Abstract
Chitosan was modified by grafting 2-pyridyl-ethyl moieties on the biopolymer backbone for the synthesis of a Platinum Group Metal (PGM) sorbent. The sorbent was tested for Pd(II) and Pt(IV) sorption from HCl solutions. Stable for HCl concentrations below 0.5 M, the sorbent reached sorption capacities as high as 3.2 and 2.6 mmol metal g−1 for Pd(II) and Pt(IV), respectively. Metal sorption mainly proceeds by electrostatic attraction in acidic solutions, though a contribution of complexation mechanism cannot be totally rejected. The resistance to intraparticle diffusion is the main controlling mechanism for uptake kinetics. While agitation speed has a limited effect on kinetics, metal concentration and sorbent dosage have a greater effect on the kinetic profiles. The intraparticle diffusivity varies between 3 × 10−11 and 4.5 × 10−10 m2 min−1. Thiourea (combined with HCl solution) is used for Pd(II) and Pt(IV) desorption. The resin could be desorbed and recycled for a minimum of five cycles maintaining high efficiencies of sorption and desorption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The extraction and the recovery of precious group metals (PGMs) is becoming a challenge for industry since metals such as palladium or platinum are: (a) widely used (for catalytic applications, and for electronic devices), (b) poorly abundant (the limited resource in a limited number of countries makes these metals very strategic), and (c) very expensive (above 540 and 1700 $/once for Pd and Pt, respectively). The raw resource being limited, the recovery of the PGMs from secondary sources is attracting a growing number of studies from academic and industrial communities. The waste materials such as spent catalysts, electronic devices may contain significant amounts of PGMs that deserve designing recovery processes (Kang and Schoenung 2005). The recovery of PGMs from waste materials is operated by a series of steps including grinding, magnetic separation, acidic leaching (for metal transfer from solid to liquid phase), and then selective precipitation, solvent extraction or sorption on resins (Brooks 1991; Barakat and Mahmoud 2004; Barakat et al. 2006; Boricha et al. 2007; Shams and Goodarzi 2006). Sorption allows concentrating the target metals in the solid phase, which can be finally valorized by desorption, thermal degradation and so on. While solvent extraction is generally designed for the extraction of metal ions from high-medium concentrated solutions (Dominguez et al. 2002; Preston and du Preez 2002; Dakshinamoorthy and Venugopal 2005; Malik and Paiva 2009; Ciezynska et al. 2007) sorption processes are preferred when the concentration is below 100 mg L−1. Sorption may proceed on ion exchange or chelating resins (Jermakowicz-Bartkowiak 2005; Venkatesan et al. 2005; Hubicki and Wołowicz 2009; Birinci et al. 2009; Venkatesan et al. 2007; Parodi et al. 2008; Hubicki et al. 2008; Cortina et al. 1998; Jermakowicz-Bartkowiak and Kolarz 2002; Qu et al. 2006; Memon et al. 2008), or extractant impregnated resins (Saitoh et al. 2007; Vincent et al. 2008a, b). However, recently a number of alternative materials have been investigated for the recovery of PGMs from dilute acid solutions. These new materials issued from renewable resources are part of the new class of biosorbents, including algal, bacterial or fungal biomass (Turner et al. 2007), biopolymers (Laudenslager et al. 2008; Dang et al. 2008; Jaworska et al. 2003; Ruiz et al. 2001, 2002; Guibal et al. 1999a, b; Kondo et al. 1997; Inoue et al. 1993), waste materials from agriculture (Parajuli et al. 2008, 2009), and even modified waste materials such as waste newspaper (Adhikari et al. 2008). These biosorbents are generally binding metal ions through sorption mechanisms comparable to those used for ion exchange and chelating resins, with the major interest of using low cost materials, with, in most cases, more environmentally friendly properties (i.e., renewable, more benign thermal degradation at the end of life cycle) than conventional resins.
Among biopolymers, chitosan has retained a great attention, due to its very high sorption properties for metal ions. Metal ions can bind to this aminopolysaccharide through chelation/complexation on amine groups in near neutral solutions (Guibal 2004). However, the unique property of this biopolymer among polysaccharides is related to its cationic behavior in acidic solutions (protonation of amine groups) that can explain its high efficiency for binding metal anions (Guibal 2004). Unfortunately, this protonation of amine groups induces its dissolving in acid solutions (with the remarkable exception of sulfuric acid solutions). It is thus necessary cross-linking the biopolymer using for example glutaraldehyde (Ruiz et al. 2002). The dialdehyde reacts on each side with amino groups. The supplementary linkages between the polymer chains increase the stability of the polymer. Glutaraldehyde cross-linked chitosan has been tested for Pd(II) and Pt(IV) sorption from HCl solutions. These metal ions appear in HCl solutions under the form of chloroanionic species (i.e., PdCl4 2− and PtCl6 2−) that can bind to protonated amine groups. The optimum pH is close to pH 2; indeed, at lower pH the competition of the counter anions of the acid (chloride anions, for example) for binding on protonated amine groups drastically reduces metal sorption capacity. Some chitosan derivatives have been synthesized with the objective of increasing sorption capacity (increase of the density of sorption sites grafting polyethyleneimine for example (Chassary et al. 2005)), enlarging the pH range for potential application (grafting new functional groups, such as sulfur compounds (Guibal et al. 2000)), or aminoacids (Ramesh et al. 2008; Fujiwara et al. 2007). The improvement of sorption properties may also consist in grafting other N-bearing groups with acid–base properties compatible with more acidic media. A good example of these modifications is given by the immobilization of pyridyl moieties on chitosan (Baba and Hirakawa 1992; Baba et al. 1996, 1998; Kagaya et al. 2000; Justi et al. 2004, 2005; Dhakal et al. 2008; Sajomsang 2010). The present study proposes a new route for the synthesis of N-(2-(2-pyridyl)ethyl)chitosan (PEC). After characterizing the structure of the chitosan derivative, it is tested for Pd(II) and Pt(IV) sorption from HCl solutions. The study first investigates the effect of HCl concentration on sorption isotherms, before carrying out the influence of several experimental parameters on uptake kinetics. Finally, the biosorbent is tested for a series of sorption/desorption cycles.
Materials and methods
Materials
Chitosan was supplied by JSC “Sonat” (Moscow, Russia). The biopolymer was characterized using 1H NMR spectroscopy for the evaluation of the degree of acetylation (DA) and by viscosimetry for the determination of average molar mass (MW). The DA was close to 16% and the MW was about 2.5 × 105 g mol−1. 2-Vinylpyridine for the synthesis of PEC was supplied by Sigma–Aldrich (Belgium); it was distillated and stabilized with 0.05% hydroquinone prior to use. Reagents for sorption experiments (i.e., palladium tetrachloroplatinic acid and platinum hexachloroplatinic acid) were supplied by Fluka Ag. (Switzerland), and Aldrich chemie/Acros (Belgium).
Synthesis of PEC
The synthesis of PEC proceeded by reaction of 2-vinylpyridine with chitosan in HCl solution. A gel was prepared by mixing 0.33 g of chitosan (i.e., 2 mmol—NH2) with 0.42 mL of 2-vinylpyridine (i.e., 4 mmol) in 1.56 mL of a 4.6% w/w HCl solution (i.e., 2 mmol). The gel was heated for 24 h at 70 °C. After cooling, a volume of 7.18 mL of HCl (0.85% w/w solution; i.e., 2 mmol) was added to the reactive suspension. The PEC hydrochloride was precipitated using acetone. Then, the wet solid was washed with isopropanol under reflux for 24 h. Finally, the polymer was dried at 50 °C until reaching a constant mass. The pyridylethylation reaction of chitosan is described on Scheme 1.
The polymer was crosslinked with glutaraldehyde to improve its stability in acidic solutions. Thirteen g of PEC (i.e., 7.62 mmol—NH2 groups) were dispersed in 50 mL of water before adding 0.13 mL of a 34% w/w HCl solution. The crosslinking bath was prepared by mixing 3.95 g of glutaraldehyde (25% solution; i.e., 10.39 mmol) with 54 mL of water. The suspension (i.e., PEC suspension in HCl solutions completed with the crosslinking agent) was maintained under agitation for 24 h at 24 °C. Finally, the product was filtered, rinsed and dried at 50 °C until reaching a constant mass. Though the synthesis procedure was not formally optimized the present conditions correspond to the best procedure found after several tests (where the temperature and the concentrations of the different reagents were varied) (Pestov et al. 2009).
Sorbent characterization
The sorbent was ground and sieved and the average size of sorbent particles that were used in this study was found to be 0.4 mm. Elemental analysis was performed using an Elemental Analyzer Perkin Elmer (Carlo Erba EA 1108). FT-IR spectra were recorded on a Spectrum One FT-IR spectrometer (Perkin Elmer), equipped with a Smart Orbit Accessory for single-reflection Attenuated Total Reflection (ATR). Fourier Transform facilities were used for subtracting the spectrum of chitosan from the spectrum of PEC to identify more properly the modifications brought by the grafting of new functional groups.
The chemical structures of the materials were determined by 1H NMR spectroscopy: the samples were dissolved in D2O/DCl (10 mg mL−1), and 3-(trimethylsilyl)-1-propanesulfonic acid was used as an external standard. The 1H NMR spectra were recorded on an Avance DRX400 Bruker NMR spectrometer at the frequency of 400 MHz. The analyses were performed at 70 °C in order to improve signal resolution.
Sorption experiments
Sorption isotherms were obtained by contact of a given amount of sorbent (i.e., 20 mg) with 25 mL of solution containing increasing concentrations of metal (in the range 0–500 mg metal L−1) at target HCl concentration (i.e., 0.1 M, 0.25 M, 0.5 M or 1 M). Samples were collected after 3 days of agitation and filtered before being analyzed by inductively coupled plasma atomic emission spectrometry (ICP-AES JY 2000, Jobin–Yvon, Longjumeau, France). The mass balance equation was used for the calculation of sorption capacity (metal concentration in the sorbent). The same experimental protocol was used for the determination of sorption performance at equilibrium when investigating the effect of HCl concentration (with concentrations varying between 0.1 and 2 M).
Kinetics were performed in batch reactor. The sorbent, at the appropriate sorbent dosage (SD), was added to 400 mL of solution with known initial concentration. Samples were regularly collected, filtered and analyzed for plotting the relative metal concentration (residual concentration divided by the initial concentration) versus time. Experimental conditions (SD, metal concentration, sorbent type, agitation speed) were varied, and the values of the parameters will be systematically reported in the caption of the figures.
Metal desorption from loaded sorbents has been studied in two steps: (a) the mass balance equation was used to evaluate the amount of metal adsorbed on the polymer at given HCl concentration (20 mg sorbent/25 mL of solution at 70 mg L−1 metal concentration); (b) the loaded sorbent (after being rinsed with water) was mixed with the eluent solutions (20 mg sorbent/25 mL of solution; contact time 3 h). The eluate was filtered and analyzed using ICP-AES. The comparison of the amounts of metal successively adsorbed and desorbed was used for the calculation of desorption efficiency. Additionally, sorption–desorption cycles were repeated five times using PEC. For this purpose 20 mg of sorbent, 25 mL of solution with 70 mg metal L−1 were used for adsorption and for desorption 25 mL of eluent (optimized composition defined by first step of the study, and depending on the metal).
Modeling of experimental results
Sorption equilibriums can be described by a number of equations depending on the sorbent and the metal considered. The most frequently used in the field of biosorption are the equations of Langmuir (2-parameters equation), Freundlich (2-parameters equation), Langmuir–Freundlich (3-parameters equation) (Tien 1994). While the Langmuir model is a “mechanistic” equation considering that the equilibrium is reached when the sorption rate equals desorption rates (with appropriate mechanisms attached to the model), the Freundlich equation is an empiric equation. The Langmuir equation assumes that: (a) the sorption occurs as a monolayer on the sorbent surface (the maximum sorption capacity corresponds to the saturation of the monolayer); (b) no interaction may exist between vicinal sorbed molecules, and (c) the sorption energy involved in the binding on the solute is homogeneous over all the sorbent (this also means that the sorbent is homogeneous).
Langmuir equation:
with qm (mg metal g−1, or mmol metal g−1) is the sorption capacity at saturation of the monolayer (maximum sorption capacity) and b is the affinity coefficient (L mg−1 metal, or L mmol−1 metal). The sorption capacity (or solute concentration in the sorbent), q (mg metal g−1, or mmol metal g−1) is plotted versus the residual metal concentration in the solution, Ceq (mg metal L−1, or mmol metal L−1).
Freundlich equation:
with kF (L1/n g−1 mg1−1/n metal, or L1/n g−1 mmol1−1/n metal) and n are the Freundlich constants.
Several steps may control sorption kinetics (Tien 1994): (a) mass transfer of the solute through the external film and in the porous network (surface and homogeneous diffusion); (b) reaction kinetics. In most cases, binding is controlled by diffusion mechanisms rather than the reaction rate. The intraparticle diffusion coefficient (De, effective diffusivity, m2 min−1) was determined using Crank’s equation, assuming the solid to be initially free of metal, and the kinetics to be controlled by intraparticle diffusion resistance (Crank 1975):
q(t) and qeq are the concentrations of the metal in the resin at time t and at equilibrium, respectively.
And qn non-zero roots of the equation:
The Mathematica™ software was used for the determination of the intraparticle diffusion coefficient, De, and for the simulation of experimental data (represented by the solid lines on the figures).
Results and discussion
Characterization of the sorbent
The grafting of 2-vinylpyridine on chitosan backbone can only occur on amine groups of chitosan. The polymer has a fraction of nitrogen sites bound with acetyl moieties (according the DA). Since the substitution of 2-pyridylethyl moieties may be incomplete a fraction of amine groups will remain under the free form. Based on these hypotheses it is possible to suggest that the PEC (before glutaraldehyde crosslinking) will have the general structure shown on Fig. 1.
The analysis of 1H NMR spectra (and more specifically the determination of the areas of the different signals, Fig. 2) served for determining the values of DA, a, and m (see Fig. 1). This first approach gave the following values: DA = 0.2, a = 0.14 and m = 0.66. This means that the substitution degree (DS defined as DS = m/(a + m)) was close to 83%. Actually, the process of pyridylethylation is quite difficult to make quantitative. It is common to use long reaction time and higher temperatures for increasing the substitution degree (Reich and Levine 1955). It takes a long reaction time in the case of 2-vinylpyridine addition on chitosan due to considerable steric hindrance effect and the rather weak nucleophilic behavior of the biopolymer (Pestov et al. 2009). Additionally, it is important to state that increasing the temperature may substantially damage the polymer, causing the hydrolysis of the basic chain in acidic medium. Under these conditions the degree of acetylation is not expected to play a significant role in the final substitution degree. Maintaining a non-reacted fraction of amine groups is also important since this free amine groups will be used for the crosslinking of the polymer with glutaraldehyde. The fraction of amine groups that remained free at the end of the synthesis (not reacted with glutaraldehyde) has not been determined.
The elemental analysis gave the following composition: C—54.67%, H—6.95% and N—9.31%. Based on the analysis of 1H NMR spectra, the expected structure is defined by the general equation: (C8H13O5N)0.2(C6H11O4N)0.8(C7H7N2)0.66 · 0.20 H2O. The theoretical composition (based on this structure) would give the elemental composition: C—54.56%, H—6.82% and N—9.58%. These theoretical values are quite close to the results found in the elemental analysis.
The FT-IR analysis shows the bands systematically found on the spectra of chitosan-based materials (glucose ring in the range 900–1200 cm−1; amine and amide bands around 1400–1600 cm−1 for example; Fig. 3a). In order to reach a better identification of the functions which were affected by the chemical modification and that even appear (pyridylethyl group, for example) the subtraction of chitosan spectrum from the spectrum of PEC was carried out (Fig. 3b). Apart of the change in the intensity of the typical chitosan bands, which were affected in intensity due to the grafting of new functional groups, new bands appeared around 1594, 1568 cm−1. This band are representative of pyridine ring, for example, N-(2-pyridyl)methylchitosan was identified through bands at 1596 and 1571 cm−1 (Dhakal et al. 2008).
Influence of HCl and chloride concentration on sorption capacity
Varying the concentration of HCl in the solution may have a dual interaction effect on sorption performance due to the influence of both the acidity and the chloride ions. Chloride ions may have a competitor effect against metal anions for binding on protonated amine groups (Guibal et al. 1999a); however, the presence of chloride anions (at appropriate concentration) may also enhance metal sorption when they contribute to the formation of adsorbable species (Guibal et al. 2000). Indeed, the speciation of metal ions in solution depends on the pH, metal concentration (in some cases more specifically when the metal forms polynuclear species) and the presence of ligands. In the case of precious metals, the presence of chloride ions induces the formation of anionic species (mainly PdCl4 2− and PtCl 2−6 ) that can bind to protonated amine groups in acidic solutions. In the case of Pt(IV) sorption, Guibal et al. (2000) compared metal sorption in hydrochloric and sulfuric acid solutions (completed with the addition of sulfate and chloride ions, respectively) with two chitosan derivatives: glutaraldehyde crosslinked chitosan (GCC) and a thiourea derivative of chitosan (TGC). In sulfuric acid solutions the concentration of chloride ions is not sufficient (coming only from the dissociation of Pt salt) to promote the formation of chloroanionic species. The addition of small amounts of chloride anions leads to the formation of chloroplatinate species that can bind to protonated amine groups (while above a limit concentration, chloride anions enter in competition with chloroplatinate anions for binding on protonated amine groups). For these reasons, the sorption profiles were compared on Fig. 4 for HCl solutions increasing acid concentration from 0.1 to 5 M and for 0.1 M HCl solutions completed with the addition of chloride ions (at total chloride concentration varying between 0.1 and 5 M). The four curves almost superimposed indicating that the two parameters HCl concentration and chloride concentration roughly play comparable role for Pd(II) and Pt(IV) sorption. Increasing chloride ion concentration (regardless of acid concentration) drastically reduced metal sorption. The protonation of the sorbent is probably the key parameter for metal binding while the presence of excessive concentrations of chloride ions induces a strong competition for the availability of the protonated groups (free amine functions, secondary amine groups and pyridine groups). Pd(II) is more sensitive than Pt(IV) to metal speciation effects: Pd(II) is suspected to form more species in solution than Pt(IV) when changing pH, metal and chloride concentrations. The comparable sorption profiles confirm that the competition effect of chloride ions is more important than speciation effects on the control of sorption performance.
The sorption of PGMs on chitosan derivatives has been widely described. On glutaraldehyde crosslinked chitosan the sorption of chloro-anions on protonated amine groups was attributed to an ion-exchange mechanism (electrostatic attraction of metal anions by protonated biopolymer). This mechanism is strongly influenced by the pH for the sorption step, and it would be expected that the desorption could proceed through pH increase (till reaching free-base form). However, the desorption is non quantitative and it is generally necessary using a strong complexing agent for fully desorbing the metals from loaded sorbent. This result suggests that metal binding proceeds through complexation reaction. However, the strong influence of competitor anions on the sorption of PGMs chloro-anions supports the hypothesis of an ion exchange mechanism. In the case of pyridine derivatives of chitosan, two possible mechanisms were identified depending on the metal (Inoue and Baba 2007). For copper, Inoue and Baba report the formation of five-membered chelates. In HCl solutions they observe that the sorbent has a high selectivity for Au(III), Pd(II), Pt(IV) and Fe(III) over Cu(II), Ni(II), Cu(II) and Cd(II). This means that the sorbent has a great affinity for metal ions that form chloro-complexes in HCl solutions over those that do not form strong chloro-complexes. Based on the effect of chloride and hydrogen ions on Pd(II) sorption, Inoue and Baba conclude that the binding occurs through an anion exchange mechanism with the formation of an ion pair between tetrachloro complex and protonated form of the pyridine derivative of chitosan. So, the mechanism involved in Pd(II) and Pt(IV) sorption remains debatable though the anion exchange mechanism can be considered a predominant step in the process, but the contribution of chelation mechanism based on the Scheme 2 cannot be totally rejected.
Influence of HCl concentration on sorption isotherms
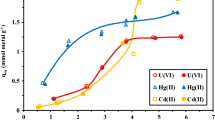
In order to confirm these trends complete sorption isotherms have been carried out for both Pd(II) and Pt(IV) at HCl concentrations of 0.1, 0.25, 1 and 2 M (Fig. 5). The sorption isotherms show remarkable differences with changing the concentration of HCl and the metal. At low HCl concentration (i.e., 0.1 and 0.25 M) the sorption isotherm for Pd(II) is almost irreversible: the sorption capacity drastically increased (almost vertical trend) and reached the saturation plateau at low residual metal concentration. For Pt(IV) despite a favorable trend the initial slopes are lower than those found with Pd(II). At higher HCl concentration (i.e., 1 and 2 M), the isotherms were defavorable (inversion of the concavity of the plot). The sorption capacities progressively increased with a much slower slope and the saturation plateau is not observed. These trends are confirmed by the modeling of the sorption isotherms (Tables 1 and 2). While for Pd(II) and Pt(IV) sorption, the Langmuir equation (solid lines) fitted well experimental data (correlation coefficient higher than 0.99) for HCl concentrations 0.1 and 0.25 M, at higher HCl concentration the Freundlich equation (discontinuous lines) gave better fits of experimental curves. At high HCl concentration, the strong competition of chloride ions induced less favorable sorption mechanism. The comparison of sorption isotherms also shows that PEC has a marked preference for Pd(II) against Pt(IV). Indeed, at both 0.1 and 0.25 M HCl concentrations, the maximum sorption capacity was higher for Pd(II) (i.e., 3.5 and 2.5 mmol Pd g−1) than for Pt(IV) (i.e., 2.6 and 2.1 mmol Pt g−1). This is also confirmed by the affinity coefficients (b) that were significantly greater (from 5 to 25 times) for Pd(II) compared to Pt(IV). At higher HCl concentration (i.e., 1 and 2 M) the sorption capacity reached appreciable levels only for very high residual metal concentrations, the initial slope of the curves was also considerably decreased making the sorbent difficult to use for the competitive recovery of PGMs at high HCl concentration. Similar trends were observed with chitosan crosslinked with glutaraldehyde: at pH below 2, sorption capacity drastically decreased. The grafting of pyridyl groups allows increasing the sorption capacity at higher acidity; however, the beneficial effect is limited for HCl concentrations greater than 0.25 M. Sulfur derivatives (based on the grafting of thiourea or rubeanic acid) also brought appreciable increase in the sorption performance for Pd(II), Pt(IV) and Au(III) binding by chitosan-based materials (Guibal et al. 2000). However, for reaching high sorption levels in very acidic solutions (above 1 M HCl concentrations) it is necessary using other systems: an example is given by the encapsulation of ionic liquids (alkyl phosphonium salts) into biopolymer capsules (Guibal et al. 2009; Vincent et al. 2008a, b), or on conventional macroporous resins (Gallardo et al. 2008). The elemental analysis has shown that the PEC (prior to glutaraldehyde crosslinking) contains up to 9.3% of nitrogen. This corresponds to 6.6 mmol nitrogen per g of sorbent, including free amine groups that could be involved in the crosslinking reaction, acetylamide groups, secondary amine potentially less reactive and pyridine nitrogen. The crosslinking treatment can induce significant variations of the elemental distribution and the relationship between sorption capacity and nitrogen content is difficult to establish. The strong effect of chloride ions confirms that the sorption involves an electrostatic attraction/ion exchange mechanism according:
Several studies have shown that the mechanism of metal sorption on pyridylmethyl chitosan (PMC) depended on the ionic character of the metal: the sorption of metal cations proceeds by chelation while metal ions were bound by an electrostatic attraction mechanism (Baba et al. 1998; Dhakal et al. 2008). This was confirmed by the positive effect of a pH increase on metal cation sorption while the sorption of metal anions (tetrachloroaurate and tetrachloropalladate anions) was enhanced when increasing the acidity of the solution.
Sorption of Pd(II) and Pt(IV) in binary component solutions
The sorption of Pd(II) and Pt(IV) from mono-component solutions has shown that PEC has a greater affinity for Pd(II) over Pt(IV); it is interesting to check if this preferential affinity is verified in metal mixtures. Figure 6 shows the sorption isotherm for Pd(II)/Pt(IV) binary solutions (molar ratio Pd(II)/Pt(IV) close to 2, in 0.1 M HCl solution). The two curves show a very favorable sorption trend: this means that the presence of the competitor metal did not drastically affect the sorption of the other metal. At equilibrium, the distribution of the molar ratio Pd(II)/Pt(IV) on the sorbent varied between 0.8 and 5.5 with a large majority of values above 3. This confirms that PEC has a preference for Pd(II) over Pt(IV) in 0.1 M HCl solutions, though this preference is not sufficient for separating the two metals from binary solutions. The extended Langmuir equation can be used for describing sorption in binary solutions according to Eq. 7a, 7b.
with qm,i, bi, are the Langmuir constants for mono component solutions. The plot of simulated curves using the constants shown on Table 1 (See Appendix, Fig. 12) shows that this simplified model is not appropriate for simulating the sorption of Pd(II) and Pt(IV) in binary component solutions. The simulated curve overestimated sorption capacity for Pt(IV) (the overestimation tended to be negligible at high metal concentration) while for Pd(II) the simulated curve underestimated sorption capacity at low metal concentration and overestimated the sorption capacity at high residual metal concentration. This evolution is consistent with the preferred sorption of Pd(II) over Pt(IV). With other chitosan derivatives (PEI-grafted chitosan, sulfur derivative of chitosan), the sorbent had also a slight preference for Pd(II) over Pt(IV), but not enough for allowing the selective separation of these PGMs (Chassary et al. 2005).
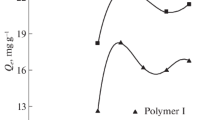
Effect of HCl concentration on Pd(II) uptake kinetics
Figure 7 shows the kinetic profiles for Pd(II) sorption on PEC at different HCl concentrations. The figure shows that under selected conditions the equilibrium was reached within a few hours (2–4 h depending on HCl concentration). As expected from previous sections the equilibrium concentration increased with HCl concentration. Another important phenomenon is identified for the uptake kinetics in 1 M HCl solutions: after 6 h of contact the concentration of Pd(II) in the solution increased. The metal previously bound was progressively released. At high HCl concentration the sorbent is partially degraded leading to the reversibility of metal binding. This phenomenon was also observed but to a lesser extent with other HCl concentrations and for greater contact times (2/3 days). The instability of the sorbent, especially in very acidic solutions, at long contact time may explain the significant decrease in the sorption capacity obtained for 1 and 2 M HCl solutions (Fig. 5) since the contact time was fixed to 3 days.
The solid lines show the modeling of the uptake kinetics with the model of resistance to intraparticle diffusion. This simplified model (the so-called Crank’s equation) fitted well experimental data. The Table 3 summarizes the values of the intraparticle diffusivity. The intraparticle diffusion coefficient roughly increased with HCl concentration. The decrease of sorption capacity with this parameter may explain that the equilibrium is reached faster.
Influence of agitation speed on Pd(II) and Pt(IV) uptake kinetics
The agitation speed may affect uptake kinetics through the effects of film diffusion. Indeed, the agitation speed contributes to controlling the thickness of the film surrounding sorbent particles. In the case of ion exchange mechanisms controlled by the resistance to film diffusion the rate varies with agitation speed, while this parameter does not influence ion exchange rate when the sorption kinetics is controlled by the resistance to intraparticle diffusion (Helfferich 1995). Figure 8 compares for both Pd(II) and Pt(IV) the uptake profiles when varying the agitation speed from 200 to 600 rpm. Surprisingly, the agitation speed 200 and 600 rpm superimposed at higher concentration levels than the curve obtained with 400 rpm. It seems that the optimum value for agitation is close to 400 rpm, regardless of metal. For other experiments, the agitation speed was systematically maintained at 400 rpm. Tables 3 and 4 show the intraparticle diffusion coefficient. In the case of Pd(II), the coefficient Deff varied between 8 × 10−11 and 9.8 × 10−11 m2 min−1; this confirms the poor effect of agitation speed and the resistance to film diffusion on the control of uptake kinetics. In the case of Pt(IV), the intraparticle diffusivity varied between 4.7 × 10−11 and 1.8 × 10−10 m2 min−1. The limited influence of agitation speed confirms that the uptake kinetics are mainly controlled by the resistance to intraparticle diffusion.
Influence of metal concentration on Pd(II) and Pt(IV) uptake kinetics
The metal concentration influences the gradient between the solution and the surface of sorbent particle (resistance to film diffusion) and between the surface of the particle and the sorption sites located in the center of the particle. Helfferich (1995), summarizing the effect of a series of experimental parameters on ion exchange rate, observed that the rate varies with the concentration in the case of a mechanism controlled by the resistance to film diffusion while for mechanisms controlled by the resistance to intraparticle diffusion the rate is independent of the concentration. The resistance to film diffusion plays generally a significant role in the initial stage of the kinetics. Since the initial slope did not appear to be controlled by metal concentration (Fig. 9), this confirms that uptake kinetics were mainly controlled by the resistance to intraparticle diffusion. The intraparticle diffusivity increased with metal concentration (Tables 3 and 4) by a factor close to 4 between 30 and 100 mg metal L−1, regardless of the metal from 8 × 1011 to 38 × 1011 m2 min−1 for Pd(II) and between 1010 and 4.4 × 1010 m2 min−1 for Pt(IV). Increasing metal concentration in the solution induces a greater concentration gradient between the solution and the center of the particle. This may contribute to increasing the diffusivity of metal ions in the porous network of the polymer.
Influence of sorbent dosage on Pd(II) and Pt(IV) uptake kinetics
When increasing sorbent dosage the residual metal concentration logically decreased. More interesting is the comparison of initial slopes. The slope of the concentration decay curve tended to increase with increasing sorbent dosage (Fig. 10). Obviously, increasing the amount of sorbent induces an increase of sorbent exchange surface (and sorption reactive groups) available for metal binding. The external surface area is correlated to resistance to film diffusion; increasing sorbent dosage will enhance uptake kinetics. The comparison of intraparticle diffusion coefficients shows a decreasing trend when the sorbent dosage increased. When increasing the amount of sorbent, the sorption capacity at equilibrium decreases; as a consequence, the concentration gradient between the solution and the internal reactive groups (i.e., the driving force for intraparticle diffusion) decreases. The intraparticle diffusion coefficients varied between 6.5 × 1011 and 2.8 × 1010 m2 min−1 for Pd(II) and between 4.7 × 1011 and 2.2 × 1010 m2 min−1 for Pd(IV). This is about four orders of magnitude lower than the values of molecular diffusivities of tetrachloropalladate and hexachloroplatinate anions in water (Marcus 1997).
Metal desorption and sorbent recycling
Several eluents have been tested for Pd(II) and Pt(IV) recovery from loaded sorbents. Most interesting results are summarized in Tables 5 and 6. Additionally, ammonia solutions (at different concentrations; i.e., between 0.5 and 3 M) have been also tested. Ammonia solutions recovered less than 10% of the metal initially sorbed. Thiourea is a ligand that was frequently used for the recovery of precious metals from loaded sorbents and resins. This ligand is generally used in acidic solutions for enhancing metal recovery (Sanchez et al. 2001; Adhikari et al. 2008; Donia et al. 2005). Tables 5 and 6 show the results obtained varying both thiourea and HCl concentration in the eluent. In the case of Pd(II), the desorption of the metal was slightly enhanced by the addition of HCl to the thiourea solution (TU): the desorption yield reached 91% for 0.05 M TU in 0.1 M HCl solution. Increasing TU concentration did not significantly improve desorption efficiency, at least in 0.1 M HCl solutions, while for 0.5 M HCl solutions, the efficiency slightly increased when the TU concentration reached 0.3 M. Optimum desorption (close to 91%) was obtained for 0.05 M TU + 0.1 M HCl solution (and for 0.3 M TU + 0.5 M HCl solutions). For Pt(IV), the desorption efficiency also exceeded 90% with 0.1 M TU + 0.5 M HCl solutions.
These solutions were used for testing the recycling of the resins. Figure 11 shows the amount of metal (Pd and Pt) adsorbed and desorbed at each sorption/desorption cycle. This figure shows that the amount of metal sorbed at each stage, regardless of the metal, decreased very slightly with the number of operations for Pt(IV) (from 1.8 to 1.5 mg) and remained roughly constant for Pd(II) (1.6 mg ± 6%). For desorption, Pd(II) recovery was also more stable along the five cycles compared to Pt(IV): the average desorbed amount was 1.3 mg (±6%), while for Pt(IV) it reached 1.2 mg (±9%). The cumulative desorption efficiency for Pd(II) reached 85% against 75% for Pt(IV). These results confirm that the desorption of both Pd(II) and Pt(IV) was not complete, slightly higher for palladium compared to platinum. Despite these decreasing trends the sorbent could be re-used for a minimum of five cycles maintaining high sorption performance.
Thiourea being a strong complexing agent for PGMs, the addition of thiourea in acidic media contributes to displace the metal from the loaded sorbent. In the perspective of sorbent recycling it is important to carefully clean the sorbent after the desorption step to remove any trace of the ligand since it could contribute to a competitive complexation of target metals for the next sorption steps.
Conclusion
The modification of chitosan with grafting pyridyl reactive groups allows extending the range of application of chitosan-based materials for the sorption of precious metals to more acid solutions (compared to glutaraldehyde cross-linked solutions). The main limitation for using these materials for more acidic media is probably due to the progressive degradation of the material at long contact time when the concentration of HCl exceeds 0.5 M. The sorption mechanism involves the interaction of chloroanionic metal species with protonated amine and the pyridyl groups in acidic media.
Sorption capacities as high as 3.5 and 2.6 mmol metal g−1 were obtained in 0.1 M HCl solutions for Pd(II) and Pt(IV), respectively. The sorption capacity decreases with increasing HCl concentration (probably due to the competition effect of high chloride ion concentration). The sorbent reveals poorly stable in very acidic solutions leading to low sorption capacities when HCl concentration reached 1 M (especially at long contact time). The Langmuir equation fits well experimental data for 0.1 and 0.25 M HCl concentrations, while for higher HCl concentrations the Freundlich equation is more appropriate for simulating experimental curves. Though the sorbent has a preference for Pd(II) over Pt(IV) (based on maximum sorption capacity and affinity coefficient) the sorption isotherm in binary solutions shows that the separation effect is not sufficient for promoting Pd(II) selective recovery.
Though the combination of several limiting processes can be involved in the control of uptake kinetics, the resistance to intraparticle diffusion seems to play the major role. The limited effect of the agitation speed confirms that the resistance to film diffusion is not the main controlling step in the mass transfer. Among tested experimental parameters, metal concentration and sorbent dosage have the major influence on the kinetic rates. The intraparticle diffusivity varies in the range 3 × 10−11 m2 min−1–4.5 × 10−10 m2 min−1.
The best desorption (close to 90%) of Pd(II) and Pt(IV) was obtained using a thiourea/HCl solutions (with respective concentrations depending on the metal). The resin was successfully carried out for five successive sorption/desorption cycles, maintaining high metal recovery levels.
References
Adhikari CR, Parajuli D, Kawakita H, Inoue K, Ohto K, Harada H (2008) Dimethylamine-modified waste paper for the recovery of precious metals. Environ Sci Technol 42(15):5486–5491
Baba Y, Hirakawa H (1992) Selective adsorption of palladium(II), platinum(IV), and mercury(II) on a new chitosan derivative possessing pyridyl group. Chem Lett 21(10):1905–1908
Baba Y, Kawano Y, Hirakawa H (1996) Highly selective adsorption resins. I. Preparation of chitosan derivatives containing 2-pyridylmethyl, 2-thienylmethyl, and 3-(methylthio)propyl groups and their selective adsorption of precious metals. Bull Chem Soc Jpn 69(5):1255–1260
Baba Y, Masaaki K, Kawano Y (1998) Synthesis of a chitosan derivative recognizing planar metal ion and its selective adsorption equilibria of copper(II) over iron(III). React Funct Polym 36(2):167–172
Barakat MA, Mahmoud MHH (2004) Recovery of platinum from spent catalyst. Hydrometallurgy 72(3–4):179–184
Barakat MA, Mahmoud MHH, Mahrous YS (2006) Recovery and separation of palladium from spent catalyst. Appl Catal A 301(2):182–186
Birinci E, Gulfen M, Aydin AO (2009) Separation and recovery of palladium(II) from base metal ions by melamine-formaldehyde-thiourea (MFT) chelating resin. Hydrometallurgy 95(1–2):15–21
Boricha AB, Bajaj HC, Ghosh PK, Jam RV (2007) Recovery of palladium from palladium phthalocyanine complex adsorbed on silica. Hydrometallurgy 87(3–4):140–147
Brooks CS (1991) Metal recovery from industrial wastes. Lewis Publishers, Chelsea
Chassary P, Vincent T, Sanchez Marcano J, MacAskie LE, Guibal E (2005) Palladium and platinum recovery from bicomponent mixtures using chitosan derivatives. Hydrometallurgy 76(1–2):131–147
Ciezynska A, Regel-Rosocka M, Wisniewski M (2007) Extraction of palladium(II) ions from chloride solutions with phosphonium ionic liquid Cyphos IL101. Pol J Chem Technol 9(2):99–101
Cortina JL, Meinhardt E, Roijals O, Marti V (1998) Modification and preparation of polymeric adsorbents for precious-metal extraction in hydrometallurgical processes. React Funct Polym 36(2):149–165
Crank J (1975) The mathematics of diffusion, 2nd edn. Oxford University Press, Oxford
Dakshinamoorthy A, Venugopal V (2005) Solvent extraction studies on the complexation of palladium with alpha benzoin oxime. J Radioanal Nucl Chem 266(3):425–429
Dang MY, Zhang TA, Wang P, Li W, Tan YK, He Y (2008) Adsorption of platinum by novel cross-linked chitosan resin particles. Chin J Process Eng 8(1):60–64
Dhakal RP, Oshima T, Baba Y (2008) Planarity-recognition enhancement of N-(2-pyridylmethyl)chitosan by imprinting planar metal ions. React Funct Polym 68(11):1549–1556
Dominguez M, Antico E, Beyer L, Aguirre A, Garcia-Granda S, Salvado V (2002) Liquid-liquid extraction of palladium(II) and gold(III) with N-benzoyl-N′,N′-diethylthiourea and the synthesis of a palladium benzoylthiourea complex. Polyhedron 21(14–15):1429–1437
Donia AM, Atia AA, Elwakeel KZ (2005) Gold(III) recovery using synthetic chelating resins with amine, thio and amine/mercaptan functionalities. Sep Purif Technol 42(2):111–116
Fujiwara K, Ramesh A, Maki T, Hasegawa H, Ueda K (2007) Adsorption of platinum(IV), palladium(II) and gold(III) from aqueous solutions onto l-lysine modified crosslinked chitosan resin. J Hazard Mater 146(1–2):39–50
Gallardo V, Navarro R, Saucedo I, Avila M, Guibal E (2008) Zinc(II) extraction from hydrochloric acid solutions using amberlite XAD-7 impregnated with Cyphos IL 101 (tetradecyl(trihexyl)phosphonium chloride). Sep Sci Technol 43(9–10):2434–2459
Guibal E (2004) Interactions of metal ions with chitosan-based sorbents: a review. Sep Purif Technol 38(1):43–74
Guibal E, Larkin A, Vincent T, Tobin JM (1999a) Chitosan sorbents for platinum sorption from dilute solutions. Ind Eng Chem Res 38(10):4011–4022
Guibal E, Larkin A, Vincent T, Tobin JM (1999b) Platinum recovery on chitosan-based sorbents. In: Amils R, Ballester A (eds) 13th International symposium on biohydrometallurgy (IBS 99), Madrid, Spain, Jun 20–23, pp 265–275
Guibal E, Vincent T, Mendoza RN (2000) Synthesis and characterization of a thiourea derivative of chitosan for platinum recovery. J Appl Polym Sci 75(1):119–134
Guibal E, Vincent T, Jouannin C (2009) Immobilization of extractants in biopolymer capsules for the synthesis of new resins: a focus on the encapsulation of tetraalkyl phosphonium ionic liquids. J Mater Chem 19(45):8515–8527
Helfferich F (1995) Ion exchange, 2nd edn. Dover Publications, Inc., Mineola
Hubicki Z, Wołowicz A (2009) Adsorption of palladium(II) from chloride solutions on Amberlyst A 29 and Amberlyst A 21 resins. Hydrometallurgy 96(1–2):159–165
Hubicki Z, Wolowicz A, Leszczynska M (2008) Studies of removal of palladium(II) ions from chloride solutions on weakly and strongly basic anion exchangers. J Hazard Mater 159(2–3):280–286
Inoue K, Baba Y (2007) Chitosan: a versatile biopolymer for separation, purification, and concentration of metal ions. In: Sengupta AK (ed) Ion exchange and solvent extraction—a series of advances, vol 18. CRC Press, Boca Raton, pp 339–374
Inoue K, Baba Y, Yoshizuka K (1993) Adsorption of metal-ions on chitosan and cross-linked copper(II)-complexed chitosan. Bull Chem Soc Jpn 66(10):2915–2921
Jaworska M, Sakurai K, Gaudon P, Guibal E (2003) Influence of chitosan characteristics on polymer properties. I: crystallographic properties. Polym Int 52(2):198–205
Jermakowicz-Bartkowiak D (2005) Preparation, characterisation and sorptive properties towards noble metals of the resins from poly(vinylbenzyl chloride) copolymers. React Funct Polym 62(1):115–128
Jermakowicz-Bartkowiak D, Kolarz BN (2002) Gold sorption on weak base anion exchangers with aminoguanidyl groups. Eur Polym J 38(11):2239–2246
Justi KC, Laranjeira MCM, Neves A, Mangrich AS, Fávere VT (2004) Chitosan functionalized with 2[-bis-(pyridylmethyl) aminomethyl]4-methyl-6-formyl-phenol: equilibrium and kinetics of copper (II) adsorption. Polymer 45(18):6285–6290
Justi KC, Fávere VT, Laranjeira MCM, Neves A, Peralta RA (2005) Kinetics and equilibrium adsorption of Cu(II), Cd(II), and Ni(II) ions by chitosan functionalized with 2[-bis-(pyridylmethyl)aminomethyl]-4-methyl-6-formylphenol. J Colloid Interface Sci 291(2):369–374
Kagaya S, Kodajima D, Takahashi Y, Kanbara T, Hasegawa K (2000) Selective sorption of gold(III) by polystyrene-supported alpha-pyridylamino oligomers. J Mater Chem 10(11):2442–2444
Kang HY, Schoenung JM (2005) Electronic waste recycling: a review of US infrastructure and technology options. Resour Conserv Recycl 45(4):368–400
Kondo K, Nakagawa SI, Matsumoto M, Yamashita T, Furukawa I (1997) Selective adsorption of metal ions on novel chitosan-supported sulfonic acid resin. J Chem Eng Jpn 30(5):846–851
Laudenslager MJ, Schiffman JD, Schauer CL (2008) Carboxymethyl chitosan as a matrix material for platinum, gold, and silver nanoparticles. Biomacromolecules 9(10):2682–2685
Malik P, Paiva AP (2009) Solvent extraction studies for platinum recovery from chloride media by a N,N′-tetrasubstituted malonamide derivative. Solvent Extr Ion Exch 27(1):36–49
Marcus Y (1997) Ion properties. Marcel Dekker, Inc., New York
Memon JR, Memon SQ, Bhanger MI, Khuhawar MY, Allen GC, Memon GZ, Pathan AG (2008) Efficiency of Cd(II) removal from aqueous media using chemically modified polystyrene foam. Eur Polym J 44(5):1501–1511
Parajuli D, Inoue K, Kawakita H, Ohto K, Harada H, Funaoka M (2008) Recovery of precious metals using lignophenol compounds. Miner Eng 21(1):61–64
Parajuli D, Khunathai K, Adhikari CR, Inoue K, Ohto K (2009) Total recovery of gold, palladium, and platinum using lignophenol derivative. Miner Eng 22(13):1173–1178
Parodi A, Vincent T, Pilsniak M, Trochimczuk AW, Guibal E (2008) Palladium and platinum binding on an imidazol containing resin. Hydrometallurgy 92(1–2):1–10
Pestov AV, Bratskaya SY, Avramenko VA, Yatluk YG (2009) N-2-(2-pyridyl)ethylchitosan—new chelate polymer. In: Rusticheli F, Caramella C, Senel S, Varum KM (eds) International conference of the european chitin society, Venice, Italy. Advances in chitin science. pp 590–594
Preston JS, du Preez AC (2002) Solvent extraction of platinum-group metals from hydrochloric acid solutions by dialkyl sulphoxides. Solvent Extr Ion Exch 20(3):359–374
Qu R, Sun C, Ji C, Xu Q, Wang C, Cheng G (2006) The sorption mechanism of Au(III) on sulfur-containing chelating resin poly[4-vinylbenzyl (2-hydroxyethyl) sulfide]. Eur Polym J 42(2):254–258
Ramesh A, Hasegawa H, Sugimoto W, Maki T, Ueda K (2008) Adsorption of gold(III), platinum(W) and palladium(II) onto glycine modified crosslinked chitosan resin. Bioresour Technol 99(9):3801–3809
Reich HE, Levine R (1955) The pyridylethylation of active hydrogen compounds. IV. The acid-catalyzed pyridylethylation of primary amines. J Am Chem Soc 77(20):5434–5436
Ruiz M, Sastre AM, Zikan MC, Guibal E (2001) Palladium sorption on glutaraldehyde-crosslinked chitosan in fixed-bed systems. J Appl Polym Sci 81(1):153–165
Ruiz M, Sastre A, Guibal E (2002) Pd and Pt recovery using chitosan gel beads. I. Influence of the drying process on diffusion properties. Sep Sci Technol 37(9):2143–2166
Saitoh T, Nakane F, Hiraide M (2007) Preparation of trioctylamine-impregnated polystyrene-divinylbenzene porous resins for the collection of precious metals from water. React Funct Polym 67(3):247–252
Sajomsang W (2010) Synthetic methods and applications of chitosan containing pyridylmethyl moiety and its quaternized derivatives: a review. Carbohydr Polym 80(3):631–647
Sanchez JM, Hidalgo M, Salvado V (2001) The selective adsorption of gold (III) and palladium (II) on new phosphine sulphide-type chelating polymers bearing different spacer arms—equilibrium and kinetic characterisation. React Funct Polym 46(3):283–291
Shams K, Goodarzi F (2006) Improved and selective platinum recovery from spent alpha-alumina supported catalysts using pretreated anionic ion exchange resin. J Hazard Mater 131(1–3):229–237
Tien C (1994) Adsorption calculations and modeling. Butterworth-Heinemann series in chemical engineering. Butterworth-Heinemann, Newton
Turner A, Lewis MS, Shams L, Brown MT (2007) Uptake of platinum group elements by the marine macroalga, Ulva lactuca. Mar Chem 105(3–4):271–280
Venkatesan KA, Selvan BR, Antony MP, Srinivasan TG, Rao PRV (2005) Extraction of palladium from nitric acid medium by commercial resins with phosphinic acid, methylene thiol and isothiouronium moieties attached to polystyrene-divinylbenzene. J Radioanal Nucl Chem 266(3):431–440
Venkatesan KA, Selvan BR, Antony MP, Srinivasan TG, Rao PRV (2007) Extraction of palladium (II) from nitric acid medium by imidazolium nitrate immobilized resin. Hydrometallurgy 86(3–4):221–229
Vincent T, Parodi A, Guibal E (2008a) Immobilization of Cyphos IL-101 in biopolymer capsules for the synthesis of Pd sorbents. React Funct Polym 68(7):1159–1169
Vincent T, Parodi A, Guibal E (2008b) Pt recovery using cyphos IL-101 immobilized in biopolymer capsules. Sep Purif Technol 62(2):470–479
Acknowledgments
L. A. S. S. acknowledges the grant from Erasmus European Program for her training period at Ecole des Mines d’Alès.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
See Fig. 12.
Simulation of Pd(II) and Pt(IV) sorption isotherms in binary solutions (0.1 M HCl solution) using the extended Langmuir equation (Eq. 7a, 7b) with the parameters of the Langmuir equation for mono-component solutions (Table 1) (simulated curves were calculated with discrete values of experimental concentrations and the trends were extrapolated joining point-to-point)
Rights and permissions
About this article
Cite this article
Santos Sopena, L.A., Ruiz, M., Pestov, A.V. et al. N-(2-(2-Pyridyl)ethyl)chitosan (PEC) for Pd(II) and Pt(IV) sorption from HCl solutions. Cellulose 18, 309–325 (2011). https://doi.org/10.1007/s10570-010-9469-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-010-9469-8