Abstract
Composite hydrogels were prepared from pineapple peel cellulose with the combinations of polyethylene glycol (PEG), polyvinyl alcohol (PVA), к-carrageenan (CN), or soluble starch (SH) in 1-allyl-3-methylimidazolium chloride solvent. Impacts of these macromolecules on the texture profile analysis (TPA) parameters, equilibrium swelling ratio (ESR), and sodium salicylate (NaSA) load of the prepared hydrogels were studied. The NaSA release kinetics of the composite hydrogels were also compared. The composite hydrogels exhibited differences in Fourier transform infrared spectroscopy (FTIR), TPA parameters, ESR, NaSA load ratio, and release kinetics. CN addition increased the hardness of the hydrogels, while PEG played an opposite role. SH and PVA could decrease hardness, gumminess, and resilience, and SH could increase the springiness and cohesiveness of the hydrogels. Most of the composite hydrogels exhibited the same basic FTIR features as the simple hydrogel. Freeze-dried composite hydrogels exhibited a markedly higher ESR than the oven-dried ones, and additions of PEG, PVA, CN, and SH showed the same effect. Addition of the PEG and PVA combination could lower the ESR of the hydrogels, whereas additions of the PEG and CN combination or PEG and SH combination could markedly increase the ESR of the hydrogels. Addition of PEG, PVA, CN, and SH respectively could increase the NaSA load ratio of the hydrogels. Oven-drying treatment, additions of the PEG and PVA combination or PEG and CN combination were propitious for extending the NaSA fast-release phase of the hydrogels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a kind of typical tropical fruit with distinctive flavor characteristics, pineapple (Ananas comosus (L.) Merr.) is widely cultivated in the tropics of the world. The relevant processed products, such as canned pineapple and pineapple juice, are favorite foods of most consumers. Another deep processing method for pineapple fruit is bromelain extraction from pineapple peel. However, both the processing of pineapple fruits and bromelain extraction produce a great quantity of peel and residue (usually accounting for 30–50 % of the total fresh fruit weight). Hence, properly processing and handling these peels and residues are of practical and academic significance, especially in the prevention of biological resource waste and environmental pollution. Pineapple peel and residue mainly consist of cellulose, hemicellulose, lignin, pectin, and other components (Bardiya et al. 1996). In spite of having less color and rich cellulose (accounting for 20–25 % of the dry weight), pineapple peel and residues are seldom utilized as an industrial product, and little research on the use of the cellulose has been carried out so far. Recently, Hu et al. (2010a, b, 2011) reported their work on the modification of pineapple peel cellulose (PPC) as hydrogels and absorbents of heavy metals in waste. Hydrogels prepared from plant fiber and the relevant cellulose derivatives have many other potential applications, such as invisible eyes, wound dressing, and super-absorbent and drug release systems because of their perfect hydrophily (Gin et al. 1990; Matthew et al. 1993; Mishra et al. 2008). Plant cellulose contains many anhydroglucopyranose units bound by β-(1 → 4)-glycosidic linkage, which is described as a linear-polymer glucan with uniform chain structure (Fengel and Wegener 1984). Due to the existence of intramolecular and intermolecular hydrogen bonds, plant cellulose is generally insoluble in most solvents and is difficult to process. Hence, the use of plant cellulose is limited in many fields. Recently, due to the innovation of some novel green solvents for many plant celluloses, such as room-temperature ionic liquid, processing of plant cellulose has become much easier than before. The characteristics of excellent solubility, zero vapor pressure, and recycling of this kind of novel green solvent make plant cellulose new potential applications (EI Seoud et al. 2007; Murugesan and Linhardt 2005). For example, 1-butyl-3-methylimidazolium chloride (BMIMCl) and 1-allyl-3-methylimidazolium chloride (AMIMCl) have been used to dissolve and turn cellulose into colloidal or composite materials for controlled release of drugs and fertilizer (Kadokawa et al. 2008a, b, 2009; Murakami et al. 2007; Murugesan and Linhardt 2005; Prasad et al. 2009a, b; Swatloski et al. 2002; Wu et al. 2004; Zhang et al. 2005). In fact, there are many kinds of ionic liquids suitable for hydrogel preparation from cellulose, mainly including four types, alkyl-substituted imidazole type ionic liquid ([R1R2 Mim]+), alkyl-substituted pyridine type ionic liquid ([RPy]+), alkyl quaternary ammonium type ionic liquid ([NRx H4−x]+), and alkyl quaternary phosphonium type ionic liquid ([PRx H4−x]+). AMIMCl and the relevant derivatives belong to alkyl-substituted imidazole type ionic liquid. When an ionic liquid is chosen, the potential toxicity, solubility, environmental impact, and economy of the ionic liquid must be considered. The purpose of this research was to prepare composite hydrogels from PPC in AMIMCl solvent and study the impacts of some macromolecule additives, such as polyethylene glycol (PEG), polyvinyl alcohol (PVA), к-carrageenan (CN), and starch (SH) on the characteristics of the prepared hydrogels. The swelling and drug-release behaviors of the prepared hydrogels were also compared.
Materials and methods
Extraction of pineapple peel cellulose
Cellulose was extracted from pineapple peel as hydrogel materials according to the method reported by Hu et al. (2010a, b). Briefly, after cleanout, pineapple peel was pulped with a beater. The pulp was then filtrated with etamine, and the residue was dried in an oven at 50 °C for 24 h. The dried residue was ground with a miller and filtrated through a fine mesh; 30 g of the dried pineapple peel powder was treated with 600 mL distilled water at 80 °C for 2 h and then was delignified with sodium chlorite at pH 3.8–4.0 and 75 °C for 2 h. After distilled water and ethanol washing, the residue was dried at 50 °C for 16 h. The dried residue was then extracted with KOH solution (10 %, w/v) at room temperature for 10 h so as to remove the semi-cellulose and other impurities, followed by water-washing filtration and ethanol (95 %, v/v) washing filtration in sequence until the filtrate became neutral. Pineapple peel cellulose was completed after drying at 50 °C for 16 h and was subjected to hydrogel preparation. The weight ratio of the PPC extracted from pineapple peel by the above processing was about 22 %.
Preparation of composite pineapple peel cellulose hydrogels
Composite PPC hydrogels were prepared mainly via heating-cooling-washing (HCW) processing and heating-cooling-freezing-thawing-washing (HCFTW) processing in AMIMCl solvent (purchased from Shanghai Cheng Jie Chemical Co., Ltd., China) according to Hu et al. (2010a, b); Kadokawa et al. (2008b, 2009); Li et al. (2009a, b) and Prasad et al. (2009a, b). Table 1 shows the proportion of different additives in PPC for the preparation of different composite hydrogels. The numbers following the abbreviations show the corresponding percentages of the materials and additive. For HCW processing, PPC was stir-treated with AMIMCl and the additives at 100 °C for 12 h in a shaker until complete dissolution. Composite hydrogels formed after the solutions were cooled to ambient temperature. The excessive ionic liquid in the prepared hydrogels was leached out via distilled water or ethanol washing. For HCFTW processing, AMIMCl, PPC, and the corresponding additives were stir-treated at 100 °C for 12 h in a shaker until complete dissolution. After cooling to ambient temperature, the formed hydrogels were subjected to a six-cycle treatment of freezing–thawing (frozen at −20 °C for 12 h and then thawed at ambient temperature for 12 h). The excessive ionic liquid in the hydrogels was leached out with distilled water or ethanol. The composite hydrogels prepared in this way were marked as FT.
Texture profile analysis (TPA) and Fourier transform infrared spectroscopy (FTIR) characterization of hydrogels
TPA on the prepared hydrogels was performed according to the method reported by Hu et al. (2010a, b), Bourne (1982), and Lin et al. (2009). A TA-XT2iR Texture Analyzer (Stable Microsystems, Surrey, UK) equipped with a 5-kg load cell was employed. Hydrogels were clipped to 0.8 cm in length and 2.5 cm in diameter as the test samples, and each hydrogel sample was subjected to compression to 30 % of their initial length at a constant pre-speed of 5 mm s−1, cross-head speed of 1 mm s−1, and post-speed of 5 mm s−1, respectively, by a cylindrical stainless-steel probe (P5: 5-mm DIA CYLinDer Stainless). All the measurements were duplicated six times. The tested TPA parameters of the prepared hydrogels including hardness, springiness, cohesiveness, gumminess, and resilience were computed via Texture Expert software. FTIR was recorded to characterize the prepared hydrogels from 4,000 to 400 cm−1 at a resolution of 2 and 32 scans per sample, were performed with a FTIR Spectrometer (Vector 33, Bruker, Germany).
Determination of the equilibrium swelling ratio (ESR) of hydrogels
The equilibrium swelling ratio of the prepared hydrogels was determined based on the routine gravimetric method according to Chen et al. (2009) and Hu et al. (2010a, b). Swelling was performed by immersing the weighed hydrogel samples in water for 3 days at room temperature so as to reach equilibrium status. The excess water on the hydrogel surface was blotted up with filter paper, and the swollen hydrogel samples were then weighed. The ESR was calculated according to the following equation:
where W s was the weight of the swollen hydrogel samples at equilibrium status, and W d was the weight of the hydrogel samples prior to swelling. All of the experiments were conducted in triplicate.
Determination of drug load ratio and release kinetics of hydrogels in vitro
The drug load and release experiments on the prepared hydrogels were performed with sodium salicylicum (NaSA) as the model drug. The dried hydrogel samples were incubated and equilibrated in 20 mL NaSA solution (10 %, w/v) in vials for 2 weeks at ambient temperature. After incubation, the drug-loaded hydrogels were removed from the NaSA solution and rinsed twice with distilled water. The drug-loaded hydrogels were then vacuum-dried to constant weight at room temperature. The residual NaSA content of the tested NaSA solution was determined according to Demir et al. (2008). NaSA load ratios (%) of the prepared hydrogels were calculated based on the following equation.
where m is the mass of hydrogel samples prior to loading. C 0 and V 0 are the concentration and volume of NaSA solution prior to loading, respectively. C t and V t are the concentration and volume of NaSA solution after loading, respectively.
Drug release kinetics experiments on the prepared hydrogels were performed by immersing the drug-loaded hydrogels in a vial filled with 200 mL distilled water (dissolution medium) at 37 °C. The tested vials were stirred in a shaker at 120 revolutions per minute. During the stir-treatment, 5 mL of the dissolution medium was sampled at intervals for NaSA concentration determination, and meanwhile an equal volume of distilled water was pipetted back to the vials so as to maintain the volume constant. The release percentage of NaSA was calculated according to the following equation:
where W t is the NaSA amount released from hydrogels at time t, and W total is the total NaSA amount loaded in hydrogel samples.
Statistical analysis
The Statistical Analysis System (SAS Institute, 1998) was employed to perform the analysis on variance and Duncan’s multiple range tests. A value of P < 0.05 was regarded as significantly different.
Results and discussion
Texture profile comparison between the composite hydrogels and the impacts of additives
Table 2 shows the TPA results of the composite hydrogels prepared from PPC and different additives. Substantially, the prepared hydrogels were the derivatives of plant cellulose belonging to the three-dimensional structure of the cross-linked polymer and possess a strong ability to imbibe and hold water within the internal structures (Gin et al. 1990; Matthew et al. 1993; Mishra et al. 2008). However, there are differences in the composition of anhydroglucopyranose units, type of β-(1 → 4)-glycosidic linkage, and branch ratio among different kinds of celluloses. Hence, each cellulose usually exhibits various characteristics, especially in mechanical properties (TPA parameters), ESR, drug load ratio, and release kinetics (Fengel and Wegener 1984). Additives make these differences more complicated. From Table 2, it was observed that gumminess showed the biggest variety (2.72–640.46) among the hydrogels, followed by hardness (22.99–1,473.08), springiness (0.27–2.02), cohesiveness (0.12–0.67), and resilience (0.18–0.55), respectively. Although prepared at the same PPC content, PPC5-FT was significantly higher in hardness and gumminess than PPC5, but showed little change in springiness, cohesiveness, and resilience, indicating that the freeze–thaw cycle enhanced the textural properties of hydrogel in hardness and gumminess. In the case of PEG addition, PEG7.5-PPC5 was significantly weakened in hardness, gumminess, and resilience as compared with PPC5, but unchanged in springiness and cohesiveness. In the case of PVA addition, PVA5-PPC5-FT declined markedly in hardness, gumminess, and resilience compared with PPC5 and PPC5-FT. In the case of CN addition, CN5-PPC5 and CN5-PPC5-FT showed a slight increase in hardness and springiness, but showed a decrease in cohesiveness, gumminess, and resilience as compared with PPC5. Like the hydrogel prepared via HCFTW processing, CN5-PPC5-FT had significantly higher gumminess than the hydrogel CN5-PPC5 prepared via HCW processing but was unchanged in hardness, springiness, cohesiveness, and resilience basically, indicating HCFTW processing can significantly improve the mechanical properties of hydrogels in gumminess. In the case of SH addition, hydrogels SH5-PPC5 and SH5-PPC5-FT decreased markedly in hardness, gumminess, and resilience, but increased significantly in springiness and cohesiveness as compared with PPC5. SH5-PPC5-FT (prepared via HCFTW processing) had enhanced hardness, springiness, and gumminess as compared with SH5-PPC5 (prepared via HCW processing). From Table 2, it was found that PVA5-PPC5-FT declined significantly in hardness and gumminess as compared with PPC5-FT, and SH5-PPC5 declined significantly in hardness and gumminess as compared with PPC5, indicating PVA and SH can decrease the hardness and gumminess of hydrogels.
In Table 2, the composite hydrogels numbered from 9 to 14 were prepared from PPC and two or three kinds of additives, among which PEG7.5-PVA5-PPC5-FT declined markedly in all TPA parameters, including hardness, springiness, cohesiveness, gumminess, and resilience, especially hardness and gumminess as compared with the hydrogels PPC5, PPC5-FT, PEG7.5-PPC5, and PVA5-PPC5-FT. PEG7.5-CN5-PPC5 also had lower hardness, gumminess, resilience, and cohesiveness than PEG7.5-PPC5 and CN5-PPC5. Although CN5-PPC5 was significantly higher than PPC5 in hardness due to the addition of CN, PEG7.5-CN5-PPC5 had lower hardness than PPC5 and CN5-PPC5, indicating that CN plays a hardness-increasing role, while PEG plays a hardness-decreasing role in composite hydrgels.
Compared with PEG7.5-PPC5, PEG7.5-SH5-PPC5 declined markedly in hardness and gumminess, but increased significantly in springiness. Similarly, PEG7.5-SH5-PPC5-FT had lower hardness and gumminess but higher springiness than PEG7.5-PPC5. SH5-PPC5-FT showed significantly lower hardness and gumminess than PPC5-FT but higher springiness. SH5-PPC5 had significantly lower hardness and gumminess but higher springiness than PPC5. These indicate that SH plays a hardness- and gumminess-decreasing role and springiness-increasing role for composite hydrogels. Compared with PEG7.5-CN5-PPC5 and PEG7.5-SH5-PPC5, PEG7.5-CN5-PPC5-FT and PEG7.5-SH5-PPC5-FT were basically unchanged in hardness, springiness, cohesiveness, and resilience, but had higher gumminess, again indicating that HCFTW processing plays little part in the improvement of hardness, springiness, cohesiveness, and resilience but can increase the gumminess of hydrogels.
The impacts of the macromolecules and the various processing methods on the characteristics of the prepared hydrogels may involve many more complicated factors, both chemically and physically, for example, the chemical crosslinking between additives and cellulose molecules, interaction between PPC and AMIMCl, the ratio of hydrophilic/hydrophobic group inside the additive molecule itself, and the residue of additives in hydrogels (Abitbol et al. 2011; Fukae et al. 2011; Hu et al. 2010a, b; Mihranyan 2013). For further rational understanding about the prepared composite hydrogels, detailed rational research needs to be carried out in this area. The work reported by Mihranyan (2013) indicated that reinforcement of PVA hydrogels could be achieved by direct chemical crosslinking of surface-modified microcrystalline cellulose whiskers with PVA. In his work, modified microcrystalline cellulose whiskers were first obtained by TEMPO-mediated oxidation of the cellulose substrate and ultrasonication followed by direct cross-linking to PVA via forming acetal bonds and freeze-thawing. The viscoelastic properties of the produced hydrogels were clearly improved following the chemical cross-linking, featuring values for viscous and elastic moduli.
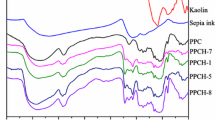
FTIR features of pineapple peel cellulose hydrogels compounded with PEG, PVA, CN, and SH
The FTIR spectrum of PPC was reported by Hu et al. (2010a, b) to compare with the hydrogel prepared from PPC and polyvinyl-polypyrrolidone. Pineapple peel cellulose exhibited the main absorption peaks at 3,411, 2,914, 1,431, 1,165, and 1,032 cm−1, respectively, which are considered as the attribution from cellulose structure, O–H stretching of polymeric compounds, and characteristic vibration of C–H stretching, respectively (During 1991; Silverstein et al. 1981; Wang et al. 2007). After PPC was prepared into hydrogel, its FTIR spectrum bands appeared at 3,425, 2,917, 1,642, 1,426, 1,374, 898, and 617 cm−1 respectively, suggesting the existence of an interaction between PPC and AMIMCl (Hu et al. 2010a, b). FTIR changes of the PPC hydrogel and its composite hydrogels prepared with the additives PEG, PVA, CN, and SH are shown in Fig. 1. As for the hydrogel of PEG7.5-PVA5-PPC5-FT, PEG and PVA were compounded. The FTIR maintained the basic features as PPC5-FT, although the characteristic peaks were slightly shifted from 3,425, 2,917, 1,642, 1,426, 1,374, 898, 617 to 3,420, 2,913, 1,646, 1,436, 1,377, 900, and 614 cm−1, respectively. The characteristic peaks of PEG on FTIR disappeared, while the characteristic peaks of PVA on FTIR appeared at 1,707, 1,143, 1,095, and 851 cm−1, respectively, indicating PEG was much easier to remove than PVA by washing after hydrogels were completed.
As for the hydrogel of PEG7.5-CN5-PPC5-FT, PEG and CN were compounded. Similarly, the FTIR maintained the basic features as PPC5-FT, although the characteristic peaks were shifted at 3,427, 2,915, 1,641, 1,428, 1,320, 1,067, and 615 cm−1, respectively. The characteristic peaks of PEG and CN on FTIR disappeared, indicating both PEG and CN were easy to remove by washing after hydrogels were completed. As for hydrogel of PEG7.5-SH5-PPC5-FT, PEG and SH were compounded. The FTIR also maintained the basic FTIR features as PPC5-FT, although the main peaks were shifted at 3,438, 2,916, 1,639, 1,427, 1,375, 1,061, and 615 cm−1, respectively. The characteristic peaks of PEG and SH on FTIR disappeared, indicating both SH and PEG were easy to remove by washing after hydrogels were completed.
Equilibrium swelling ratios of the composite pineapple peel cellulose hydrogels
Table 3 shows the measured ESR of the hydrogels prepared from PPC hydrogels and different additives, also involving the oven-dried and freeze-dried processing forms. Compared with the oven-dried forms, each of the freeze-dried composite hydrogels exhibited a markedly higher ESR, regardless of addition of PEG, PVA, CN, or SH. This maybe suggests that freeze-drying processing is beneficial for construction of fine intrinsic hydrogel structures. Primary FESEM (field emission scanning electron microscope) image observation proved that the hydrogels prepared via freeze-drying processing usually showed smoother surface, evenly distributed micro-apertures than those hydrogels prepared via oven-drying processing. Table 3 also shows that the freeze-dried PEG7.5-PPC5, CN5-PPC5, and SH5-PPC5 exhibited a higher ESR than the freeze-dried PPC5. The oven-dried PEG7.5-PPC5 and SH5-PPC5 also had a higher ESR than the oven-dried PPC5. Both the freeze-dried PVA5-PPC5-FT and oven-dried PVA5-PPC5-FT had a higher ESR than the freeze-dried PPC5 and oven-dried PPC5. These results indicate that freeze-drying processing and additions of PEG, PVA, CN, and SH can increase the ESR of the hydrogels, respectively. However, both the freeze-dried CN5-PPC5-FT and SH5-PPC5-FT showed a lower ESR than the freeze-dried CN5-PPC5 and SH5-PPC5 and the oven-dried SH5-PPC5-FT had a lower ESR than the oven-dried SH5-PPC5, suggesting that the freeze–thaw treatment had slight effect on the ESR of the hydrogels. The oven-dried PEG7.5-PVA5-PPC5-FT had a lower ESR than the oven-dried PVA5-PPC5-FT, suggesting that simultaneous additions of PEG and PVA can decrease the ESR of hydrogels. However freeze-dried PEG7.5-CN5-PPC5-FT and PEG7.5-SH5-PPC5-FT had higher ESRs than freeze-dried CN5-PPC5-FT and SH5-PPC5-FT, suggesting that addition of the PEG and CN combination or PEG and SH combination can markedly increase the ESR of hydrogels. In fact, more complicated factors may possibly be involved in the ESR of the hydrogels, such as the ratio of the hydrophilic/hydrophobic group inside the additive molecules, washing method, and additive residue in the hydrogels. For further rational understanding, further research needs to be carried out in this area.
NaSA load ratio and release behaviors of the prepared composite hydrogels
In the present research, NaSA was used as a model drug to study the drug load ratio and controlling release kinetics of the composite hydrogels. The load ratios of the prepared composite hydrogels at room temperature are listed in Table 3. Similarly to the ESR, all the freeze-dried composite hydrogels had a significantly higher NaSA load ratio than the oven-dried composite hydrogels. This is perhaps the contribution of freeze-drying treatment to the fine structure of the hydrogels (smoother surface, evenly distributed micro-apertures) Table 3 also shows that all the freeze-dried composite hydrogels involving the freeze-dried PEG7.5-PPC5, freeze-dried CN5-PPC5, and freeze-dried SH5-PPC5 had higher NaSA load ratios than the freeze-dried simple hydrogel PPC5. Similarly, all the oven-dried composite hydrogels, involving the oven-dried PEG7.5-PPC5, oven-dried CN5-PPC5, and oven-dried SH5-PPC5 similarly had higher NaSA load ratios than the oven-dried simple hydrogel PPC5. Both freeze-dried and oven-dried PVA5-PPC5-FT showed relatively high NaSA load ratios. These results suggest that the addition of PEG, PVA, CN, and SH can increase the NaSA load ratio of hydrogels.
NaSA release kinetics of the main composite hydrogels prepared, including the freeze-dried and oven-dried forms, are shown in Figs. 2, 3, 4, and 5. It was known that some parameters, such as the swelling behavior of hydrogels, drug affinity to hydrogels, drug solubility in water, and so on, are involved in the controlling release (Brazel and Peppas 1999; Chen et al. 2009). Basically, the release behaviors of all the prepared hydrogels can be divided into two phases: a linear steep release phase and a slow release phase. At the end, most of the loaded NaSA was released out of the hydrogels. Figure 2 shows the NaSA release kinetics of the freeze-dried and oven-dried PPC5, PEG7.5-PPC5, and PVA5-PPC5-FT. The fast NaSA release phases of the freeze-dried PPC5, PEG7.5-PPC5, and PVA5-PPC5-FT existed for 90, 60 and 60 min, respectively, while those of the oven-dried PPC5, PEG7.5-PPC, and PVA5-PPC5-FT were extended to 180, 180, and 120 min, respectively. In Fig. 3, the fast NaSA release phases of the freeze-dried CN5-PPC5 and CN5-PPC5-FT ended at about the 60th and 30th min, respectively, while those of the oven-dried CN5-PPC5 and CN5-PPC5-FT basically ended at about the 120th and 90th min, respectively. These results indicate that oven-drying treatment is propitious to extending the fast release phase for hydrogels. In Fig. 4, the fast NaSA release phases of freeze-dried PEG7.5-PVA5-PPC5-FT and PEG7.5-CN5-PPC5-FT existed for 180 and 120, min respectively, and were significantly higher than the freeze-dried PEG7.5-SH5-PPC5-FT (60 min), the freeze-dried PPC5, PEG7.5-PPC5, PVA5-PPC5-FT, CN5-PPC5, CN5-PPC5-FT, SH5-PPC5, and SH5-PPC5-FT, respectively (Figs. 2, 5), suggesting additions of the PEG and PVA combination or PEG and CN combination can significantly extend NaSA release of the hydrogels. From Fig. 5, it was found that the oven-dried SH5-PPC5 and SH5-PPC5-FT showed the fast NaSA release phases within 60 and 90 min, respectively, while the freeze-dried SH5-PPC5 showed a very short fast NaSA release phase only within 2 min. At the end of the fast release phase, about 99 % of NaSA was released. A similar phenomenon was also found for the freeze-dried SH5-PPC5-FT. The difference in the prepared hydrogels in drug (NaSA) release might be highly correlated with some factors including cellulose characteristics, and additive and formation method. Also, the ratio of the hydrophilic/hydrophobic group inside the additive molecules, washing method, and the residue of additives in hydrogels might be involved (Abitbol et al. 2011; Hu et al. 2010a, b; Brazel and Peppas 1999; Chen et al. 2009; Fukae et al. 2011). From the application point of view, these differences in drug release are of significance and affect practice in many areas, because it can meet the different requirements for controlling release via varying additives and processing methods during hydrogel preparation, especially for the applications of medicinal release and absorption in the pharmacology field, farm land fertilization in agriculture, drought resistance for horticulture, and so on.
NaSA release kinetics of hydrogels PEG7.5-PVA5-PPC5-FT, PEG7.5-CN5-PPC5-FT, and PEG7.5-SH5-PPC5-FT (filled square) for freeze-dried; (open circle) for oven-dried; a for PEG7.5-PVA5-PPC5-FT; b for PEG7.5-CN5-PPC5-FT; c for PEG7.5-SH5-PPC5-FT. Each point is the mean of three determinations, and each bar is equal to 1 standard error
Conclusion
Composite hydrogels prepared from PPC and additives exhibited differences in TPA parameters, ESR, drug load ratio, and release kinetics. The freeze–thaw cycle could enhance the textural properties of the prepared hydrogels, especially in hardness and gumminess. Additives showed significant impacts on the TPA parameters, ESR, drug load ratio, and release kinetics of the hydrogels. CN could increase the hardness of the hydrogels, while PEG played an opposite role. Additions of SH and PVA could decrease the hardness, gumminess, and resilience, but SH could increase the springiness and cohesiveness of the hydrogels. Freeze-drying processing and the addition of PEG, PVA, CN, and SH could markedly increase the ESR of the hydrogels. Freeze–thaw treatment had a slight effect on the ESR of the hydrogels. The addition of the PEG and PVA combination could lower the ESR of the hydrogels, but additions of the PEG and CN combination and PEG and SH combination could markedly increase the ESR of the hydrogels. Addition of PEG, PVA, CN, and SH, respectively, could increase the NaSA load ratio of the hydrogels. Additions of the PEG and PVA combination or PEG and CN combination played an extension effect on NaSA release. Freeze-drying could significantly increase the NaSA load ratio. Oven-drying treatment was propitious to extending the NaSA fast release phase of the prepared hydrogels.
References
Abitbol T, Johnstone T, Quinn TM, Gray DG (2011) Reinforcement with cellulose nanocrystals of poly(vinyl alcohol) hydrogels prepared by cyclic freezing and thawing. Soft Matter 7:2373–2378
Bardiya N, Somayaji D, Khanna S (1996) Biomethanation of banana peel and pineapple waste. Bioresour Technol 58:73–76
Bourne MC (ed) (1982) Viscosity and consistency. In: Food texture and viscosity. Concept and measurement. Academic Press, New York, pp 199–246
Brazel CS, Peppas NA (1999) Mechanisms of solute and drug transport in relaxing, swellable, hydrophilic glassy polymers. Polymer 40:3383–3398
Chen J, Liu M, Liu H, Ma L (2009) Synthesis, swelling and drug release behavior of poly(N, N-diethylacrylamide-co-N-hydroxymethyl acrylamide) hydrogel. Mater Sci Eng, C 29:2116–2123
Demir S, Kahraman MV, Bora N, Apohan NK, Scedil A, Ogan E (2008) Preparation, characterization, and drug release properties of poly(2-hydroxyethyl methacrylate) hydrogels having beta-cyclodextrin functionality. J Appl Polym Sci 109:1360–1368
During JR (1991) Vibrational spectra and structure. Elsevier, New York
EI Seoud OA, Koschella A, Fidale LC, Dorn S, Heinze T (2007) Applications of ionic liquids in carbohydrate chemistry: a window of opportunities. Biomacromolecules 8:2629–2647
Fengel D, Wegener G (1984) Wood: chemistry, ultrastructure, reactions. Walter de Gruyter, Berlin, pp 613–635
Fukae R, Yoshimura M, Yamamoto T, Nishinari K (2011) Effect of stereoregularity and molecular weight on the mechanical properties of poly(vinyl alcohol) hydrogel. J Appl Polym Sci 120(1):573–578
Gin H, Dupuy B, Baquey A, Baquey CH, Ducassou D (1990) Lack of responsiveness to glucose of microencapsulated islets of Langerhans after three weeks’ implantation in the rat—influence of the complement. J Microencapsul 7:341–346
Hu XY, Zhao MM, Huang HH (2010a) Modification of pineapple peel fiber as metal ion adsorbent through reaction with succinic anhydride in pyridine and dimethyl sulfoxide solvents. Water Environ Res 82:733–741
Hu XY, Hu K, Zeng LL, Zhao MM, Huang HH (2010b) Hydrogels prepared from pineapple peel cellulose using ionic liquid and their characterization and primary sodium salicylate release study. Carbohydr Polym 82:62–68
Hu XY, Zhao MM, Song GS, Huang HH (2011) Modification of pineapple peel fibre with succinic anhydride for Cu(2 +), Cd(2 +) and Pb(2 +) removal from aqueous solutions. Environ Technol 32:739–746
Kadokawa J, Murakami M, Kaneko Y (2008a) A facile method for preparation of composites composed of cellulose and a polystyrene-type polymeric ionic liquid using a polymerizable ionic liquid. Compos Sci Technol 68:493–498
Kadokawa J, Murakami M, Kaneko Y (2008b) A facile preparation of gel materials from a solution of cellulose in ionic liquid. Carbohydr Res 343:769–772
Kadokawa J, Murakami M, Takegawa A, Kaneko Y (2009) Preparation of cellulose-starch composite gel and fibrous material from a mixture of the polysaccharides in ionic liquid. Carbohydr Polym 75:180–183
Li L, Lin Z, Yang X, Wan Z, Cui S (2009a) A novel cellulose hydrogel prepared from its ionic liquid solution. Chin Sci Bull 54:1622–1625
Li Y, Wu M, Liu R, Huang Y (2009b) Cellulose-based solid–solid phase change materials synthesized in ionic liquid. Sol Energy Mater Sol Cells 93:1321–1328
Lin C, Wang YT, Wu JS (2009) Modification in physical properties of rice gel by microbial transglutaminase. J Sci Food Agric 89:477–481
Matthew HW, Salley SO, Peterson WD, Klein MD (1993) Complex coacervate microcapsules for mammalian cell culture and artificial organ development. Biotechnol Prog 9:510–519
Mihranyan A (2013) Viscoelastic properties of cross-linked polyvinyl alcohol and surface-oxidized cellulose whisker hydrogels. Cellulose 20:1369–1376
Mishra RK, Datt M, Pal K, Banthia AK (2008) Preparation and characterization of amidated pectin based hydrogels for drug delivery system. J Mater Sci Mater Med 19:2275–2280
Murakami M, Kaneko Y, Kadokawa J (2007) Preparation of cellulose-polymerized ionic liquid composite by in situ polymerization of polymerizable ionic liquid in cellulose-dissolving solution. Carbohydr Polym 69:378–381
Murugesan S, Linhardt R (2005) Ionic liquids in carbohydrate chemistry-current trends and future directions. Curr Org Synth 2:437–451
Prasad K, Kaneko Y, Kadokawa J (2009a) Novel gelling systems of k-, i- and l-carrageenans and their composite gels with cellulose using ionic liquid. Macromol Biosci 9:376–382
Prasad K, Murakami M, Kaneko Y, Takada A, Nakamura Y, Kadokawa J (2009b) Weak gel of chitin with ionic liquid, 1-allyl-3-methylimidazolium bromide. Int J Biol Macromol 45:221–225
Silverstein RM, Bassler GC, Morrill TC (1981) Spectrometric identification of organic compounds. Wiley, New York
Swatloski RP, Spear SK, Holbrey JD, Rogers RD (2002) Dissolution of cellose with ionic liquids. J Am Chem Soc 124:4974–4975
Wang L, Han G, Zhang Y (2007) Comparative study of composition, structure and properties of Apocynum venetum fibers under different pretreatments. Carbohydr Polym 69:391–397
Wu J, Zhang J, Zhang H, He J, Ren Q, Guo M (2004) Homogeneous acetylation of cellulose in a new ionic liquid. Biomacromolecules 5:266–268
Zhang H, Wu J, Zhang J, He J (2005) 1-Allyl-3-methylimidazolium chloride room temperature ionic liquid: a new and powerful nonderivatizing solvent for cellulose. Macromolecules 38:8272–8277
Acknowledgments
The authors thank The National Natural Science Fundation of China (project grant no. 31271978) and the Ministry of Education PRC (project grant no. 20120172110017) for the financial support, which made this work possible.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, X., Wang, J. & Huang, H. Impacts of some macromolecules on the characteristics of hydrogels prepared from pineapple peel cellulose using ionic liquid. Cellulose 20, 2923–2933 (2013). https://doi.org/10.1007/s10570-013-0075-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-013-0075-4