Abstract
A mechanism for chemical attachment and growth of a Cu-BTC Metal–Organic Framework, also known as MOF-199 or HKUST-1, onto cellulosic substrates is reported. Four different experimental procedures were attempted in order to elucidate the role of carboxylate groups on the anionic cellulose’s surface. The order of addition of Cu(OAc)2—copper acetate, BTH3, 1,3,5-benzenetricarboxylic acid and TEA—Triethylamine was found to be a critical factor for the attachment and growth of the MOF-199 crystals onto anionic cellulose. The presence of MOF-199 crystals was probed using XRD and XPS spectra and a strong chemical interaction to the carboxymethylated cellulose fibers was confirmed by intense and vigorous washing of the specimens with water, DMF and methanol. Based on the recognized ability of MOF-199 to capture gases and toxic chemicals, combined with the availability of cellulose-based fibrous materials, the described procedure provides the basis for future fabrication of functionalized fibers and active filtration media.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulose is the most abundant renewable polymer in nature and cellulose-based fibers have found several industrial applications such as coatings, laminates, optical films, pharmaceuticals, foods and textiles (Kim et al. 2006). Due to the abundance of hydroxyl groups on cellulose a myriad of chemistries have been attempted making cellulose a very versatile substrate. Some of these modifications include the introduction of stable negative or positive electrostatic charges via carboxymethylation and cationization procedures (Habibi et al. 2010, Hyde et al. 2007).
Metal–Organic Frameworks (MOFs) are a recently-identified class of crystalline nanoporous materials built of small metal-containing clusters connected three-dimensionally by polyfunctional organic ligands (Eddaoudi et al. 2002; Bordiga et al. 2007; Prestipino et al. 2006). MOFs exhibit large surface areas and are the focus of intense research for a range of applications such as serving as host molecules for gases and other molecules (Britt et al. 2008; 2009; Furukawa and Yaghi 2009).
The gas sorption properties of MOFs have been extensively reported in literature suggesting many practical applications such as gas storage and active filtration media (James 2003; Fletcher et al. 2005; Dincă and Long 2008). For example, Cui et al. (2009) reported the utilization of MOF films on stainless steel fibers for the solid-phase microextraction of volatile and harmful benzene homologues. Yehia et al. (2004) prepared mixed matrix membranes by mixing MOFs with poly(3-acetoxyethylthiophene) (PAET) and reported that MOFs selectively adsorbed methane. Keskin and Sholl (2010) also reported that the addition of particles to a polymeric matrix represents an important method for enhancing the performance of these polymeric materials for potential applications in gas separation processes. More recently, Kaskel et al. (2011) immobilized MOF-199 in polymer (polystyrene, polyvinylpyrrolidone and polyacrylonitrile) by electrospinning to obtain composite fibers with MOF particles. However, as far as the authors know there are no reports on the chemical attachment of MOFs to polymeric substrates or cellulosic fibers. Creating a chemical interaction (i.e. mixing of supramolecular forces and covalent bonds) has the advantage of extending the shelf-life of the attached compounds allowing their continued use in devices (Goddard and Hotchkiss 2007). In this manuscript we present a method to chemically attach and grow MOF 199 to the surface of cellulosic fibers. While the method hereby described can be extrapolated to other MOFs, MOF 199 was selected due to its easy synthesis and the presence of open metal sites which are important in order to achieve high storage capacities of gases such as hydrogen and methane (Eddaoudi et al. 2002; Rowsell and Yaghi 2005; Tranchemontagne et al. 2008; Chui et al. 1999). MOF 199 is composed of dimeric cupric tetracarboxylate units with a short Cu–Cu internuclear separation and because it exhibits a unique turquoise-blue color, confirmation of its attachment to the cellulosic substrates could be confirmed optically.
Experimental section
Materials
Natural cellulose fabrics (TIC/400R standard woven cotton fabrics) were purchased from SD Atlas and used as received. Copper (II) acetate, 1,3,5-benzenetricarboxylic acid, methanol, N,N-dimethylformamide (DMF), sodium hydroxide, sodium chloroacetate and ethanol were obtained from Sigma-Aldrich Company (St. Louis, MO). All chemicals and reagents used were analytical grade and used as received.
Instrumentation
Scanning electron microscopy (SEM) micrographs were obtained on a LEO 1550 FE-SEM at 5 kV with the specimens being carbon-coated before imaging. X-ray diffraction patterns were collected with a Rigaku SmartLab X-Ray Diffractometer. Fourier Transform Infrared (FTIR) spectroscopy was carried out in a Nicolet Magna 760 FTIR spectrometer (Thermo Fisher Scientific Inc., Waltham, MA) in single attenuated total reflectance (ATR) mode. X-ray Photoelectron Spectroscopy (XPS) spectra were obtained on a Surface Science Instruments model SSX-100, using a monochromated Al KR X-ray source.
Carboxymethylation of cellulose substrates
In order to create a suitable substrate where Cu ions could be easily anchored, as a first step of the MOF199 synthesis, natural cellulosic substrates (cotton) were rendered anionic as shown in the upper section of Fig. 1. The procedure for carboxymethylation of cellulose was based on the classical reaction between cellulose and chloroacetate salt under the presence of sodium hydroxide as a catalyst (Wang et al. 2011). In this study, solutions of 1 M sodium chloroacetate and 5 % sodium hydroxide were left reacting with cellulose for 1 h. Carboxymethylated fibers were vigorously washed with distilled water for removal of residual reactants.
Experimental procedures (A, B, C, and D) followed for the in situ formation of Metal–organic Framework MOF199 onto anionic cellulose substrates using as Cu(OAc)2—copper acetate, BTH3, 1, 3, 5-benzenetricarboxylic acid and TEA—triethylamine. The anionization of the cellulosic substrates is shown in the upper section of the figure and it was common for all experimental procedures. The fabrics were washed with water, DMF (dimethylformamide) and MeOH (methanol) to detach any loosely MOF and only specimens from procedures A and C exhibited the blue color characteristic of MOF 199. (Color figure online)
Attachment of MOF 199 to the anionic modified cellulose
The synthesis of MOF 199 was performed following a modified methodology based on a method described by Tranchemontagne et al. (2008). Four different experimental procedures (lower section of Fig. 1) were pursued in order to determine the effect of the order of addition on the attachment and synthesis of MOF 199 as well as to elucidate the role of the carboxylic groups grafted on the surface of the anionic cellulose during carboxymethylation. The four experiments pursued are described as follows:
Procedures A and C
Copper acetate (860 mg) was mixed in 12 mL of a solvent mixture DMF:ethanol:water (1:1:1) and left overnight reacting in the presence of an anionic cellulose fabric swatch (0.17 g). Then, 1,3,5-benzenetricarboxylic acid (500 mg) previously dissolved in 12 mL of the same solvent mixture was added dropwise and kept stirring for another 24 h. In the case of experimental procedure A, 0.5 mL of triethylamine were added to the solution immediately after the addition of 1,3,5-benzenetricarboxylic acid while in experimental procedure C, triethylamine was not used.
Procedures B and D
Copper acetate (860 mg), 1,3,5-benzenetricarboxylic acid (500 mg) and a swatch of anionic cellulose (0.17 g) were mixed in 24 mL of a solvent mixture DMF:ethanol:water (1:1:1) and left overnight under a vigorous stirring. For experimental procedure B, 0.5 mL of triethylamine were added to the solution immediately after addition of 1,3,5-benzenetricarboxylic acid. Procedure D did not use triethylamine.
In all cases, after procedures A, B, C and D the modified cellulose specimens were washed subsequently with distilled water, DMF and methanol. The specimens were washed in these three solvents, 5 h in each, in order to remove MOF-199 crystals that were not chemically attached to the cellulose fibers.
Results and discussion
After following experimental procedures A, B, C and D as well as intensive and vigorous washing of the specimens in three different solvents, it was observed that samples from experiments A and C obtained a turquoise-blue color which was of a similar shade than the characteristic color reported for MOF-199 crystals (Bordiga et al. 2007). In order to validate this indirect evidence of the formation of MOF-199 crystals on the surface of the cellulose substrates, X-ray diffraction (XRD) analyses were performed as shown in Figs. 2 and 3. Figure 2 (top) shows the XRD pattern of MOF-199 crystals synthesized without the presence of cellulose substrates and strictly following the procedure reported by Tranchemontagne et al. (2008). The arrows on the spectrum, matching some of the characteristic peaks, correspond to simulated XRD patterns indicating a quantitative agreement between the pattern of our synthesized crystals and those of MOF-199. Figure 2 (bottom) corresponds to the XRD spectrum of a sample of anionic cotton showing a characteristic broad peak of crystalline cellulose at 2θ = 22.5° which is in accordance with previous reports including the latest by Sun et al. (2007). Once the spectra of pristine MOF-199 and that of anionic cellulose were obtained (Fig. 2), XRD spectra of specimens that underwent experimental procedures A, B, C, and D were obtained and are shown in Fig. 3 along with photographs of the specimens. As illustrated in the photographs, only samples from procedures A and C showed a turquoise-blue coloration even after the intensive washing with water, DMF and ethanol. The thorough washing procedure was aimed at removing MOF-199 crystals that were loosely attached to the substrates. Samples from procedures B and D showed massive discoloration and just small traces of blue. XRD spectra confirmed the optical observations as specimens undergoing procedures A and C exhibited XRD patterns that can be construed as a superposition of the spectra of MOF-199 and cellulose shown in Fig. 2. This superposition is more evident a smaller values of 2θ where MOF-199 characteristic peaks are clearly shown and not overshadow by the broad and large peak of crystalline cellulose. In sharp contrast, the XRD spectra for samples undergoing experimental procedures B and D were very similar to that of pristine anionic cellulose and no characteristic peaks indicating the formation of MOF-199 were observed. Since the main difference among the experimental procedures was the order of addition of the reactants, we could assume from the XRD and optical analyses, that the order of addition was a critical and determinant factor.
XRD spectra of synthesized MOF 199 (top). The peaks highlighted with the red point indicate agreement between the experimental measurements and the peaks from a simulated pattern reported by Tranchemontagne et al. (2008). The XRD spectra of anionic cotton is presented in the bottom of the figure. (Color figure online)
XRD patterns and optical images of anionic cellulose specimens exposed to different experimental procedures (A, B, C and D) and further washing with water, DMF and EtOH. The optical images for specimens exposed to procedures A and C show a blue shade after the three successive washing treatments corroborating indicating the successful attachment and growth of MOF199 crystals onto the cellulose specimens. (Color figure online)
Incorporating Cu(II) to the modified cellulose specimens using copper acetate was designed as the first and anchoring step for all experimental procedures aimed at attaching and growing MOF-199 crystals on the surface of anionic cellulose. Copper ions were expected to be captured by the carboxylate groups on the surface of the anionic cellulose via an ion exchange mechanism. These trapped copper ions were expected to act as nucleation centers and anchors for the later formation of MOF-199 crystals. The positive formation of bonded MOF-199 for the samples undergoing procedures A and C could be explained via the pKa of the acetic acid (pKa = 4.76) and the pKa of the benzenetricarboxylic acid (pKa = 3.16). For each copper ion trapped by the modified cellulose, one acetate ion gets free, which in turn might steal the more acidic hydrogen from the benzenetricarboxylic molecule, yielding a benzenecarboxylate ion which is needed for the formation of the MOF-199 structure. This also explains why the addition of TEA in procedure C is no needed. The overnight time given to the ion exchange process between the copper acetate and the cellulosic substrates was expected to yield a high density of copper ions on the surface of the specimens. The more copper trapped the more tricarboxylate that can be formed and hence a higher yield of MOF-199 formation.
However, for the samples exposed to procedures B and D, since all the reactants are mixed together at the beginning of the experiments, there is a competition between the aliphatic carboxylate ions (those in the cellulosic substrates and those in the copper acetate) for the more acidic hydrogen present in the tricarboxylic acid. The carboxylate ions on the modified cellulose appear to have prevailed, leading to a very acidic cellulose that does not trap copper, hence favoring to the formation of free MOF-199 crystals in the solvent. Due to sedimentation, some of these MOF-199 crystals deposited on the surface of the cellulosic substrates but they were easily removed during the intense washing of the samples with three different solvents.
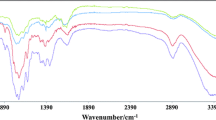
Evidence of a higher density of copper in the samples undergoing experimental procedures A and C is available from XPS analysis as shown in Fig. 4. As expected, for cellulose based fibers the spectra shows mainly signals from carbon (C1s) and oxygen (O1s). These results are in quantitative agreement with those reported by Matuana et al. (2001) when they probed the surface of esterified cellulosic fibers via XPS. Atomic analysis also shows that the percentage of copper (Cu 2p) for the sample in procedure B was 0.25 % while samples from procedures A and C exhibited almost twice that value (0.49 and 0.54 %, respectively). The chemical attachment formed under the procedure hereby reported, consisting of metal coordination forces among the metal carbonyl clusters that form the MOF and covalent bonds between the cellulose back bone and at least one carboxylate pendant groups from the MOF was verified using several techniques. FTIR-ATR experiments were performed in samples exposed to experimental procedures A and C and compared to the spectrum of pristine anionic cellulose as shown in Fig. 5. Although there were no carbonyl signal shifts between the anionic cellulose and the cellulose-MOF specimens, an increase in the intensity signal at 1,645 cm−1 was observed which is possibly due to a greater amount of carbonyl groups added by the MOF. An increase in the intensity signal at 2,891 cm−1 was also noted. While results from XRD, XPS and FTIR–ATR analytical techniques are in agreement, they only provide an indirect evidence of the synthesis of the MOF-199 crystals on the surface of the fibers. FESEM imaging of the specimens was performed in order to gather direct evidence of the presence of the MOF-199 crystals as shown in the micrographs in Fig. 6. Figure 6 (above) shows crystals of MOF-199 formed during experimental procedure A. The crystals appear to have a broad distribution of sizes varying from 200 nm to a few microns in characteristic length. The shapes of some of the larger crystals are very distinctive for MOF-199. The inset of Fig. 6 (above) shows an energy dispersive X-ray spectroscopy (EDX) analysis of the area being imaged by the FESEM corroborating the presence of copper. Figure 6 (below) shows MOF-199 crystals over the surface of cellulose fibers that underwent experimental procedure C and the size, size distribution and geometries are very similar to those in Fig. 6 (above).
XPS spectra of three different specimens after being exposed to experimental procedures (A, B, and C) aimed at growing MOF 199 crystals on the surface of anionic cellulose. The elemental composition (%) for copper (Cu 2p) appears to be in agreement with the shades of blue of the specimens and the XRD patterns shown in Fig. 3. Samples from procedures A and C contain almost twice the amount of copper than the sample from procedure B hence highlighting the critical step of copper attachment to the cellulosic substrates in the in situ synthesis of MOF 199
Conclusions
The present study reports on methods related to the fabrication of functionalized fibers possibly capable of selectively absorbing gases and toxic chemicals. The results obtained indicate that the attachment of MOF-199 to the carboxylate groups from the anionic cellulose is indeed possible and that the presence of the carboxyl groups in the modified cellulose plays a stabilizing role in the formation of MOF-199 crystals.
References
Bordiga S, Regli L, Bonino F, Groppo E, Lamberti C, Xiao B, Wheatley PS, Morris RE, Zecchina A (2007) Adsorption properties of HKUST-1 toward hydrogen and other small molecules monitored by IR. Phys Chem Chem Phys 9:2676–2685
Britt D, Tranchemontagne D, Yaghi OM (2008) Metal-organic frameworks with high capacity and selectivity for harmful gases. PNAS 105(33):11623–11627
Britt D, Furukawa H, Wang B, Glover TG, Yaghi OM (2009) Highly efficient separation for carbon dioxide by a metal-organic framework replete with open metal sites. PNAS 106(49):20637–20640
Chui SSY, Lo SMF, Charmant JPH, Orpen AG, Williams ID (1999) A chemically functionalizable nanoporous material [Cu3(TMA)2(H2O)3]n. Science 283(5405):1148–1150
Cui XY, Gu ZY, Jiang DQ, Li Y, Wang HF, Yan XP (2009) In situ hydrothermal growth of metal-organic framework 199 films on stainless steel fibers for solid-phase microextraction of gaseous benzene homologues. Anal Chem 81:9771–9777
Dincă M, Long JR (2008) Hydrogen storage in microporous metal-organic frameworks with exposed metal sites. Angew Chem Int Ed 47:6766–6779
Eddaoudi M, Kim J, Rosi N, Vodak D, Watcher J, O’Keeffe M, Yaghi OM (2002) Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 295:469–472
Fletcher AJ, Thomas MK, Rosseinsky MJ (2005) Flexibility in metal-organic framework materials: impact on sorption properties. J Solid State Chem 178:2491–2510
Furukawa H, Yaghi OM (2009) Storage of hydrogen, methane, and carbon dioxide in highly porous covalent organic frameworks for clean energy applications. J Am Chem Soc 131:8875–8883
Goddard JM, Hotchkiss JH (2007) Polymer surface modification for the attachment of bioactive compounds. Prog Polym Sci 32:698–725
Habibi Y, Lucia LA, Rojas OJ (2010) Cellulose nanocrystals: chemistry, self-assembly, and applications. Chem Rev 110:3479–3500
Hyde K, Dong H, Hinestroza JP (2007) Effect of surface cationization on the conformal deposition of polyelectrolytes over cotton fibers. Cellulose 14(6):615–623
James SL (2003) Metal-organic frameworks. Chem Soc Rev 32:276–288
Kaskel S, Rose M, Bohringer B, Jolly M, Fischer R (2011) MOF processing by electrospinning for functional textiles. Adv Eng Mater 13:356–360
Keskin S, Sholl DS (2010) Selecting metal-organic frameworks as enabling materials in mixed membranes for high efficiency natural gas purification. Energy Environ Sci 3:343–351
Kim J, Yun S, Ounaies Z (2006) Discovery of cellulose as a smart material. Macromolecules 39:4202–4206
Matuana LM, Balatinecz JJ, Sodhi RNS, Park CB (2001) Surface characterization of esterified cellulosic fibers by XPS and FTIR spectroscopy. Wood Sci Technol 35:191–201
Prestipino C, Regli L, Vitillo JG, Bonino F, Damin A, Lamberti C, Zecchina A, Solari PL, Kongshaug KO, Bordiga S (2006) Local structure of framework Cu(II) in HKUST-1 metallorganic framework: spectroscopic characterization upon activation and interaction with adsorbates. Chem Mater 18:1337–1346
Rowsell JLC, Yaghi OM (2005) Strategies for hydrogen storage in metal-organic frameworks. Angew Chem Int Ed 44:4670–4679
Sun Y, Lin L, Pang C, Deng H, Peng H, Li J, He B, Liu S (2007) Hydrolysis of cotton fiber cellulose in formic acid. Energy Fuels 21(4):2386–2389
Tranchemontagne DJ, Hunt JR, Yaghi OM (2008) Room temperature synthesis of metal-organic frameworks: MOF-5, MOF-74, MOF-177, MOF-199, and IRMOF-0. Tetrahedron 64:8553–8557
Wang Z, Hauser PJ, Laine J, Rojas OJ (2011) Multilayers of low charge density polyelectrolytes on thin films of carboxymethylated and cationic cellulose. J Adhes Sci Technol 25:643–660
Yehia H, Pisklak TJ, Ferraris JP, Balkus KJ, Musselman IH (2004) Methane facilitated transport using copper (II) biphenyl dicarboxylate-triethylenediamine poly(3-acetoxyethylthiophene) mixed matrix membranes. Polym Prepr 45:35–36
Acknowledgments
Financial support for this work via grant HDTRA1-08-1-0023 from the Defense Threat Reduction Agency (DTRA) is acknowledged and highly appreciated. Special thanks to Mick Thomas and John Hunt (FESEM), Jonathan Shu (XPS), and Maura Weathers (XRD). This work made use of the electronic microscopy facility of the Cornell Center for Materials Research (CCMR) with support from the National Science Foundation Materials Research Science and Engineering Centers (MRSEC) program (DMR 1120296). Special Thanks to Professor Omar Yaghi at UCLA and Dr. Hiroyasu Furukawa for introducing the authors to the wonderful world of reticular chemistry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Silva Pinto, M., Sierra-Avila, C.A. & Hinestroza, J.P. In situ synthesis of a Cu-BTC metal–organic framework (MOF 199) onto cellulosic fibrous substrates: cotton. Cellulose 19, 1771–1779 (2012). https://doi.org/10.1007/s10570-012-9752-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-012-9752-y