increasing.
Changes to cellulose morphology occur only in the heating phase, whereas changes in lignin morphology occur in the holding and cooling phases. Figure 4 summarizes the process, during the heating stage water is expelled from cellulose microfibrils as the elementary fibers coalesce. After water has been expelled there is little further change in cellulose during the holding and cooling phases, and this agrees with our previous MD simulations in which it was found that water expulsion is an irreversible process (Langan et al. 2014). On the other hand, there is little change to lignin during the heating phase, but aggregates begin to appear and increase in number during the holding phase. Lignin aggregates continue to appear and increase in size during the cooling phase. Our previous MD simulations suggest that lignin aggregation occurs during SEP as soon as lignin has phase separated from hemicellulose. The results presented here indicate that this phase separation starts at or above ~140 °C during SEP.
This experiment is one of the first to directly probe morphological changes in biomass chips during SEP and it provides new insights into how the cellulose and lignin components change at different stages of the pretreatment. Having demonstrated the usefulness of this approach, we plan to carry out further studies to address several questions that arise from these initial results. In particular, we will extend the heating phase to higher temperatures (temperatures as high as 180 °C have been used for SEP) to verify that lignin aggregation starts during the heating phase after the temperature has reached 140 °C. We will examine dilute acid pretreatments, which involve more pronounced hydrolysis of lignin-hemicellulose bonds, to see if lignin aggregation starts during the heating phase at lower temperatures. We will also examine different types of biomass. Our hope is that this approach will allow us to optimize the heating, holding and cooling stages of pretreatments of different types of biomass to allow the exact size and nature of lignin aggregates to be controlled in order to enhance enzyme accessibility to cellulose and therefore the efficiency of biomass conversion.
References
Cheng G, Varanasi P, Li C, Liu H, Melnichenko YB, Simmons BA, Kent MS, Singh S (2011) Transition of cellulose crystalline structure and surface morphology of biomass as a function of ionic liquid pretreatment and its relation to enzymatic hydrolysis. Biomacromolecules 12:933–941
Langan P, Evans BR, Foston M, Heller WT, O’Neill HM, Petridis L, Pingali SV, Ragauskas AJ, Smith JC, Davison B (2012) Neutron technologies for bioenergy research. Ind Biotechnol 8(4):209–216
Langan P, Petridis L, O’Neill HM, Pingali SV, Foston M, Nishiyama Y, Schulz R, Lindner B, Hanson BL, Harton S, Heller WT, Urban V, Evans BR, Gnanakaran S, Ragauskas AJ, Smith JC, Davison B (2014) Common Processes Driving the Thermochemical Pretreatment of Lignocellulosic Biomass. Green Chemistry 16:63–68
Li C, Cheng G, Balan V, Kent MS, Ong M, Chundawat SPS, daCosta Sousa L, Melnichenko YB, Dale BE, Simmons BA, Singh S (2011) Influence of physico-chemical changes on enzymatic digestibility of ionic liquid and AFEX pretreated corn stover. Bioresour Technol 102:6928–6936
Melnichenko YB, Kiran E, Wignal GD, Heath KD, Salaniwal S, Cochran HD, Stamm M (1999) Pressure- and temperature-induced transitions in solutions of poly(dimethylsiloxane) in supercritical carbon dioxide. Macromolecules 32:5344–5347
Nishiyama Y, Langan P, O’Neill HM, Pingali SV, Harton S (2013) Structural coarsening of aspen wood by hydrothermal pretreatment monitored by small- and wide-angle scattering of X-rays and neutrons on oriented specimens. Cellulose. doi:10.1007/s10570-013-0069-2
Pingali SV, Urban VS, Heller WT, McGaughey J, O’Neill HM, Foston M, Myles DA, Ragauskas A, Evans BR (2010) Breakdown in cell wall nanostructure in dilute acid pretreated biomass. Biomacromolecules 11:2329–2335
Sannigrahi P, Ragauskas AJ, Tuskan GA (2010) Poplar as a feedstock for biofuels: a review of compositional characteristics, Biofuels. Bioprod Biorefin 4:209–226
Acknowledgments
This research is funded by the Genomic Science Program, Office of Biological and Environmental Research, U.S. Department of Energy, under FWP ERKP752. The Center for Structural Molecular Biology (CSMB) and the Bio-SANS beam line is supported by the Office of Biological and Environmental Research, using facilities supported by the U. S. Department of Energy, managed by UT-Battelle, LLC under contract No. DE-AC05-00OR22725. This Research on Bio-SANS and GP-SANS at Oak Ridge National Laboratory’s High Flux Isotope Reactor was sponsored by the Scientific User Facilities Division, Office of Basic Energy Sciences, US Department of Energy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pingali, S.V., O’Neill, H.M., Nishiyama, Y. et al. Morphological changes in the cellulose and lignin components of biomass occur at different stages during steam pretreatment. Cellulose 21, 873–878 (2014). https://doi.org/10.1007/s10570-013-0162-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-013-0162-6