Abstract
Oxidized regenerated cellulose (ORC) has been used as an absorbable hemostat since World War II. In the present study, hemostasis time was determined in a spleen incision model in swine. The effect of mass on absorbable hemostat efficacy and hemostasis time was evaluated by standardizing the ORC materials on a mass basis. The median hemostasis time for a single layer of the new nonwoven ORC was as much as 51 % shorter than woven ORC (P < 0.001). The mean hemostasis time for nonwoven ORC was not affected by the mass of hemostat applied to the wound. The hemostatic efficacy of woven ORC increased with the mass (layers) of hemostat applied to the wound. Nonwoven ORC is significantly faster in achieving hemostasis than woven ORC, and its hemostatic efficacy is not influenced by the mass of material applied. Tissue reaction was minimal and the material was fully absorbed by 14 days.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oxidized regenerated cellulose (ORC) is used to manufacture a family of absorbable hemostatic agents that have been in clinical use for more than 5 decades. (Cronkite et al. 1944; Franz et al. 1944; Putnam 1943; Gabay 2006) ORC hemostats are indicated for use in surgical procedures when conventional methods of hemostasis, such as pressure and ligature, are ineffective or impractical. ORC may be used for endoscopic procedures, and it may be cut to size to conform to the bleeding site. (Gabay 2006) ORC formulations have been used to control bleeding in a wide variety of open and endoscopic procedures, including laparoscopic partial nephrectomy, (Breda et al. 2007) laparoscopic cholecystectomy, (Rastogi and Dy 2002) liver and spleen trauma, (Theuer and Imagawa 1999; Trooskin et al. 1989) and sternotomy (Mair et al. 2005); and ear/nose/throat, (Bhatnagar and Berry 2004; Shinkwin et al. 1996) obstetrics/gynecology, (Awonuga et al. 2006; Sharma et al. 2003; Sharma and Malhotra 2006) orthopedic, (Sabel and Stummer 2004) urologic, (Abou-Elela et al. 2009; Bollens et al. 2007) and plastic surgery. (Bassetto et al. 2008)
The mechanism of action for ORC hemostats is believed to start with the material absorbing water and then swelling slightly to provide tamponade at the bleeding site. The ORC fibers initially entrap fluid, blood proteins, platelets and cells forming a gel-like “pseudo-clot” which acts as a barrier to blood flow, and subsequently as a matrix for solid fibrin clot formation. Fiber density and fabric weave or knit patterns are important features in hemostatic efficacy.
The synthesis and production of commercial ORC hemostats involve many steps that include dissolving cellulosic material in a solvent, extrusion and coagulation of the solution as continuous fibers, and oxidation by dinitrogen tetroxide under closely regulated conditions. The regenerated cellulose is obtained through the viscose process, which affords very pure cellulosic fibers as the end products. Compared with cotton cellulose, regenerated cellulose is of lower molecular weight and lower crystallinity. In addition, cotton cellulosic fibers have tapered shapes, and therefore have different diameters along the fibers. In contrast, regenerated cellulose fibers are continuous and have uniform diameters throughout the fiber length. These properties of regenerated cellulose fibers are critical to achieving subsequent uniform and consistent oxidation. The regenerated cellulose fibers are twisted into yarns that can be knitted into fabrics with different basis weights. Oxidation is then carried out on the fabrics to render them bioabsorbable when implanted into human body. (Stillwell et al. 1997)
Regenerated cellulose can be oxidized by nitrogen dioxide (NO2) or dinitrogen tetroxide (N2O4), nitric acid, periodic acid, and hypochlorite with different final products. Oxidation reaction of cellulose with NO2 transforms the primary hydroxyl groups on carbon 6 (C6) into carboxyl groups as the result of main reaction, and the end product is a copolymer of anhydroglucuronic acid and anhydroglucose. Due to the presence of carboxyl groups, ORC becomes acidic after contacting body fluids. (Stillwell et al. 1997)
The secondary reactions at C2 and C3 lead to the ketone group formation at these positions, and the ketone groups are believed to be responsible for the degradation of ORC. The combination of cellulose regeneration and uniform NO2 oxidation results in consistent absorbability and tissue reaction to the commercially available products. Implanted, ORC is fully absorbed in 7–14 days with minimal tissue reaction, and it has in vitro and in vivo antimicrobial activity against a wide range of gram-positive and gram-negative organisms, including methicillin-resistant Staphylococcus aureus (MRSA). (Johnson and Johnson 2007; Dineen 1976, 1977a, b; Kuchta and Dineen 1983; Spangler et al. 2003).
The proposed mechanism for absorption of ORC is via phagocytosis by macrophage cells. Hydrolytic enzymes in macrophage such as ß-D-glucosidase and,ß-D- glucuronidase degrade oligomers. In bench top and animal studies, the size distribution and the quantity of the oligomers decreases over time. Ultimately glucose and glucuronic acid are generated. Degradation products do not accumulate in blood or urine which is consistent with a cellular elimination mechanism (Dimitrijevich et al. 1990a, b; Stillwell et al. 1997).
Materials and methods
All test animals were handled and maintained in accordance with the current standards promulgated in the Guide for the Care and Use of Laboratory Animals. (National Academy Press 1996) This study was conducted following IACUC review and approval. Commercially available material was provided in the manufacturers’ sterile packaging. These were: original Surgicel® Absorbable Hemostat (W8), Surgicel® Nu-Knit® Absorbable Hemostat (W20), and Surgicel® Fibrillar® Absorbable Hemostat (NW35). The premarketing product was Surgicel® SNoW® Absorbable Hemostat (NW11), a nonwoven fabric with a density of 100 to 110 g/m2 made from 150 denier fiber yarn, packaged in foil and gamma sterilized to a minimum of 30 kGy. (Table 1)
Hemostasis time
The first stage of testing compared original W8 with the new nonwoven material (NW11). The second stage compared the hemostatic efficacy on a weight basis among four commercially available ORC formulations.
Experimental animals
Female crossbred Yorkshire swine, weight range 94–131 pounds, were used for hemostasis time testing. Animals were individually housed in stainless-steel cages, fed once a day with a standard pig chow and provided water ad libitum, and kept on a 12-h light/dark cycle at 71° ± 7 °F and 30–70 % relative humidity.
Porcine surgical procedure
Anesthesia was induced with an intramuscular injection of 5 mg/kg tiletamine, 5 mg/kg xylazine, and 0.011 mg/kg glycopyrrolate. An intravenous catheter was then placed in the marginal ear vein. An endotracheal tube was inserted and attached to a veterinary anesthesia machine; anesthesia was maintained by a semi–closed-circuit inhalation of isoflurane and oxygen at a flow rate of 1–2 L/min. Assisted ventilation was accomplished with a mechanical ventilator set at 8–12 respirations/min and a tidal volume of approximately 5 mL/lb body weight. Ophthalmic ointment was applied to both eyes of each anesthetized animal. Lactated Ringer’s solution was administered intravenously at approximately 5–10 mL/h. Vital signs and oxygenation were monitored throughout.
The spleen was located, incrementally externalized as needed, and kept moist with saline-soaked gauze. Beginning at the distal tip of the ventral side of the spleen, proceeding proximally, 15-mm long × 3-mm deep incisions were made with a #11 scalpel blade that had a Kelly forceps clamped onto it, thereby limiting the incision depth to 3 mm. One new site was initiated for each sample tested; 12 sites per test article were used in stage 1, and 10 sites in stage 2.
Each spleen had 2 negative control sites; the first was incised on the distal tip of the spleen at the beginning of the study, the second was incised on the proximal end of the spleen at the end of the study. By confirming that gauze/tamponade was not able to control bleeding from these 2 sites within 10 min, the consistency of the experimental model over the testing was confirmed. Some investigators quantify injury bleeding rate, however we did not. Rather, by confirming that the negative control gauze/tamponade alone did not control the bleeding in a reasonable period of time we have demonstrated adequate control of variability in injury across animals and experiments. The injury and treatment procedures for the negative controls were identical to the test articles.
Hemostat allocation and application
In stage 1, W8 and NW11 were applied as either a single or double layer to 12 incision sites. In stage 2, the test compounds were applied in layers as follows: W8, 1–4 layers; NW11, 1–3 layers; W20, 1 or 2 layers; and NW35, 1 layer. Layering enabled the comparison of each ORC formulation on a weight basis.
Hemostasis timing
The ORC hemostats were applied to a freshly created wound site, followed by dry gauze, and occlusive digital pressure (tamponade) was applied for 1 min (stage 1) or 2 min (stage 2). Following the initial tamponade, digital pressure was discontinued, the gauze pad was removed, and a 30-s hemostasis evaluation period was performed. If hemostasis was achieved within 30 s, the time to hemostasis was noted and testing was concluded for that article. The model is intended to be a highly reproducible situation which allows efficient comparison between materials. The model does not simulate complex or higher blood-flow wounds although the trends measured in this model are expected to be the same for more complicated wounds.
If hemostasis was not achieved, pressure and gauze were reapplied for additional 30-s tamponade and observation periods until hemostasis was achieved, or until the testing period reached 10 min. At 10 min, the trial was aborted as a failure and recorded as “greater than 10 min.” The hemostatic material was graded as a “pass” if hemostasis was achieved in 10 min or less, and “fail” if hemostasis was not achieved in 10 min from test article application time.
Hemostasis was defined as the absence of free-flow bleeding, which was specified as any new appearance of blood or blood flow from the incision site, or any new appearance of blood or blood flow from within, bleeding through, under, or out of the test site. Pinpoint or petechial bleeding that appeared but did not grow, or saturation of blood into the hemostat that may have occurred prior to or during tamponade application that did not spread during the observation period, was not considered free-flow bleeding.
Sites that failed to achieve hemostasis by 10 min were treated by remedial measures to stop the bleeding before proceeding to the next test site.
Tissue reaction and absorption
Experimental animals
Forty female Long-Evans rats, approximately 10 weeks old (237–277 g), were used in this study. The rats were individually housed in suspended closed-bottomed cages maintained at 70 °F and 50 % relative humidity on a 12-h light–dark cycle, and provided food and water ad libitum with wood blocks and nylon bones as environmental enrichment.
Test materials
Using aseptic technique, W8 and NW11 were cut with scissors to provide an implant of approximately 28 mg/275 g rat (i.e. 102 mg/kg), which measured 1.25 × 2 cm for NW11 and 1 × 2 cm for W8. A worst case human exposure for NW 11 is considered to be two 4 × 4 inch pieces (216 cm2) or 2376 mg (216 cm2 × 11 mg/cm2). The implant mass was proportional to approximately 2.5 times the worst-case exposure of 2376 mg in a 58 kg human (i.e. 40 mg/kg). Implant assignment was generated by the randomization function of Microsoft® Excel 2000.
Implant procedure
Anesthesia was induced with 3–5 % isoflurane at 1–2 l/min and ophthalmic ointment applied. The entire dorsum, from neck to base of tail, was shaved, scrubbed with chlorhexidine diacetate, rinsed with alcohol, and painted with an aqueous iodophor solution. A sterile drape was applied prior to surgery.
On the dorsum of each animal, parallel 3.5-cm incisions were made 0.5 cm from the vertebral column midline in the thoraco-lumbar region. A subcutaneous pocket large enough to contain a relatively flat implant was made by blunt dissection and the test materials were placed in each pocket. To serve as a location marker, an interrupted stitch of 4-0 dyed polypropylene suture was placed inside the pocket adjacent to the implants. The incision sites were closed with surgical wound clips.
Implant evaluation
Animals were humanely euthanized at 7, 14, and 28 days post implantation (N = 12, 12, and 16, respectively).
Macroscopic
At each study interval, implant sites were examined for residual hemostat and evaluated for hematoma, infection, wound dehiscence, immunologic response, or abnormal wound healing. When present, macroscopic alterations of the viscera or tissues were reported and tissue sections containing the lesions (or portions of the lesions) were collected for microscopic evaluation. All collected tissue sections were immersed in 10 % neutral buffered formalin. After adequate fixation, all collected tissues were trimmed and macroscopically evaluated. Encapsulation of the implant sites was graded using a 0–4 scale (0 = no capsule or adverse reaction area; 1 = 0–0.5 mm capsule or reaction area; 2 = 0.6–1.0 mm; 3 = 1.1–2.0 mm; and 4 = 2.1 mm or greater). A score below 3 was considered acceptable. The difference between the average scores for the NW11 and W8 at each study interval were ranked as not significant (0–0.5); trace (0.6–1.0); slight (1.1–2.0); moderate (2.1–3.0); or marked (greater than 3.0). A score below 3 was considered acceptable (Table 2).
Microscopic
After macroscopic evaluation, tissue sections were placed into tissue cassettes, processed, and stained with hematoxylin and eosin. Microscopic evaluation utilized an ISO grading scale in which a severity score of 0–4 was assigned to 9 measures of inflammation and tissue response. (ISO 2007) At each study interval, the average scores for W8 were subtracted from the average scores for NW11 and ranked as follows: nonirritant (0.0–2.9); slight irritant (3.0–8.9); moderate irritant (9.0–15.0); or severe irritant (15.1 or greater).
Absorption of the implant material was graded on the following scale: not absorbed (>10 % of the material remained); essential absorption (<10 % of the material remained); complete absorption (no material remained, but implant site identified); implantation site not present (no material remained and there was no evidence of the implant site); return to normal (implant site identified, article completely absorbed, and the tissue response resolved).
Results
Hemostasis time stage 1
Gauze (negative control) passed 0 of 12 times. W8 1 layer (positive control) passed 4 of 12 times. W8 2 layer passed 12 of 12 times. Single or double layers of NW11 passed 12 of 12 times (see Table 2).
A Kruskal–Wallis test was used to compare the time to hemostasis for W8 versus NW11, as shown in Table 3. NW11 was significantly faster than W8, regardless of the number of layers or formulation, as shown in Table 3.
Hemostasis time stage 2
The 4 ORC formulations each had a different basis mass (Table 4) and were applied in layers to normalize the hemostat mass for comparison. The hemostasis time and the effect of layering (increasing the mass of hemostat) are shown in Table 5.
As shown in Table 5, when W8 was applied in 1, 2, or 3 layers (8–24 mg/cm2), hemostasis within 10 min was achieved in 50 % or fewer sites. When W8 was applied in 4 layers (32 mg/cm2), hemostasis was achieved in 75 % of sites. NW11, W20, and NW35 achieved hemostasis within 10 min regardless of the number of layers used (11–40 mg/cm2). The hemostatic effectiveness of W20 was improved by adding more layers over the wound site.
A Chi square test for trend on the 4 W8 groups did not find a significant linear trend among the success rate for 1, 2, or 3 layers (Graphpad Instat v. 3.10). ANOVA on all other groups (NW11, W20, and NW35) did not find any significant differences at p < 0.05 (Graphpad Prism 5.03).
Tissue reaction and absorption
There were no biologically relevant treatment-related in-life incidents during the duration of this study.
Absorption
Both W8 and NW11 were present at day 7 in all test sites and were considered not absorbed. On day 14 the implants were essentially (i.e., <10 % remaining) or fully absorbed in 7 NW11 and 9 W8 sites. Both implants were completely absorbed by day 28. Under the conditions of this study, NW11 and W8 had similar absorption profiles and were considered fully absorbed by 14 days.
Tissue reaction
The macroscopic tissue response to NW11 was not significantly different than the response to W8. The difference between the encapsulation grading scores for NW11 versus W8 groups was 0.0, 0.25, and 0.06 at days 7, 14, and 28, respectively. In implant sites with encapsulation scores above 0, there were no microscopic findings that corresponded directly with the encapsulation score.
The microscopic tissue response to NW11 was not significantly different than the response to W8 at any of the study intervals. NW11 was ranked as a nonirritant compared to W8 using the ISO scale. The average tissue response grading score at days 7, 14, and 28 for NW11 versus W8 were 7.6 versus 6.3, 6.6 versus 6.4, and 3.8 versus 3.0, respectively. At days 7 and 14 the overall tissue response to either implant was considered to be minimal to mild and characterized by limited to low numbers of macrophages, lymphocytes, and fibroblasts and lower numbers of giant cells. At day 28 the tissue response was resolving. Because the implants were absorbed, and because the inflammatory reaction was so slight at 28 days it is expected that the sites would return to a normal steady state within weeks. There was no evidence of hematoma, infection, wound dehiscence, immunologic response, or abnormal wound healing at any time. (Table 6)
Discussion
The splenic laceration model was used to compare several forms of oxidized regenerated cellulose hemostats for use on wounds with mild to moderate bleeding.
In the first stage of hemostasis testing, single and double layers of NW11 successfully achieved hemostasis, as did double layers of original W8. The time to achieve hemostasis was significantly less for NW11 than for W8. In fact, single layers of NW11 were faster than double layers of original W8, and double layers of NW11 achieved hemostasis in half the time of original W8. With NW11 the reduction in hemostasis time ranged from 31 to 51 % compared to original W8.
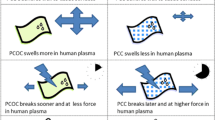
The general observation that hemostasis time decreased as the number of layers of hemostat (and therefore hemostat mass) increased led us to compare the hemostasis time for several ORC formulations that were normalized on a mass basis by layering. Table 4 shows the basis mass of the 4 ORC formulations (ranging from 8 mg/cm2 to 35 mg/cm2) and the number of layers required to deliver approximately equivalent amounts of hemostat to the wound site. As seen in Fig. 1, non-woven materials have a much higher surface area as compared to woven materials. We speculate that additional layers of non-woven material compensate for the relatively lower surface area of these materials. If this speculation is accurate, it would explain why additional layers of non-woven hemostats are not necessary to achive similar hemostatic efficacy.
The original W8 formulation appeared to work better by adding more layers, as the success ratio increased when more layers were applied; likewise the hemostasis time decreased with increased layers. In contrast, NW11 was 100 % successful and the hemostasis time was consistent (5.4–5.9 min) regardless of the number of layers. The hemostatic performance of W20 was somewhat in-between W8 and NW11; its hemostatic effectiveness was 100 % regardless of the mass, but hemostasis time was shorter with 2 layers compared to 1 layer (3.7 min vs 5.6 min). Lastly, NW35, the most dense formulation, was 100 % effective and had a hemostasis time of 5.7 min. These data suggest that hemostatic performance of nonwoven ORC materials (NW11 and NW35) does not depend on the mass of the implant, but mass may be important for knitted forms (W8 and W20).
Hemostasis time is one of the important considerations when choosing an absorbable hemostat. Although hundreds of articles have been published on absorbable hemostats, relatively few address hemostasis time, and even fewer compare hemostasis time between products. Among studies that evaluate hemostasis time, accurate comparison is complicated by the variety of methods and models used to evaluate hemostatic control. In the present study 4 varieties of ORC were directly compared in a swine splenic injury model and the hemostatic performance was carefully related to the mass of the hemostat. NW11 was found to be effective in a single-layer application and could reduce hemostasis time by more than 50 % relative to original ORC.
Original ORC has also been compared to commercial (Schwartz et al. 2004; Kheirabadi et al. 2002) and patient-derived (Davidson et al. 2000; Hanks et al. 2003) fibrin sealants. In a human trial Schwartz and colleagues reported that hemostasis with an investigational fibrin sealant was approximately twice as fast as all other commercial hemostats combined; however, comparison between fibrin sealant and original ORC is precluded because the hemostasis times for 7 different commercial hemostats were combined and averaged as a single group. (Schwartz et al. 2004) Kheirabadi and coworkers compared fibrin sealant with ORC, microfibrillar collagen, and gelatin sponge hemostats in sealing an aortic anastomosis in heparinized rabbits. (Kheirabadi et al. 2002) While this model found fibrin sealant effective, performance was measured in volume of blood loss through a suture line rather than hemostasis time of an open wound, which impedes comparison with the present study. Davidson and colleagues compared original ORC with autologous fibrin sealant in a swine model of partial hepatectomy and found that fibrin sealant and ORC both significantly reduced bleeding volume and hemostasis time compared to untreated controls. (Davidson et al. 2000) Hanks and coworkers compared original ORC with autologous fibrin sealant in 69 patients in a broad range of surgical procedures. They found the hemostasis time for fibrin sealant was approximately half that of ORC, but digital compression was not used and the amount of ORC was unspecified. (Hanks et al. 2003)
The tissue response was acceptable and considered equivalent for NW11 and original W8 at 7, 14, and 28 days post implantation. At necropsy, there was no microscopic or macroscopic evidence of treatment-related dehiscence of skin incisions, biologically relevant hematoma formation, failure to absorb, or delayed wound healing. Under the conditions of this study, NW11 and W8 had similar absorption profiles and were considered absorbed by 14 days. These data demonstrate the biocompatibility of this new form of ORC hemostat.
Because many surgeons report frustration that hemostatic agents target mild to moderate bleeding and call for more advanced hemostats that can be used to treat more difficult bleeding situations (i.e. where clotting factors and platelets may be deficient) further studies comparing the efficacy of hemostatic products in severe bleeding models may be pursued next.
Conclusion
In conclusion, under the conditions of this study, the hemostatic performance of nonwoven ORC materials does not depend on the mass of the implant, but mass may be important for knitted forms. We speculate that additional layers of non-woven material compensate for the relatively lower surface area of these materials. ORC was well tolerated and essentially absorbed by 14 days after implantation. To our knowledge this is the only publication comparing the hemostatic performance and local tolerance of these four materials. As such these data provide guidance to users of these products to select the best material to balance acceptable hemostatic performance with minimal implanted material.
References
Abou-Elela A, Morsy A, Badawy H, Fathy M (2009) (2009) Use of oxidized cellulose hemostats (Surgicel®) to support parenchymal closure and achieve hemostasis following partial nephrectomy. Surg Technol Int 18:75–79
Awonuga AO, Merhi ZO, Khulpateea N (2006) Abdominal packing for intractable obstetrical and gynecologic hemorrhage. Int J Gynaecol Obstet 93:160–163
Bassetto F, Vindigni V, Scarpa C, Botti C, Botti G (2008) Use of oxidized regenerated cellulose to stop bleeding after a facelift procedure. Aesthetic Plast Surg 32:807–809
Bhatnagar RK, Berry S (2004) Selective Surgicel packing for the treatment of posterior epistaxis. Ear Nose Throat J 83:633–634
Bollens R, Rosenblatt A, Espinoza BP, De Groote A, Quackels T, Roumeguere T et al (2007) Laparoscopic partial nephrectomy with “on-demand” clamping reduces warm ischemia time. Eur Urol 52:804–809
Breda A, Stepanian SV, Lam JS, Liao JC, Gill IS, Colombo JR et al (2007) Use of haemostatic agents and glues during laparoscopic partial nephrectomy: a multi-institutional survey from the United States and Europe of 1347 cases. Eur Urol 52:798–803
Cronkite EP, Deaver JM, Lozner EL (1944) Experiences with use of thrombin with and without soluble cellulose for local hemostasis, War Medicine V5 p. 80–82
Davidson BR, Burnett S, Javed MS, Seifalian A, Moore D, Doctor N (2000) Experimental study of a novel fibrin sealant for achieving haemostasis following partial hepatectomy. Br J Surg 87:790–795
Dimitrijevich D, Tatarko M, Gracy RW (1990a) Biodegradation of oxidized regenerated cellulose. Carbohydr Res 195:247–256
Dimitrijevich SD, Tatarko M, Gracy RW, Wise GE, Oakford LX (1990b) In vivo degradation of oxidized, regenerated cellulose. Carbohydr Res 198:331–341
Dineen P (1976) Antibacterial activity of oxidized regenerated cellulose. Surg Gynecol Obstet 142:481–486
Dineen P (1977a) The effect of oxidized regenerated cellulose on experimental intravascular infection. Surgery 82:576–579
Dineen P (1977b) The effect of oxidized regenerated cellulose on experimental infected splenotomies. J Surg Res 23:114–116
Franz VK, Clarke HT, Lattes R (1944) Hemostasis with Absorbable Gauze (Oxidized Cellulose), Annals of Surgery p. 181–198
Gabay M (2006) Absorbable hemostatic agents. Am J Health-Syst Pharm 63:1244–1253
Hanks JB, Kjaergard HK, Hollingsbee DA (2003) A comparison of the haemostatic effect of Vivostat® patient-derived fibrin sealant with oxidised cellulose (Surgicel®) in multiple surgical procedures. Eur Surg Res 35:439–444
ISO (2007) ISO 10993–6 Biological evaluation of medical devices. Tests for local effects after implantation. International Organization for Standardization, Geneva
Johnson and Johnson (2007), Prescribing Information, Surgicel Original, Surgicel Nu-Knit and Surgicel Fibrillar absorbable hemostats (oxidized regenerated cellulose). Johnson and Johnson Wound Management, a Division of Ethicon, Inc, Somerville
Kheirabadi BS, Field-Ridley A, Pearson R, MacPhee M, Drohan W, Tuthill D (2002) Comparative study of the efficacy of the common topical hemostatic agents with fibrin sealant in a rabbit aortic anastomosis model. J Surg Res 106:99–107
Kuchta N, Dineen P (1983) Effects of absorbable hemostats on intraabdominal sepsis. Infect in Surg 2:441–444
Mair H, Kaczmarek I, Oberhoffer M, Groetzner J, Daebritz S, Reichart B (2005) Surgicel Nu-Knit hemostat for bleeding control of fragile sternum. J Thorac Cardiovasc Surg 130:605–606
Press National Academy (1996) Guide for the care and use of laboratory animals. Institute for Laboratory Animal Research (ILAR). National Academy Press, Washington
Putnam TJ (1943) The use of thrombin on soluble cellulose in neurosurgery. Ann Surg 118:127–130
Rastogi V, Dy V (2002) Control of port-site bleeding from smaller incisions after laparoscopic cholecystectomy surgery: a new, innovative, and easier technique. Surg Laparosc Endosc Percutan Tech 12:224–226
Sabel M, Stummer W (2004) Haemostasis in spine surgery. The use of local agents: surgicel and Surgifoam. Eur Spine J 13(suppl 1):S97–S101
Schwartz M, Madariaga J, Hirose R, Shaver TR, Sher L, Chari R (2004) Comparison of a new fibrin sealant with standard topical hemostatic agents. Arch Surg 139:1148–1154
Sharma JB, Malhotra M (2006) Successful management of uterine incision hemorrhage in caesarean section with topical oxidized regenerated cellulose (Surgicel Nu Nnit): a case report. Arch Gynecol Obstet 274:115–116
Sharma JB, Malhotra M, Pundir P (2003) Laparoscopic oxidized cellulose (Surgicel) application for small uterine perforations. Int J Gynaecol Obstet 83:271–275
Shinkwin CA, Beasley N, Simo R, Rushton L, Jones NS (1996) Evaluation of Surgicel Nu-knit, Merocel and Vasolene gauze nasal packs: a randomized trial. Rhinology 34:41–43
Spangler D, Rothenburger S, Nguyen K, Jampani H, Weiss S, Bhende S (2003) In vitro antimicrobial activity of oxidized regenerated cellulose against antibiotic-resistant microorganisms. Surg Infect (Larchmt) 4:255–262
Stillwell L, Marks MG, Safertsein L, Wiseman DM (1997) Oxidized cellulose: chemistry, processing and medical applications. In: Domb AJ, Kost J, Wiseman DM (eds) Handbook of biodegradable polymers. Harwood Academic Publishers, Amsterdam, pp 291–306
Theuer CP, Imagawa DK (1999) Use of knitted oxidized cellulose (Nu-knit) for the definitive packing of grade III liver fracture. Injury 30:137–140
Trooskin SZ, Flancbaum L, Boyarsky AH, Greco RS (1989) A simplified approach to techniques of splenic salvage. Surg Gynecol Obstet 168:546–548
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hutchinson, R.W., George, K., Johns, D. et al. Hemostatic efficacy and tissue reaction of oxidized regenerated cellulose hemostats. Cellulose 20, 537–545 (2013). https://doi.org/10.1007/s10570-012-9828-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-012-9828-8