Abstract
This systematic review and meta-analysis examined the efficacy of adolescent cognitive–behavioral sleep interventions. Searches of PubMed, PsycINFO, CENTRAL, EMBASE, and MEDLINE were performed from inception to May 1, 2016, supplemented with manual screening. Nine trials were selected (n = 357, mean age = 14.97 years; female = 61.74%). Main outcomes were subjective (sleep diary/questionnaire) and objective (actigraphy) total sleep time (TST), sleep onset latency (SOL), sleep efficiency (SE), and wake after sleep onset (WASO). There were a small number of randomized controlled trials (RCTs; n = 4) and a high risk of bias across the RCTs; therefore, within sleep condition meta-analyses were examined (n = 221). At post-intervention, subjective TST improved by 29.47 min (95% CI 17.18, 41.75), SOL by 21.44 min (95% CI −30.78, −12.11), SE by 5.34% (95% CI 2.64, 8.04), and WASO by a medium effect size [d = 0.59 (95% CI 0.36, 0.82)]. Objective SOL improved by 16.15 min (95% CI −26.13, −6.17) and SE by 2.82% (95% CI 0.58, 5.07). Global sleep quality, daytime sleepiness, depression, and anxiety also improved. Gains were generally maintained over time. Preliminary evidence suggests that adolescent cognitive–behavioral sleep interventions are effective, but further high-quality RCTs are needed. Suggestions for further research are provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is growing recognition that many adolescents obtain insufficient and/or poor-quality sleep, which is increasingly being regarded as an epidemic of sleep deprivation among adolescents and an important public health problem (Adolescent Sleep Working Group 2014; American Medical Association 2010; Millman 2005; Office of Disease Prevention and Health Promotion 2011). The American Academy of Sleep Medicine, the National Sleep Foundation, and the American Center for Disease Control and Prevention have suggested that adolescents optimally require between 8 and 10 h of sleep per night (Hirshkowitz et al. 2015; Paruthi et al. 2016). For example, longitudinal studies have shown that adolescents sleep around 9.2 h per night under ad lib (unrestricted) sleep conditions (Carskadon and Acebo 2002). However, recent systematic reviews and meta-analyses have demonstrated that many adolescents obtain insufficient sleep (i.e., <8 h), especially on school nights (Gradisar et al. 2011b). Sixty-five percent of adolescents report sleep onset latencies exceeding 30 min (Hysing et al. 2013), more than half report the need for more sleep (Wolfson and Carskadon 1998), and most (59%) wake feeling unrefreshed at least a few times per week (National Sleep Foundation 2011).
Prevalence estimates of sleep disorders in adolescence vary considerably, but according to studies that have incorporated rigorous criteria, approximately 30% of adolescents suffer from a sleep disorder (Ohayon and Roberts 2001). Insomnia is the most prevalent sleep disorder among adolescents (Johnson et al. 2006; Roberts et al. 2009). Insomnia is defined as chronic dissatisfaction with sleep quantity and/or quality, despite adequate opportunity to sleep (American Psychiatric Association 2013). It is associated with difficulty initiating and/or maintaining sleep, early morning awakening, and unrefreshing sleep (Johnson et al. 2006). Approximately 8–11% of young people meet diagnostic criteria for insomnia at any one time (Dohnt et al. 2012), which tends to persist over time (Roberts et al. 2009).
Delayed sleep phase disorder (DSPD) is defined as normal sleep that is delayed in its timing with respect to the individual’s sleep onset and rising times (American Psychiatric Association 2013). Seventeen percent of adolescents report difficulties falling asleep before 2 a.m. at least three times per week (Saxvig et al. 2012). Furthermore, between 1 and 7% of adolescent’s meet diagnostic criteria for DSPD (Johnson et al. 2006; Ohayon et al. 2000; Pelayo et al. 1988), with the majority (51%) reporting at least one symptom (Lovato et al. 2013). This delay in sleep onset may lead to significant difficulty in rising for school in the morning, school non-attendance, daytime sleepiness, chronic sleep reduction, and poor school performance (Gradisar and Crowley 2013).
A number of factors interact to make sleep susceptible to disturbance in adolescence. First, children and adolescents are subject to the same physiological susceptibilities and psychological and environmental vulnerabilities that cause insomnia in adults (Keller & El-Sheikh 2011), such as predisposition to cognitive–emotional hyperarousal (Fernandez-Mendoza et al. 2011). Second, sleep during adolescence is affected by physiological development (Colrain and Baker 2011). Adolescence is associated with a progressive reduction in the accumulation of homeostatic sleep pressure during wakefulness, which leads to a reduction in sleep drive (Feinberg et al. 2006). Adolescence is also associated with a delay in the timing of sleep, which is related to a lengthening of the intrinsic period of the endogenous circadian oscillator (Carskadon et al. 2004). Melatonin is released later in the evening among adolescents than children, which also delays the onset of evening sleepiness (Carskadon et al. 1993). Third, parental control over bedtime lessens during adolescence (Short et al. 2011). Fourth, adolescents develop responsibilities and social interests (e.g., homework, employment, friendships) that encourage remaining awake later into the evening (Adam et al. 2007; Maume 2013). Finally, electronic devices have a deleterious impact on sleep in adolescence, including delaying sleep onset and reducing sleep duration (Bartel et al. 2015; Hale and Guan 2015). These physiological maturational processes and social and cultural factors have been described as a “perfect storm” of factors in adolescence (Carskadon 2011), so that reduced sleep propensity in the late evening becomes permissive of continued waking activities and delayed bedtimes (Carskadon 2011; Jenni and LeBourgeois 2006). This delay in sleep has two potential sleep-related consequences: (a) sleep restriction, because school starts early in the morning, and (b) reduced restorative value of sleep, because recovery sleep tends to occur at an inappropriate circadian phase (Carskadon et al. 2004).
Adolescent Sleep and Mental Health
Sleep disturbance is an important contributor to, and maybe even cause of, cycles of increasing vulnerability and risk among young people (Harvey 2015). Sleep problems are pervasive in psychiatric disorders and precipitate and maintain many emotional and behavioral problems (Dahl and Harvey 2007; Harvey et al. 2011). Indeed, there is emerging evidence that sleep may qualify as a transdiagnostic process (Harvey 2015; Harvey et al. 2011). Sleep problems are a highly relevant “bridge symptom” (Baglioni et al. 2014) and share the highest percentage of connected symptoms within all symptoms in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association 1994; Borsboom et al. 2011). In particular, there is a strong relationship between sleep disturbance and internalizing problems in adolescence. Recent evidence suggests that sleep problems, particularly wakefulness in bed (e.g., prolonged sleep onset latency and poor sleep efficiency), precede the development of anxiety and depression in adolescence more than the reverse (Lovato and Gradisar 2014; McMakin and Alfano 2015).
Although the specific mechanisms are not fully understood, research suggests that shared biological, cognitive, and interpersonal risk factors may underlie the relationship between sleep disturbance and mental health problems in adolescence. In particular, several modifiable risk mechanisms appear to underlie the relationship between sleep disturbance and internalizing problems, including stress/arousal, emotion processing, and cognitive factors (Cowie et al. 2014). To the extent that sleep and mental health problems in youth share common etiological underpinnings, early treatment programs for sleep problems might reduce the risk of developing mental health problems and can be considered a helpful general preventive strategy (Baglioni et al. 2011; Dahl and Harvey 2007).
Cognitive–Behavioral Sleep Interventions
Cognitive–behavioral sleep interventions are short-term, multicomponent, goal-oriented psychotherapeutic treatments. They aim to modify the patterns of thinking and behavior that may be underlying an individuals sleep disturbance, such as poor sleep hygiene, irregular sleep–wake schedules, delayed bedtimes, pre-sleep hyperarousal, and maladaptive sleep-related cognitions. Before reviewing adolescent cognitive–behavioral sleep interventions, we will review adult cognitive–behavioral sleep interventions, as the adult literature is more extensive.
Research Among Adults
Cognitive–Behavioral Therapy for Insomnia
Cognitive–behavioral therapy for insomnia (CBT-I) involves behavioral techniques such as sleep education, sleep hygiene instruction, stimulus control, sleep restriction, and relaxation training, but also addresses negative thought processes and unhelpful beliefs about sleep through a focus on bedtime worry and rumination (for a review, see Edinger and Means 2005). The components of CBT-I are described in Table 1. Empirical support for the effectiveness of CBT-I has grown steadily since the 1960s, especially over the past 15 years. CBT-I is recommended as an effective therapy for insomnia by the Standards of Practice Committee of the American Academy of Sleep Medicine (Morgenthaler et al. 2006). The American College of Physicians recently recommended that all adult patients should receive CBT-I as an initial treatment for chronic insomnia (Qaseem et al. 2016).
There is strong evidence from multiple systematic reviews and meta-analyses that CBT-I improves sleep in adults, usually with medium–large effect sizes (Koffel et al. 2015; Murtagh and Greenwood 1995; Morin et al. 1994; Trauer et al. 2015; van Straten et al. 2017). Effects tend to be strongest for wakefulness-in-bed variables compared to sleep duration variables and subjective sleep variables compared to objective sleep variables. Effects also tend to persist over time. For example, a recent systematic review and meta-analysis of 20 randomized controlled trials (RCTs) among adults diagnosed with insomnia [1162 patients (64% female; mean age = 56 years), range 20–201 patients] found that face-to-face CBT-I (delivered individually or in groups on at least two occasions) produced marked and statistically significant improvements in sleep diary measured sleep onset latency [SOL; 19.03 min (95% CI 14.12–23.93 min)], wake after sleep onset [WASO; 26.00 min (95% CI 15.48–36.52 min)], and sleep efficiency [SE; 9.91% (95% CI 8.09, 11.73%)], but not total sleep time [TST; 7.61 min (95% CI −0.51–15.74 min)] at post-treatment time points compared to control conditions (Trauer et al. 2015). Improvements were maintained at follow-up, suggesting that treatment gains persist over time. The effect sizes were similar in size to those seen in meta-analyses of hypnotics, such as benzodiazepines (Huedo-Medina et al. 2012; Nowell et al. 1997). However, different to hypnotics, CBT-I effects are likely to continue after treatment cessation (Sivertsen et al. 2006) and to have fewer side effects, such as adverse effects and rebound insomnia after discontinuation (Buscemi et al. 2007; Kales et al. 1991). For polysomnography-measured sleep, only five studies were available for meta-analysis, and effect sizes were similar to the sleep diary outcomes. For actigraphy-measured sleep (a device that measures the degree and intensity of motion), only three studies were analyzed, and the effect size estimates were markedly lower than for the sleep diary outcomes. However, a major limitation of the meta-analysis was that only one study included in the review had low risk of bias across all domains—while most studies were methodologically rigorous in regard to patient follow-up and outcome reporting, allocation concealment and blinding were rarely reported or were not undertaken. Furthermore, approximately half of the studies used a waiting list or placebo tablet comparator, rather than an active control condition. Wait list control groups do not account for treatment expectation effects, demand characteristics, and non-specific therapeutic factors, such as the quality of the therapeutic alliance and relationship. Studies with wait list control groups also tend to observe larger effect sizes than those using a psychological placebo (Furukawa et al. 2014).
Regarding the comparative efficacy of different modalities, three meta-analyses of psychological interventions for sleep problems have suggested that individual and group treatments are equally effective (Murtagh and Greenwood 1995; Trauer et al. 2015; van Straten et al. 2017), while another indicated that individual treatment is most effective (Morin et al. 1994). Three studies have directly compared individual and group CBT-I. Two of the studies found that both forms of CBT-I were equally effective at improving sleep (Bastien et al. 2004; Verbeek et al. 2006), while the third study found that individual CBT-I resulted in greater improvement on several sleep variables compared to group CBT-I, including SOL and sleep quality (Yamadera et al. 2013). However, emerging evidence suggests that face-to-face treatments delivered over at least four sessions are more effective than self-help interventions (either though books, audio, or Internet) or face-to-face interventions with fewer sessions (van Straten et al. 2017). For example, Lancee et al. (2016) recently conducted a randomized controlled non-inferiority trial comparing an online treatment program (dCBT-I) to standard CBT-I (6 weekly, 45-min sessions) and a wait list control among 90 (30 per group) individuals (mean age = 41.6 years) who were diagnosed with insomnia. Although dCBT-I and face-to-face CBT-I both significantly improved many features of sleep relative to wait list controls, face-to-face CBT-I treatment was found to be superior to online treatment. Patient adherence to online interventions also represents a major challenge, as indicated by the 50% completion rate for computerized CBT-I in two recent studies (Lancee et al. 2016; Christensen et al. 2016).
There is also emerging evidence that CBT-I leads to improvements in physical and mental health symptoms, including reductions in anxiety and depression, with small–medium effect sizes (Ballesio et al. 2017; Belleville et al. 2011; Taylor and Pruiksma 2014). For example, a recent systematic review of 16 RCTs that assessed the efficacy of in-person CBT-I among psychiatric populations [571 patients (aged 20–70 years), range 17–63 patients] found that CBT-I resulted in significant small-to-medium effects in depression symptomatology [mean effect size = 0.51 (95% CI 0.31–0.70)] and marginal improvements in anxiety symptomatology [mean effect size = 0.21 (95% CI −0.08, 0.50)] relative to control conditions (Taylor and Pruiksma 2014). However, again, more than half of the studies included in the review used wait list control conditions.
Other important findings from meta-analyses of adult CBT-I are that samples recruited from the community and that have shorter duration of insomnia diagnoses tend to show greater improvements in sleep variables post-treatment than do clinical samples (Koffel et al. 2015; Murtagh and Greenwood 1995). This is probably related to chronicity of sleep disturbance, suggesting that those beginning treatment with fewer sleep problems may do better in treatment (Koffel et al. 2015), highlighting the importance of early intervention. For example, several commentators have argued that future research is needed to determine whether cognitive–behavioral sleep interventions can be delivered prophylactically to at-risk, pre-insomniac psychiatric populations (Vitiello et al. 2013).
Mindfulness-Based Sleep Interventions
Despite the effectiveness of CBT-I, many patients have not achieved clinically significant outcomes (Morin et al. 2006), suggesting that there is room for improvement among cognitive and behavioral approaches to sleep problems. There is emerging evidence that sleep problems can also be treated successfully using protocols that include a mindfulness component. Mindfulness can be defined as “the awareness that emerges through paying attention on purpose, in the present moment, and non-judgmentally to the unfolding of experience” and is considered a “third-wave” cognitive–behavioral therapy (Kabat-Zinn 2003, p. 145). Several RCTs have evaluated in person mindfulness-based therapies in adults with sleep problems (Britton et al. 2010, 2012; Garland et al. 2014; Gross et al. 2011; Ong et al. 2014) and have found significant improvements within active treatment conditions for subjective and objective measures of sleep, especially SOL and SE, but not beyond active control conditions (Garland et al. 2014; Gross et al. 2011). However, many of these studies were limited by small sample sizes (mean = 49, range 23–111).
Cognitive–Behavioral Therapies for DSPD
Finally, there is accumulating evidence that DSPD can be treated successfully using cognitive–behavioral therapies. Along with timed melatonin administration, timed light exposure and chronotherapy are recommended as effective therapies for DSPD by the Standards of Practice Committee of the American Academy of Sleep Medicine (Morgenthaler et al. 2006), based on a systematic review of the literature (Sack et al. 2007). Bright light therapy (Lewy and Sack 1996) consists of scheduled natural sunlight and/or broad-spectrum white light from a specialized lamp at usual wake time, while incrementally advancing light exposure each day. The goal of morning light exposure is to shift the circadian rhythm earlier and therefore correct the pathological phase delay. Chronotherapy (Czeisler et al. 1981) involves a prescribed progressive delay in the schedule of sleep time until the desired sleep schedule is reached. The treatment is based on the premise that patients with DSPD may have greater difficulty shifting their rhythm in an advance direction, and that the timing of sleep is the main synchronizer of the circadian system.
Research Among Adolescents
Despite the fact that (1) cognitive–behavioral therapies are firmly established as frontline treatments for many adult sleep problems/disorders, and (2) and there is high prevalence, high chronicity, and serious consequences associated with adolescent sleep problems/disorders, research on adolescent cognitive–behavioral sleep interventions is not as developed as the adult literature. No previous systematic reviews and meta-analyses have been conducted to provide a synthesis of this developing field, a lacuna in the literature that we address here.
The research on adolescent cognitive–behavioral sleep interventions may be less developed compared to the adult literature for a number of reasons. First, adolescent sleep intervention research has tended to focus on school-based sleep education programs, which have the potential to reach a large number of adolescents (i.e., whole school classes) and are relatively easy to implement (e.g., “train the teacher”). However, recent reviews and large-scale RCTs have found that while these psychoeducational programs are effective in increasing students’ knowledge about sleep and insomnia, they are less effective in improving sleep behavior or mental health (Blunden et al. 2012; Blunden and Rigney 2015; Gruber 2016; Kira et al. 2014; Wing et al. 2015). These findings are consistent with recent reviews suggesting that targeted interventions are more effective than universal interventions in preventing child and adolescent depression (Horowitz and Garber 2006; Rohde 2015), and that simple sleep hygiene education does not guarantee positive outcomes in adults (Irish et al. 2015). In sum, these findings suggest that active sleep interventions that incorporate cognitive–behavioral principles demonstrated to be effective in adult populations (e.g., CBT-I, mindfulness-based sleep interventions, bright light therapy, and chronotherapy), and that are delivered to students who are already experiencing early signs of sleep and/or mental health problems, may be more effective (Wensing et al. 2010). Second, the later chronophase of adolescents may mask insomnia symptoms and/or reduce motivation to seek help. Many clinicians and researchers have reported an overlap between DSPD and sleep onset insomnia symptoms, especially on school nights (Gradisar and Crowley 2013). However, adolescents may experience delayed bedtimes and wakefulness in bed as egosyntonic, for both biological and social reasons. For example, delayed bedtimes and prolonged SOL may afford adolescents with opportunities to complete homework and/or use electronic devices in bed without parental supervision. Furthermore, adolescents may be reluctant to seek help for sleep inertia, daytime sleepiness, and fatigue, as they may see these experiences as normative parts of development and/or embarrassing/shameful. Finally, parents, teachers, and health professionals may focus on addressing the consequences of adolescent sleep disturbance, such as anxiety, depression, difficulty concentrating, and poor school performance, rather than the underlying causes (i.e., insomnia or DSPD).
Methods
A systematic review and meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al. 2015). The primary aim of the systematic review and meta-analysis was to answer the following research question: What is the efficacy of adolescent cognitive–behavioral sleep interventions for subjective (e.g., questionnaire/sleep diary) and objective (e.g., actigraphy) sleep outcomes, compared with baseline and control, for adolescents with self-identified sleep problems and/or a diagnosis of a sleep disorder? A secondary aim of the review was to examine the effects of the interventions on self-reported global sleep quality, daytime sleepiness, anxiety, and depression.
Study Selection
Eligible studies were trials of cognitive–behavioral sleep interventions among adolescents. Inclusion criteria were:
-
1.
Participants were adolescents aged between 10 and 19 years. The age range was based on the definition of adolescence adopted by the World Health Organization (2015).
-
2.
Studies were published in peer-review journals.
-
3.
Studies included more than three participants (i.e., were not case studies).
-
4.
The primary aim/focus was the use of a multimodal sleep intervention based on cognitive and behavioral principles.
-
5.
Interventions incorporated at least two behavioral components (e.g., sleep hygiene, stimulus control, sleep restriction, and/or bright light therapy) and at least one cognitive component (e.g., identifying and challenging dysfunctional beliefs and attitudes about sleep and/or pre-sleep worry). A previous systematic review and meta-analysis evaluated behavioral interventions for pediatric insomnia (Meltzer and Mindell 2014), but did not include cognitive–behavioral interventions, and focused on both children and adolescents (the review identified 16 controlled trials and 12 within-subject studies, but only one study was conducted among adolescents). Furthermore, the package of CBT-I has been shown to be more effective than separate delivery of the behavioral or cognitive components for improving insomnia among adults. Harvey et al. (2014) found that cognitive therapy resulted in slower, but more long-lasting, improvements in insomnia symptoms compared with behavior therapy. However, the full complement of CBT-I produced the largest short- and long-term effects on insomnia symptoms.
-
6.
Interventions were delivered on at least four occasions. Adult CBT-I is usually conducted over the course of four to eight sessions (Edinger and Means 2005), and a recent meta-analysis found that adult CBT-I delivered on at least four occasions is more effective than CBT-I delivered on fewer occasions (van Straten et al. 2017).
-
7.
Interventions were delivered individually or in small groups. School-based sleep education programs were excluded because they are delivered to entire school classes without regard to individual risk factors or symptoms, are didactic rather than therapeutic, and do not include cognitive restructuring.
-
8.
Interventions were delivered in person. Interventions delivered online were excluded because the aim of the review was to evaluate the efficacy of face-to-face interventions, and recent evidence suggests that face-to-face CBT-I treatment is superior to online treatment (e.g., Lancee et al. 2016; van Straten et al. 2017).
-
9.
Participants were identified as having self-reported symptoms of sleep disturbance (e.g., high scores on a screening questionnaire) or a diagnosis of a sleep disorder (e.g., insomnia or DSPD).
-
10.
To increase the generalizability of findings, studies evaluating participants with comorbid medical or psychiatric problems were not excluded, as adolescents with sleep problems and disorders often have related physical and mental health problems.
-
11.
Finally, studies that combined traditional CBT-I therapies with “third-wave” therapies (e.g., mindfulness) or other treatment modules (e.g., anxiety-/depression-specific components, bright light therapy), were not excluded, as adult studies suggest the efficacy of such combined approaches (Britton et al. 2010, 2012; Garland et al. 2014; Gross et al. 2011; Ong et al. 2014), and the aim of the review was to evaluate the broad-based and transdiagnostic effects of adolescent cognitive–behavioral sleep interventions.
Data Sources and Searches
Online searches of the PubMed (United States Library of Medicine), PsycINFO (Wolters Kluwer Health OvidSP), CENTRAL (Cochrane Central Register of Controlled Trials), EMBASE, and MEDLINE (Thomson Reuters Web of Knowledge) databases were performed on May 1, 2016, by the first author using several relevant search terms [e.g., (sleep OR insomnia) AND (intervention OR therapy OR treatment) AND (cognitive–behavior OR cognitive–behaviour OR CBT-I OR mindfulness OR multimodal OR multicomponent)] and search limits (e.g., “Ages: Adolescent”). Full data sources and search terms are provided in Online Resource 1. Abstracts were examined for references to cognitive–behavioral sleep interventions and adolescence, and if the study appeared relevant, then the full text was retrieved. “Pearling,” the process of manually scanning the reference list of identified articles, was used for additional relevant studies not identified in the electronic database search. Examination of abstracts and reference lists for selection of appropriate articles was conducted independently by two authors (MB, LS).
Data Extraction
Pertinent details from each study were recorded using standardized forms, including methodological information regarding study design, sample characteristics, intervention framework, program delivery, and outcome measures. These details are described in the study characteristics section and Table 3. MB and LS independently extracted the data.
Quality Assessment
To conduct an appraisal of the studies’ methodological quality, each of the trials was evaluated according to the quality index for randomized and non-randomized studies proposed by Downs and Black (1998). The index was adapted to be consistent with a procedure used by Blunden et al. (2012) in their review of school-based sleep education programs. The original quality index is a 27-item checklist assessing five subscales: (1) reporting whether the information provided in the paper is sufficient to allow the reader to make an unbiased assessment of the findings; (2) external validity: the extent to which the findings from the study could be generalized to the population from which the study subjects were derived; (3) bias: the extent of bias in the measurement of the program and the outcome; (4) confounding: addressing the bias in the selection of study subjects, and (5) power: whether the negative findings from a study could be due to chance. Item descriptions are provided in Table 2. Answers are scored 0 or 1, except for one item in the reporting subscale that scored 0–2, and the single item on power, which scored 0–5. The total maximum score for quality is 32. However, because most studies included in the review did not report statistical power, and there was not enough data to calculate power, the power score was excluded. Therefore, 26 of the possible 27 items were evaluated, giving a maximum score of 27 points.
The Downs and Black (1998) quality index has demonstrated high internal consistency (Kuder–Richardson 20: 0.89), good test–retest (r = 0.88) and inter-rater (r = 0.75) reliability, and high correlations (r = 0.90) with other validated quality assessment instruments (r = 0.90) for non-RCTs. It has been recommended as a strong instrument for assessing the methodological quality of interventions (Deeks et al. 2003; Higgins and Green 2011).
Additionally, all controlled trials were reviewed for risk of bias using the recommended Cochrane guidelines (Higgins and Green 2011) with ratings for randomization, allocation concealment (selection bias), blinding of outcome assessment (detection bias), and selective reporting (reporting bias).
LS and MR independently completed the study quality assessments and risk-of-bias ratings. Given that one of these raters (MR) was an author on one of the studies, an independent rater (AP; see Acknowledgements) performed an additional rating of the study by Blake et al. (2016).
Data Synthesis and Analysis
The main outcomes of interest were subjective (e.g., sleep diary/questionnaire) and objective (e.g., actigraphy) measures of TST, SOL, SE, and WASO. Secondary outcomes of interest were questionnaire-based ratings of global sleep quality, daytime sleepiness, anxiety, and depression. Primary and secondary endpoints were assessed at two time points: immediately after the treatment and at follow-up.
All analyses used random-effects models. Heterogeneity was assessed using the I 2 statistic (25% = low, 50% = moderate, 75% = high; Higgins et al. 2003). Additionally, all trials were assessed for publication bias using funnel plots and the Egger test (Egger et al. 1997; Higgins et al. 2003). Publication bias was only assessed when there were three or more studies for the relevant analysis. When variables were defined consistently across studies (i.e., objective and subjective SOL, TST, SE), effects were based on unstandardized raw mean differences. When variables were not defined consistently across studies (i.e., objective and subjective WASO), or when different questionnaires were used to assess the same construct (i.e., global sleep, daytime sleepiness, anxiety, and depression), data were pooled using standardized mean differences using change score standardization. For all analyses, we calculated effects based on an assumed correlation between measurements over time of r = 0.5, which was based on results obtained in Blake et al. (2016). Effect sizes were based on Cohen’s d and interpreted with the following: 0.2 = small, 0.5 = medium, 0.8 = large (Cohen 1992).
Four primary sensitivity analyses were performed. First, we restricted analyses to studies with high methodological quality, defined as a score of 17 or above on the Downs and Black (1998) instrument. We chose 17 as the cutoff because all RCTs scored above 16, while all uncontrolled trials scored below 17 (see Table 4). Second, we limited analyses to studies that identified adolescents through clinical diagnosis, rather than based on self-report symptoms, as adolescents with more severe problems may benefit more from intervention. Third, we restricted analyses to studies that evaluated adolescents with “pure” sleep problems, rather than adolescents with concomitant sleep and psychiatric problems, as concomitant problems may interfere with sleep treatment. Fourth, we limited analyses to studies that incorporated sleep restriction because this may be among the most effective component of CBT-I (Fernando et al. 2013). Sensitivity analyses were only conducted at the post-intervention time point, as few trials included follow-up time points. Finally, we conducted a separate set of sensitivity analyses to explore whether increasing or decreasing the assumed correlation between measurements over time (from the assumed r = 0.5) influenced the results.
We did not conduct sensitivity analyses on length of treatment (i.e., short vs. long), type of facilitator (i.e., psychologist vs. experienced CBT-I sleep therapist), type of delivery (i.e., individual vs. group), or type of intervention (i.e., pure CBT-I vs. added components), as there was little variation across trials. Several authors have recommended limiting the number of sensitivity analyses in meta-analyses with relatively few studies (Cuijpers 2016).
All statistical tests were two-tailed, with p values <0.05 considered statistically significant. For effect size and I 2 estimates, 95% confidence intervals were calculated. Meta-analyses were performed using the metafor package v1.9.8 (Viechtbauer 2010), in R software v 3.3.1 (R Core Team 2015).
Results
Study Selection
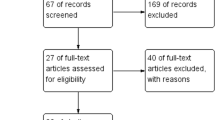
One hundred and eighty-nine articles were retrieved from the database searches, of which 10 met inclusion criteria. The study flow diagram is presented in Fig. 1. Two articles (Bootzin and Stevens 2005; Britton et al. 2010) reported data from the same trial (pre- and post-intervention and follow-up). Therefore, nine trials were included in the final analysis. One trial (Clarke et al. 2015) included adolescents aged 12–20 years. However, because only 5% of the participants (n = 2) were aged over 19 years, this study was included in the final analysis.
Study Characteristics
Table 3 shows the general characteristic of the nine selected trials. Three trials were large-scale RCT’s (Blake et al. 2016; De Bruin et al. 2015; Gradisar et al. 2011a), one was a prospective RCT (Clarke et al. 2015), and five were uncontrolled feasibility trials (Bei et al. 2013; Bootzin and Stevens 2005; De Bruin et al. 2014; Roeser et al. 2016; Schlarb et al. 2011). Two RCTs used active controls (Blake et al. 2016; Clarke et al. 2015), and two used wait list comparators (De Bruin et al. 2015; Gradisar et al. 2011a). Three trials were conducted in Australia, two in the USA, two in the Netherlands, and two in Germany. Two hundred and twenty-one participants (mean age = 14.89 years; female = 62.27%; range 11–20 years) completed the sleep intervention conditions (average per study = 25 participants, range 9–63 participants), and three hundred and fifty-seven participants (mean age = 14.97 years; female = 61.74%; range 11–20 years) completed the trials overall (average per study = 40 participants, range 9–123 participants). Seven out of nine interventions were delivered in groups (ranging from 2 to 9 participants), two individually, and two included parents. Psychologists delivered seven out of the nine interventions and experienced CBT-I sleep therapists the other two. Eight out of nine interventions were delivered over six/seven weekly 90- to 100-min sessions and one in 10 weekly sessions over 12 weeks. Three trials evaluated adolescents with insomnia disorder, one DSPD, and four with sleep/insomnia complaints. Four trials included participants with comorbid mental health disorders/symptoms (substance abuse, depression, anxiety), and three trials excluded participants with non-sleep disorders. Six trials included longitudinal follow-up, ranging from 2 to 12 months.
The content of the interventions reflects the range of choices facing clinicians (Harvey 2015). Only two studies evaluated manualized CBT-I (De Bruin et al. 2014, 2015); the other interventions included added treatment components. All nine interventions incorporated cognitive restructuring, eight sleep education, eight sleep hygiene, eight stimulus control, five sleep restriction, four relaxation techniques, two bright light therapy, two mindfulness-based cognitive therapy, one mindfulness-based stress reduction, two savoring, two hypnotherapy, two anxiety-specific modules, and one depression-specific modules. The mean number of treatment components was 6 (range 4–7).
Study Quality
The methodological quality of the reviewed studies is presented in Table 4. The average total score for the nine reviewed trials was 16 out of a possible 27 (59%), ranging from 11 to 19 (41–70%). All of the trials scored poorly on the external validity subscale, and most of the trials scored poorly on the confounding subscale, suggesting that the trials had low generalizability. Furthermore, few trials attempted to blind those measuring the main outcomes of the intervention (item 15) or measured compliance with the intervention (item 19).
Risk of Bias
The risk of bias in the controlled studies is presented in Table 5. Only two RCTs were universally assessed as having low risk of bias across all domains. Sequence generation and allocation concealment generally followed accepted methods. However, many RCTs did not report blinding techniques in detail. No RCTs described blinding of participants and personnel, possibly because of the difficulty of doing so with behavioral interventions. Moreover, two RCTs did not describe blinding of outcome assessors. Additionally, one RCT included incomplete outcome data (i.e., did not use intent-to-treat analysis and/or appropriate data imputation methods). Furthermore, selective outcome reporting was generally unclear, because most RCTs failed to publish a methods protocol paper prior to the commencement of the interventions. In sum, there was a high risk of bias across the RCTs. That is, the proportion of information from the RCTs at a high risk of bias was sufficient to affect the interpretation of the results (Higgins and Green 2011).
Meta-analyses
Because a small number of RCTs were available for meta-analyses (n = 4), and there was a high risk of bias across the RCTs, meta-analyses comparing sleep and control condition were considered unreliable. Nevertheless, these results are presented in Online Resource 2, but should be interpreted with caution. Within sleep condition meta-analyses were examined instead (Table 6).
Primary Endpoints
All nine trials measured subjective sleep using sleep diaries or self-report questionnaires, six measured objective sleep using actigraphy, and five included a follow-up time point. While most trials reported TST, not all reported SOL, SE, or WASO.
There were marked and statistically significant improvements in subjective TST [29.47 min (95% CI 17.18–41.75 min; I 2 = 26.62)], SOL [−21.44 min (95% CI −30.78 to −12.11 min; I 2 = 85.94)], SE [5.34% (95% CI 2.65, 8.04%; I 2 = 69.60)], and WASO [d = 0.59 (95% CI 0.36, 0.82; I 2 = 28.64)], at the post-intervention time point. Improvements were generally maintained at the follow-up time point and increased for subjective TST and SOL. Moreover, there were marked and statistically significant improvements in objective SOL [−16.15 min (95% CI −26.13 to −6.17 min; I 2 = 72.87)] and SE [2.82% (95% CI 0.58, 5.07%; I 2 = 66.06)], at the post-intervention time point. Improvements were maintained at follow-up and increased for SE, but decreased for SOL. There was also a marginal improvement in objective TST [9.19 min (95% CI −0.65 to 19.04 min; I 2 = 10.40)] at the post-intervention time point although there was only weak evidence to reject the null hypothesis of no effect (p = 0.07), and there was no statistically significant support that this improvement was maintained at follow-up, possibly because fewer studies were available for this meta-analysis (i.e., n = 3). Objective WASO did not improve at the post-intervention or follow-up time points.
It should be noted, however, that there was evidence of moderate–high heterogeneity between trials in SOL and SE outcomes, as well as considerable uncertainty in the heterogeneity estimates (shown by the wide I 2 confidence intervals).
Secondary Endpoints
Five trials measured global sleep quality. There was a significant improvement at the post-intervention time point, with large effect sizes [d = −0.92 (95% CI −1.52, −0.32; I 2 = 86.89)]. Improvements were maintained at follow-up. Four trials measured daytime sleepiness. There was a significant improvement at the post-intervention time point, with a moderate effect size [d = −0.63 (95% CI −1.07, −0.18; I 2 = 79.34)]. Improvements increased at follow-up. Three trials measured depression symptoms. There were marginal improvements at post-intervention, with a large effect size [d = −1.22 (95% CI −2.47, 0.01; I 2 = 94.56)]. Improvements increased at follow-up. Two studies measured anxiety symptoms. There was a marginal improvement at the post-intervention time point, with a small effect size [d = −0.33 (95% CI −0.67, −0.00; I 2 = 23.93)]. Only one trial measured anxiety at the follow-up time point, so a meta-analysis was not conducted on this outcome. Here again, there was evidence of moderate–high heterogeneity between trials in outcomes and/or considerable uncertainty in heterogeneity estimates.
Sensitivity Analyses and Publication Bias
None of the sensitivity analyses described earlier showed consistent differences in effect sizes across subjective or objective outcomes (shown in Online Resources 3–6). This suggests that there were no consistent differences in efficacy between studies with high- and low-quality, clinical diagnoses versus self-report symptoms, pure versus comorbid sleep problems, or sleep restriction components. To assess for publication bias, funnel plots were constructed and Egger tests were performed for each outcome variable. No statistically significant results were found, apart from objective TST and depression at the post-intervention time point. However, the publication bias results should be interpreted with caution, as funnel plots require a considerable number of studies, generally at least 30 (Cuijpers 2016; Lau et al. 2006). Additionally, the size of the effects was largely robust to the decision to use an estimated within-participant correlation of r = 0.5 between measurements over time. This was evidenced by consistency in findings when sensitivity analyses were conducted with a stronger (r = 0.7) and weaker (r = 0.3) assumed correlation between measurement time points. The only changes to interpretation were that the effect for objective TST (p = 0.049) and anxiety symptoms (p = 0.01) became statistically significant from baseline to post-intervention, when using a stronger and weaker correlation, respectively.
Program Acceptability
Five studies reported data on sleep condition program acceptability (Bei et al. 2013; Blake et al. 2016; Bootzin and Stevens 2005; De Bruin et al. 2014; Schlarb et al. 2011). In general, the sleep programs were well accepted. Average rating of the programs and components was above 70%. Sleep education (Bei et al. 2013; Blake et al. 2016; Schlarb et al. 2011), sleep scheduling (Bei et al. 2013; Blake et al. 2016), personalized bedtimes (De Bruin et al. 2014), relaxation (De Bruin et al. 2014), and mindfulness (Blake et al. 2016) were rated as the most helpful components.
Discussion
Despite the fact that (1) cognitive–behavioral therapies are firmly established as frontline treatments for many adult sleep problems/disorders; (2) there is high prevalence, high chronicity, and serious consequences associated with adolescent sleep problems/disorders; and (3) there is a growing body of research on adolescent cognitive–behavioral sleep interventions, this was the first systematic review and meta-analysis to provide a synthesis of this emerging field. From the data presented here, it is not possible to make firm conclusions about the efficacy of adolescent cognitive–behavioral sleep interventions. There was evidence of considerable heterogeneity between the trials, which might have been due to the small number of trials, the small number of participants per trial (Cuijpers 2016), and/or the differences in the approaches to delivering the interventions, including variation in participants, treatment components, and comparators. Moreover, the methodological quality of the trials varied, and there was high risk of bias across the RCTs.
The within sleep condition results were promising. There was evidence that adolescent cognitive–behavioral sleep interventions produce meaningful improvements in subjectively and objectively measured TST, SOL, and SE. As with adults, improvements tended to be stronger for wakefulness-in-bed variables compared to sleep duration variables and subjective sleep variables compared to objective sleep variables. Improvements also tended to persist over time, particularly for wakefulness-in-bed variables. Furthermore, the interventions were associated with meaningful improvements in perceived sleep quality and functional outcomes (daytime sleepiness, depression, and anxiety). The programs were also well-accepted, particularly the stimulus control, relaxation, and mindfulness components.
As described by Koffel et al. (2015), the smaller improvements in TST compared with wakefulness-in-bed variables were most likely related to the techniques used in cognitive–behavioral sleep interventions. The goals of stimulus control and sleep restriction are to initially limit sleep opportunities to increase the sleep drive. As a result, one would expect more immediate improvements in SOL and SE, and delayed improvements in TST, as participants gradually increase their opportunity for sleep. The findings are also consistent with large epidemiologic studies showing that in adolescents, evening chronotype and increased daytime sleepiness are associated with deficits in self-regulation, while short sleep duration is not (Anderson et al. 2009; Owens et al. 2016; Perez-Lloret et al. 2013). These findings suggest that circadian misalignment, wakefulness in bed, and daytime sleepiness, more than sleep duration, may increase the risk of mental health problems during adolescence.
In general, the effects sizes were smaller in magnitude than those reported in meta-analyses of CBT-I among adults with chronic insomnia (Koffel et al. 2015; Trauer et al. 2015; van Straten et al. 2017). However, the baseline deficits were also smaller than those reported in adult trials. For example, at baseline, the mean subjective TST, SOL, and SE in the included trials were 448.25 min, 46.05 min, and 83.72%, respectively, whereas they were 334.1 min, 57.6 min, and 71.8%, respectively, in the meta-analysis by Trauer et al. (2015). Other studies have found that patients with insomnia diverge from healthy control participants in subjectively assessed TST, SOL, and SE by around 95 min, 23 min, and 16%, respectively (Buysse et al. 2007), and Scholle et al. (2011) found that normal polysomnography assessed TST, SOL, and SE among adolescents are around 473 min, 24 min, and 89%, respectively. Therefore, improvements in subjective TST, SOL, and SE of 29.47 min, 21.44 min, and 5.34%, respectively, and objective TST, SOL, and SE of 9.19 min, 16.15 min, and 2.82%, respectively, represent major increases in these variables, especially SOL.
These results are particularly promising for three reasons. First, there are numerous barriers to sleep intervention in adolescence, including powerful physiological and social/cultural factors that combine to delay sleep onset and restrict sleep opportunities (Adam et al. 2007; Carskadon et al. 2004; Feinberg and Campbell 2010; Hale and Guan 2015; Maume 2013; Short et al. 2011). Second, school-based sleep education programs have been shown to be largely ineffective for improving objective and subjective sleep among adolescents (Blunden et al. 2012; Blunden and Rigney 2015; Gruber 2016; Kira et al. 2014; Wing et al. 2015). Third, wakefulness in bed during adolescence is a consistent indicator of current and future internalizing problems (Alfano et al. 2013; Forbes et al. 2009; Lovato and Gradisar 2014; McMakin and Alfano 2015).
Suggestions for Further Research
Trials of adolescent cognitive–behavioral sleep interventions have been limited in several ways. First, more than half of the trials were uncontrolled feasibility trials evaluating a small number of participants (Bei et al. 2013; Bootzin and Stevens 2005; De Bruin et al. 2014; Roeser et al. 2016; Schlarb et al. 2011). Second, two of the RCTs used wait list controls (De Bruin et al. 2015; Gradisar et al. 2011a). It is generally accepted that for a trial to achieve a high standard of evidence, it must include adequate statistical power to detect significant effects and an active control condition or psychological placebo (Furukawa et al. 2014). The two RCTs that used active control conditions (Blake et al. 2016; Clarke et al. 2015) found smaller effects. Future adolescent cognitive–behavioral sleep intervention trials should recruit larger sample sizes and use high-quality active control conditions (Morin et al. 2006; Riemann and Perlis 2009; Vitiello et al. 2013).
Third, two RCTs did not describe blinding of outcome assessors (De Bruin et al. 2015; Gradisar et al. 2011a). Empirical evidence shows that lack of reporting of blinding is associated with biased estimates of treatment effect and more positive outcomes (Boutron et al. 2008; Higgins and Green 2011; Moher et al. 2012). Blinding is an especially important issue for non-pharmacologic trials, where outcome assessors can have an important influence of the treatment effect (Boutron et al. 2008). Studies are also needed that measure treatment expectancy and credibility. Beliefs and expectations about behavioral and psychological treatments play an important role in determining experiences and outcomes of treatment (Devilly and Borkovec 2000).
Fourth, most of the studies did not include trial protocol papers. Reporting and publishing trial protocol papers prior to the commencement of interventions is an important step in increasing the transparency of research and the reliability of outcome papers (Cybulski et al. 2016). Furthermore, although all of the RCTs were registered as clinical trials, only one was registered prospectively (Clarke et al. 2015). Retrospective registration makes it impossible to determine whether aims and methods were determined prior to data analyses being conducted. Additionally, while most of the studies included in the meta-analysis reported TST, not all reported SOL, SE, and WASO, and few studies controlled for familywise false rejection error rates. Although there is no consensus about optimal outcomes when treating sleep problems, most adult CBT-I trials include TST, SOL, SE, and WASO as dependent variables. Morin (2003) has described recommendations for measuring outcomes in insomnia trials.
Fifth, most of the studies failed to measure compliance with the intervention. When evaluating new interventions, if significant results are found, but treatment fidelity is not monitored and optimized, the outcome could be due to an effective treatment or to unknown factors unintentionally added or omitted from the treatment (Bellg et al. 2004). On the other hand, if nonsignificant results are found, the outcome could be due to an ineffective treatment or a lack of treatment fidelity (Bellg et al. 2004). Improving treatment fidelity can also increase statistical power, by reducing random and unintended variability (i.e., drift in interventionist adherence). Furthermore, translating clinical interventions from research to practice can be improved when treatments are well defined and there are guidelines for implementing them (Bellg et al. 2004).
Sixth, all of the studies were limited by low generalizability. For example, the RCT by De Bruin et al. (2015) included adolescents diagnosed with insomnia but excluded adolescents diagnosed with other psychiatric disorders. Between 40 and 92% of all cases of insomnia occur in the context of psychiatric disorders (Breslau et al. 1996; Kessler et al. 2012). The RCT by Gradisar et al. (2011a) evaluated adolescents with DSPD, which is a low prevalence disorder (between 1 and 7%; Johnson et al. 2006; Ohayon et al. 2000; Pelayo et al. 1988). The RCTs by Blake et al. (2016) and Clarke et al. (2015) evaluated adolescents with specific comorbidities. Trials among adolescents experiencing subclinical and generalized levels of sleep and mental health problems are needed (Chase and Pincus 2011; Johnson et al. 2000; Moore et al. 2009; Warner et al. 2008).
Seventh, a diverse range of methods has been used to assess sleep. Adolescent sleep interventions have tended to use subjective sleep measures more consistently than objective sleep measures. Most studies have used sleep diaries to measure subjective sleep, but other studies have relied on retrospective questionnaires (Bei et al. 2013; Blake et al. 2016). Sleep diaries are considered the gold standard of subjective self-report (Buysse et al. 2006; Morin and Espie 2003) but are vulnerable to poor compliance (missing data and entry errors), and all subjective measures of sleep are subject to the limits of introspection and memory bias. In general, there have been discrepancies in results between studies comparing subjective and objective sleep. This may be due to self-report bias, the limits of introspection, and poor compliance, but also to the limits of currently available objective measures of sleep. While wrist actigraphy shows high sensitivity (to detect sleep), it demonstrates lower specificity (to detect wakefulness in bed; Meltzer et al. 2012). Therefore, differences between subjective and objective SE may be due to actigraphy algorithms scoring epochs as sleep, whereas the individual perceives it as wake. Removal of the actigraph, technical failures, and other measurement errors, can also distort sleep behaviors. Human scoring of actigraphy, using collateral information (e.g., light and event markers), may improve its accuracy. Web or mobile platforms, with built-in reminders and error checking, may improve sleep diary accuracy. However, no adolescent sleep intervention studies have measured sleep with polysomnography, which is widely considered to be the gold standard of objective sleep measurement. Overall, a combination of subjective and objective assessments should be used to assess outcomes in trials of adolescent cognitive–behavioral sleep interventions.
Eighth, cognitive–behavioral sleep intervention studies conducted among adolescents have not comprehensively assessed functional outcomes, such as anxiety and depression. Studies that have assessed mental health have tended to use generic measures of emotional distress (Bootzin and Stevens 2005; Schlarb et al. 2011) or have not examined heterogeneous anxiety and depressive symptoms (Blake et al. 2016; Clarke et al. 2015; Gradisar et al. 2011a), limiting specificity of findings for any symptom cluster. Morin (2003) has suggested that it is critical to document efficacy beyond the simple reduction of insomnia symptoms in CBT-I trials and to incorporate additional indicators of efficacy, such as daytime functioning, fatigue, mood, anxiety, quality of life, school attendance, academic performance, and physical health.
Ninth, most of the studies included in the meta-analysis did not report whether interventions and assessments were conducted during school term or school holiday periods. If pre-intervention assessments were conducted during school term and post-intervention assessments during school holiday periods, effect sizes could have been overestimated for TST and underestimated for wakefulness in bed. There is strong evidence that adolescent sleep is characterized by sleep restriction and high sleep drive during the school week and sleep saturation and lower sleep drive during vacation periods (Bei et al. 2014). Studies are also needed that differentiate between outcomes on school days and weekend/vacations. Adolescent sleep is strongly affected by school schedules (Bei et al. 2014), and outcomes of interventions may differ across the week.
Tenth, adolescent cognitive–behavioral sleep intervention studies have rarely considered intra-individual variability of sleep. Implementing different techniques to reduce sleep variability, such as establishing regular sleep–wake times, sleep hygiene instruction, stimulus control therapy, and cognitive–behavioral therapy, is a common goal of sleep interventions, and regularity and variability of sleep behaviors have been recommended as measures of treatment adherence and non-adherence, respectively, in behavioral insomnia treatments (Buysse et al. 2010). A systematic review of daily sleep variability among adults found that higher variability in sleep is associated with sleep and mental health problems (including insomnia, depression, anxiety), and that CBT-I reduces such variability (Bei et al. 2016). With respect to adolescents, a number of studies have found that variability in sleep schedules is associated with poor functional outcomes among adolescents, including anxiety, depression, daytime sleepiness, behavioral problems, and poor school performance (Biggs et al. 2011; Fuligni and Hardway 2006; Pasch et al. 2010; Wolfson and Carskadon 1998), as well as altered brain development (Telzer et al. 2015). In sum, these findings suggest that variability in sleep patterns should be considered as an additional dimension of sleep in trials of adolescent cognitive–behavioral sleep interventions.
Eleventh, studies are needed that examine mechanisms of change during adolescent cognitive–behavioral sleep interventions (Morin et al. 2006; Schwartz and Carney 2012; Vitiello et al. 2013). In order for such an intervention approach to reach the highest standards of evidence, studies must not only demonstrate that it is effective, but also show that the intervention works via its purportedly active mechanism (Garratt et al. 2007; Insel 2015). Treatment mechanism research can lead to a number of positive outcomes, including clarifying the nature and etiology of disorders, elucidating the processes leading to therapeutic change, identifying active treatment components, and refining current treatment protocols (Kazdin 2007; Kraemer et al. 2002). A number of mediators may account for therapeutic change in adolescent cognitive–behavioral sleep interventions, including improvements in sleep–wake variability, pre-sleep hyperarousal, and maladaptive beliefs and attitudes about sleep (Schwartz and Carney 2012). Studies are also needed that examine moderators of change (e.g., age and gender), predictors of treatment adherence (e.g., baseline symptoms (short sleep duration, anxiety, depression), self-efficacy, and attitude to treatment; Matthews et al. 2013), dose–response relationships (i.e., high- vs. low-intensity treatments), and temporal relations between variables (Morin et al. 2014).
Twelfth, studies are needed that examine specificity of effects of treatment components. Across studies included in the meta-analysis, adolescents rated behavioral treatments as more helpful than cognitive treatments, consistent with results from the adult literature—a recent meta-analysis found that relaxation treatments are particularly effective for reducing SOL in adults (van Straten et al. 2017). However, no adolescent studies have compared the efficacy of different treatment components using dismantling designs (Harvey et al. 2014). In particular, studies are needed that examine the therapeutic efficacy and specificity of different treatment components for adolescents with different symptom profiles. A personalized approach to adolescent sleep interventions (i.e., tailoring the interventions based on biopsychosocial symptom profiles) may improve their therapeutic efficacy and cost-effectiveness. On the other hand, it is also possible that transdiagnostic approaches may solve the “too many empirically supported treatments problem” that can impede the dissemination and uptake of treatments (Weisz et al. 2014, p. 68). Because comorbidity is the norm (Kessler et al. 2005, 2007), clinicians are often faced with the dilemma of which disorder/disorders to prioritize for treatment. Treating transdiagnostic processes may reduce the burden on clinicians, who are often required to learn multiple disorder-focused protocols (e.g., CBT-I, mindfulness-based sleep interventions, bright light therapy, and chronotherapy), with shared theoretical underpinnings (Harvey 2004, 2015; Mansell et al. 2009). Harvey (2015) recently proposed a transdiagnostic intervention for youth sleep and circadian problems (TranS-C-Youth), which is informed by basic sleep/circadian principles, and aims to address the broad range of sleep disturbances that adolescent’s experience, including pre-sleep arousal, maladaptive sleep-related cognitions, prolonged SOL, poor SE, delayed bedtimes, inadequate opportunity to sleep, irregular sleep–wake schedules, difficulty waking up/getting out of bed in the morning, and daytime sleepiness. However, RCTs evaluating TranS-C-Youth are yet to be published.
Thirteenth, many of the studies included in the meta-analysis did not include follow-ups (Bei et al. 2013; Blake et al. 2016; Schlarb et al. 2011) or used short follow-ups (i.e., up to 14 weeks; Clarke et al. 2015; De Bruin et al. 2014, 2015). Studies are needed that investigate durability and progression of change over time. Finally, studies are needed that evaluate the broad-based effectiveness and acceptance of adolescent cognitive–behavioral sleep interventions in the community (e.g., primary care, mental health, adolescent health, sleep medicine, and schools), using different modes of delivery (e.g., non-specialist practitioners, group settings, individual settings, Internet, and other e-health modes of delivery). As described by Gradisar and Richardson (2015), “the list of research questions is extensive and highlights that our field has only begun to evaluate treatment for adolescent sleep disturbance, which may or may not present and respond to treatment like adult insomnia” (p. 1841).
Conclusion
Our meta-analysis provides preliminary evidence that adolescent cognitive–behavioral sleep interventions are an efficacious treatment for sleep and mental health problems, producing clinically meaningful responses within active treatment conditions. Their efficacy seems to be maintained over time and result in significant alleviation of sleep problems and improvement in functional outcomes. However, it is not possible to make definitive conclusions about the efficacy of adolescent cognitive–behavioral sleep interventions from the data presented here. Further large-scale controlled treatment outcome studies are needed to confirm these findings.
References
Adam, E. K., Snell, E. K., & Pendry, P. (2007). Sleep timing and quantity in ecological and family context: A nationally representative time-diary study. Journal of Family Psychology, 21, 4–19.
Adolescent Sleep Working Group. (2014). School start times for adolescents. Pediatrics, 134(3), 642–649.
Alfano, C. A., Reynolds, K., Scott, N., Dahl, R. E., & Mellman, A. (2013). Polysomnographic sleep patterns of non-depressed, non-medicated children with generalized anxiety disorder. Journal of Affective Disorders, 147, 379–384.
American Medical Association. (2010). Resolution 503: Insufficient sleep in adolescents. Chicago, IL: American Medical Association, American Academy of Sleep Medicine.
American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: American Psychiatric Publishing.
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: American Psychiatric Publishing.
Anderson, B., Storfer-Isser, A., Taylor, H. G., Rosen, C. L., & Redline, S. (2009). Associations of executive function with sleepiness and sleep duration in adolescents. Pediatrics, 123(4), e701–e707.
Baglioni, C., Battagliese, G., Feige, B., Spiegelhalder, K., Nissen, C., Voderholzer, U., et al. (2011). Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. Journal of Affective Disorders, 135, 10–19.
Baglioni, C., Spiegelhalder, K., Feige, B., Nissen, C., Berger, M., & Riemann, D. (2014). Sleep, depression and insomnia—A vicious circle? Current Psychiatry Reviews, 10, 202–213.
Ballesio, A., Aquino, M. R. J. V., Feige, B., Johann, A. F., Kyle, S. D., Spiegelhalder, K., et al. (2017). The effectiveness of behavioural and cognitive behavioural therapies for insomnia on depressive and fatigue symptoms: A systematic review and network meta-analysis. Sleep Medicine Reviews. doi:10.1016/j.smrv.2017.01.006.
Bartel, K. A., Gradisar, M., & Williamson, P. (2015). Protective and risk factors for adolescent sleep: A meta-analytic review. Sleep Medicine Reviews, 21, 72–85.
Bastien, C. H., Morin, C. M., Ouellet, M.-C., Blais, F. C., & Bouchard, S. (2004). Cognitive-behavioral therapy for insomnia: Comparison of individual therapy, group therapy, and telephone consultations. Journal of Consulting and Clinical Psychology, 72(4), 653–659.
Bei, B., Allen, N. B., Nicholas, C. L., Dudgeon, P., Murray, G., & Trinder, J. (2014). Actigraphy-assessed sleep during school and vacation periods: A naturalistic study of restricted and extended sleep opportunities in adolescents. Journal of Sleep Research, 23, 107–117.
Bei, B., Byrne, M., Ivens, C., Waloszek, J., Woods, M., Dudgeon, P., et al. (2013). Pilot study of a mindfulness-based, multi-component, in-school group sleep intervention in adolescent girls. Early Intervention in the Real World, 7, 213–220.
Bei, B., Wiley, J. F., Trinder, J., & Manber, R. (2016). Beyond the mean: A systematic review on the correlates of daily intraindividual variability of sleep/wake patterns. Sleep Medicine Reviews, 28, 108–124.
Belleville, G., Cousineau, H., Levrier, K., & St-Pierre-Delorme, M.-È. (2011). Meta-analytic review of the impact of cognitive-behavior therapy for insomnia on concomitant anxiety. Clinical Psychology Review, 31, 638–652.
Bellg, A. J., Borrelli, B., Resnick, B., Hecht, J., Minicucci, D. S., Ory, M., et al. (2004). Enhancing treatment fidelity in health behavior change studies: Best practices and recommendations from the NIH Behavior Change Consortium. Health Psychology, 23, 443–451.
Biggs, S. N., Lushington, K., van den Heuvel, C. J., Martin, A. J., & Kennedy, J. D. (2011). Inconsistent sleep schedules and daytime behavioral difficulties in school-aged children. Sleep Medicine, 12, 780–786.
Blake, M. J., Waloszek, J. M., Schwartz, O., Raniti, M. B., Simmons, J. G., Blake, L., et al. (2016). The SENSE Study: Post intervention effects of a randomized controlled trial of a cognitive-behavioral and mindfulness-based group sleep improvement intervention among at-risk adolescents. Journal of Consulting and Clinical Psychology, 84(12), 1039–1051.
Blunden, S., Chapman, J., & Rigney, G. (2012). Are sleep education programs successful? The case for improved and consistent research efforts. Sleep Medicine Reviews, 16, 355–370.
Blunden, S., & Rigney, G. (2015). Lessons learned from sleep education in schools: A review of dos and don’ts. Journal of Clinical Sleep Medicine, 11, 671–680.
Bootzin, R. R., & Stevens, S. J. (2005). Adolescents, substance abuse, and the treatment of insomnia and daytime sleepiness. Clinical Psychology Review, 25, 629–644.
Borsboom, D., Cramer, A. O. J., Schmittmann, V. D., Epskamp, S., & Waldorp, L. J. (2011). The small world of psychopathology. PLoS ONE, 6, e27407.
Boutron, I., Moher, D., Altman, D. G., Schulz, K. F., & Ravaud, P. (2008). Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: Explanation and elaboration. Annals of Internal Medicine, 148, 295–309.
Breslau, N., Roth, T., Rosenthal, L., & Andreski, P. (1996). Sleep disturbance and psychiatric disorders: A longitudinal epidemiological study of young adults. Biological Psychiatry, 39, 411–418.
Britton, W. B., Bootzin, R. R., Cousins, J. C., Hasler, B. P., Peck, T., & Shapiro, S. L. (2010). The contribution of mindfulness practice to a multicomponent behavioral sleep intervention following substance abuse treatment in adolescents: A treatment-development study. Substance Abuse, 31, 86–97.
Britton, W. B., Haynes, P. L., Fridel, K. W., & Bootzin, R. R. (2012). Mindfulness-based cognitive therapy improves polysomnographic and subjective sleep profiles in antidepressant users with sleep complaints. Psychotherapy and Psychosomatics, 81, 296–304.
Buscemi, N., Vandermeer, B., Friesen, C., Bialy, L., Tubman, M., Ospina, M., et al. (2007). The efficacy and safety of drug treatments for chronic insomnia in adults: A meta-analysis of RCTs. Journal of General Internal Medicine, 22(9), 1335–1350.
Buysse, D., Ancoli-Israel, S., Edinger, J., Lichstein, K., & Morin, C. (2006). Recommendations for a standard research assessment of insomnia. Sleep, 29, 1155–1173.
Buysse, D., Cheng, Y., Germain, A., Moul, D. E., Franzen, P. L., Fletcher, M., et al. (2010). Night-to-night sleep variability in older adults with and without chronic insomnia. Sleep Medicine, 11, 56–64.
Buysse, D., Thompson, W., Scott, J., Franzen, P. L., Germain, A., Hall, M., et al. (2007). Daytime symptoms in primary insomnia: A prospective analysis using ecological momentary assessment. Sleep Medicine, 8, 198–208.
Carskadon, M. (2011). Sleep in adolescents: The perfect storm. Pediatric Clinics of North America, 58, 637–647.
Carskadon, M., & Acebo, C. (2002). Regulation of sleepiness in adolescents: Update, insights, and speculation. Sleep, 25(6), 606–614.
Carskadon, M., Acebo, C., & Jenni, O. (2004). Regulation of adolescent sleep: Implications for behavior. Annals New York Academy of Sciences, 1021, 276–291.
Carskadon, M. A., Vieira, C., & Acebo, C. (1993). Association between puberty and delayed phase preference. Sleep, 16, 258.
Chase, R. M., & Pincus, D. B. (2011). Sleep-related problems in children and adolescents with anxiety disorders. Behavioral Sleep Medicine, 9, 224–236.
Christensen, H., Batterham, P. J., Gosling, J. A., Ritterband, L. M., Griffiths, K. M., Thorndike, F. P., et al. (2016). Effectiveness of an online insomnia program (SHUTi) for prevention of depressive episodes (the GoodNight Study): A randomised controlled trial. The Lancet Psychiatry, 0366(15), 333–341.
Clarke, G., McGlinchey, E., Hein, K., Gullion, C., Dickerson, J., Leo, M., et al. (2015). Cognitive-behavioral treatment of insomnia and depression in adolescents: A pilot randomized trial. Behaviour Research and Therapy, 69, 111–118.
Cohen, J. (1992). A power primer. Psychological Bulletin, 112, 155.
Colrain, I. M., & Baker, F. C. (2011). Changes in sleep as a function of adolescent development. Neuropsychology Review, 21, 5–21.
Cowie, J., Alfano, C. A., Patriquin, M., Reynolds, K. C., Talavera, D., & Clementi, M. (2014). Addressing sleep in children with anxiety disorders. Sleep Medicine Clinics, 9, 137–148.
Cuijpers, P. (2016). Meta-analyses in mental health research. A practical guide. https://indd.adobe.com/view/5fc8f9a0-bf1e-49d3-bf5f-a40bfe5409e0.
Cybulski, L., Mayo-Wilson, E., & Grant, S. (2016). Improving transparency and reproducibility through registration: The status of intervention trials published in clinical psychology journals. Journal of Consulting and Clinical Psychology, 84(9), 753–767.
Czeisler, C. A., Richardson, G. S., Coleman, R. M., Zimmerman, J. C., Moore-Ede, M. C., Dement, W. C., et al. (1981). Chronotherapy: Resetting the circadian clocks of patients with delayed sleep phase insomnia. Sleep, 4(1), 1–21.
Dahl, R. E., & Harvey, A. G. (2007). Sleep in children and adolescents with behavioral and emotional disorders. Sleep Medicine Clinics, 2, 501–511.
De Bruin, E. J., Bögels, S. M., Oort, F. J., & Meijer, A. M. (2015). Efficacy of cognitive behavioral therapy for insomnia in adolescents: A randomized controlled trial with internet therapy, group therapy and a waiting list condition. Sleep, 38, 1913–1926.
De Bruin, E. J., Oort, F. J., Bogels, S. M., & Meijer, A. M. (2014). Efficacy of internet and group-administered cognitive behavioral therapy for insomnia in adolescents: A pilot study. Behavioral Sleep Medicine, 12, 235–254.
Deeks, J. J., Dinnes, J., D’Amico, R., Sowden, A. J., Sakarovitch, C., Song, F., et al. (2003). Evaluating non-randomised intervention studies. Health Technology Assessment, 7(27), 3–10.
Devilly, G. J., & Borkovec, T. D. (2000). Psychometric properties of the credibility/expectancy questionnaire. Journal of Behavior Therapy and Experimental Psychiatry, 31, 73–86.
Dohnt, H., Gradisar, M., & Short, M. A. (2012). Insomnia and its symptoms in adolescents: Comparing DSM-IV and ICSD-II diagnostic criteria. Journal of Clinical Sleep Medicine, 8, 295–299.
Downs, S. H., & Black, N. (1998). The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. Journal of Epidemiology and Community Health, 52, 377–384.
Edinger, J. D., & Means, M. K. (2005). Cognitive-behavioral therapy for primary insomnia. Clinical Psychology Review, 25, 539–558.
Egger, M., Smith, G. D., Schneider, M., Minder, C., & Berne, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ, 315, 629–634.
Feinberg, I., & Campbell, I. G. (2010). Sleep EEG changes during adolescence: An index of a fundamental brain reorganization. Brain and Cognition, 72, 56–65.
Feinberg, I., Higgins, L. M., Khaw, W. Y., & Campbell, I. G. (2006). The adolescent decline of NREM delta, an indicator of brain maturation, is linked to age and sex but not to pubertal stage. American Journal of Physiology, Regulatory, Integrative and Comparative Physiology, 291, R1724–R1729.
Fernandez-Mendoza, J., Calhoun, S. L., Bixler, E. O., Karataraki, M., Liao, D., Vela-Bueno, A., et al. (2011). Sleep misperception and chronic insomnia in the general population: The role of objective sleep duration and psychological profiles. Psychosomatic Medicine, 73, 88.
Fernando III, A., Arroll, B., & Falloon, K. (2013). A double-blind randomised controlled study of a brief intervention of bedtime restriction for adult patients with primary insomnia. Journal of Primary Health Care, 5(1), 5–10.
Forbes, E. E., Bertocci, M. A., Gregory, A. M., Ryan, N. D., Axelson, D. A., et al. (2009). Objective sleep in pediatric anxiety disorders and major depressive disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 47, 148–155.
Fuligni, A. J., & Hardway, C. (2006). Daily variation in adolescents’ sleep, activities, and psychological well-being. Journal of Research on Adolescence, 16, 353–378.
Furukawa, T. A., Noma, H., Caldwell, D. M., Honyashiki, M., Shinohara, K., Imai, H., et al. (2014). Waiting list may be a nocebo condition in psychotherapy trials: A contribution from network meta-analysis. Acta Psychiatrica Scandinavica, 130(3), 181–192.
Garland, S. N., Carlson, L. E., Stephens, A. J., Antle, M. C., Samuels, C., & Campbell, T. S. (2014). Mindfulness-based stress reduction compared with cognitive behavioral therapy for the treatment of insomnia comorbid with cancer: A randomized, partially blinded, noninferiority trial. Journal of Clinical Oncology, 32, 449–457.
Garratt, G., Ingram, R. E., Rand, K., & Sawalani, G. (2007). Cognitive processes in cognitive therapy: Evaluation of the mechanisms of change in the treatment of depression. Clinical Psychology: Science and Practice, 14, 224–239.
Gradisar, M., & Crowley, S. J. (2013). Delayed sleep phase disorder in youth. Current Opinion in Psychiatry, 26(6), 580.
Gradisar, M., Dohnt, H., Gardner, G., Paine, S., Starkey, K., Menne, A., et al. (2011a). A randomized controlled trial of cognitive-behavior therapy plus bright light therapy for adolescent delayed sleep phase disorder. Sleep, 34, 1671–1680.
Gradisar, M., Gardner, G., & Dohnt, H. (2011b). Recent worldwide sleep patterns and problems during adolescence: A review and meta-analysis of age, region, and sleep. Sleep Medicine, 12, 110–118.
Gradisar, M., & Richardson, C. (2015). CBT-I cannot rest until the sleepy teen can. Sleep, 38, 1841–1842.
Gross, C. R., Kreitzer, M. J., Reilly-Spong, M., Wall, M., Winbush, N. Y., Patterson, R., et al. (2011). Mindfulness-based stress reduction versus pharmacotherapy for chronic primary insomnia: A randomized controlled clinical trial. Explore, 7, 76–87.
Gruber, R. (2016). School-based sleep education programs: A knowledge-to-action perspective regarding barriers, proposed solutions, and future directions. Sleep Medicine Reviews. doi:10.1016/j.smrv.2016.10.001.
Hale, L., & Guan, S. (2015). Screen time and sleep among school-aged children and adolescents: A systematic literature review. Sleep Medicine Reviews, 21, 50–58.
Harvey, A. G. (2004). Cognitive behavioural processes across psychological disorders: A transdiagnostic approach to research and treatment. Oxford: Oxford University Press.
Harvey, A. G. (2015). A transdiagnostic intervention for youth sleep and circadian problems. Cognitive and Behavioral Practice, 23(3), 341–355.
Harvey, A. G., Bélanger, L., Talbot, L., Eidelman, P., Beaulieu-Bonneau, S., Fortier-Brochu, E., et al. (2014). Comparative efficacy of behavior therapy, cognitive therapy, and cognitive behavior therapy for chronic insomnia: A randomized controlled trial. Journal of Consulting and Clinical Psychology, 82, 670–683.
Harvey, A. G., Murray, G., Chandler, R. A., & Soehner, A. (2011). Sleep disturbance as transdiagnostic: Consideration of neurobiological mechanisms. Clinical Psychology Review, 31, 225–235.
Higgins, J., & Green, S. (2011). Handbook for systematic reviews of interventions version 5.1. 0 [updated March 2011]. The Cochrane Collaboration.
Higgins, J., Thompson, S., Deeks, J., & Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ, 327, 557–560.
Hirshkowitz, M., Whiton, K., Albert, S. M., Alessi, C., Bruni, O., DonCarlos, L., et al. (2015). National sleep foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health, 1(1), 40–43.
Horowitz, J. L., & Garber, J. (2006). The prevention of depressive symptoms in children and adolescents: A meta-analytic review. Journal of Consulting and Clinical Psychology, 74(3), 401.
Huedo-Medina, T. B., Kirsch, I., Middlemass, J., Klonizakis, M., & Siriwardena, A. N. (2012). Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: Meta-analysis of data submitted to the Food and Drug Administration. BMJ, 345, e8343.
Hysing, M., Pallesen, S., Stormark, K. M., Lundervold, A. J., & Sivertsen, B. (2013). Sleep patterns and insomnia among adolescents: A population-based study. Journal of Sleep Research, 22, 549–556.
Insel, T. R. (2015). The NIMH experimental medicine initiative. World Psychiatry, 14, 151–153.
Irish, L. A., Kline, C. E., Gunn, H. E., Buysse, D. J., & Hall, M. H. (2015). The role of sleep hygiene in promoting public health: A review of empirical evidence. Sleep Medicine Reviews, 22, 23–36.
Jenni, O. G., & LeBourgeois, M. K. (2006). Understanding sleep-wake behavior and sleep disorders in children: The value of a model. Current Opinion in Psychiatry, 19, 282–287.
Johnson, E., Chilcoat, H. D., & Breslau, N. (2000). Sleep quality and elevated blood pressure in adolescents. Psychiatry Research, 94, 93–102.
Johnson, E., Roth, T., Schultz, L., & Breslau, N. (2006). Epidemiology of DSM-IV insomnia in adolescence: Lifetime prevalence, chronicity, and an emergent gender difference. Pediatrics, 117, e247–e256.
Kabat-Zinn, J. (2003). Mindfulness-based interventions in context: Past, present, and future. Clinical Psychology: Science and Practice, 10, 144–156.
Kales, A., Manfredi, R. L., Vgontzas, A. N., Bixler, E. O., Vela-Bueno, A., & Fee, E. C. (1991). Rebound insomnia after only brief and intermittent use of rapidly eliminated benzodiazepines. Clinical Pharmacology and Therapeutics, 49(4), 468–476.
Kazdin, A. E. (2007). Mediators and mechanisms of change in psychotherapy research. Annual Review of Clinical Psychology, 3, 1–27.
Keller, P., & El-Sheikh, M. (2011). Children’s emotional security and sleep: Longitudinal relations and directions of effects. Journal of Child Psychology and Psychiatry and Allied Disciplines, 52, 64–71.
Kessler, R. C., Avenevoli, S., Costello, E. J., Georgiades, K., Green, J. G., Gruber, M. J., et al. (2012). Prevalence, persistence, and sociodemographic correlates of DSM-IV disorders in the National Comorbidity Survey Replication Adolescent Supplement. Archives of General Psychiatry, 69, 372–380.
Kessler, R. C., Chiu, W. T., Demler, O., & Walters, E. E. (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry, 62, 617–709.
Kessler, R. C., Merikangas, K. R., & Wang, P. S. (2007). Prevalence, comorbidity, and service utilization for mood disorders in the United States at the beginning of the twenty-first century. Annual Review of Clinical Psychology, 3, 137–158.
Kira, G., Maddison, R., Hull, M., Bluden, S., & Olds, T. (2014). Sleep education improves the sleep duration of adolescents: A randomized controlled pilot study. Journal of Clinical Sleep Medicine, 10, 787–792.
Koffel, E. A., Koffel, J. B., & Gehrman, P. R. (2015). A meta-analysis of group cognitive behavioral therapy for insomnia. Sleep Medicine Reviews, 19, 6–16.
Kraemer, H. C., Wilson, T., Fairburn, C. G., & Agras, S. (2002). Mediators and moderators of treatment effects in randomized clinical trials. Archives of General Psychiatry, 59, 877–883.
Lancee, J., van Straten, A., Morina, N., Kaldo, V., & Kamphuis, J. H. (2016). Guided online or face-to-face cognitive behavioral treatment for insomnia? A randomized wait-list controlled trial. SLEEP, 39(1), 183–191.
Lau, J., Ionnidis, J. P., Terrin, N., Schmid, C. H., & Olkin, I. (2006). The case of the misleading funnel plot. BMJ, 333, 596–597.
Lewy, A. J., & Sack, R. L. (1996). The role of melatonin and light in the human circadian system. Progress in Brain Research, 111, 205–216.
Lovato, N., & Gradisar, M. (2014). A meta-analysis and model of the relationship between sleep and depression in adolescents: Recommendations for future research and clinical practice. Sleep Medicine Reviews, 18, 521–529.
Lovato, N., Gradisar, M., Short, M., Dohnt, H., & Micic, G. (2013). Delayed sleep phase disorder in an Australian school-based sample of adolescents. Journal of Clinical Sleep Medicine, 9, 939–944.
Mansell, W., Harvey, A., Watkins, E., & Shafran, R. (2009). Conceptual foundations of the transdiagnostic approach to CBT. Journal of Cognitive Psychotherapy, 23, 6–19.
Matthews, E. E., Arnedt, J. T., McCarthy, M. S., Cuddihy, L. J., & Aloia, M. S. (2013). Adherence to cognitive behavioral therapy for insomnia: A systematic review. Sleep Medicine Reviews, 17(6), 453–464.
Maume, D. J. (2013). Social ties and adolescent sleep disruption. Journal of Health and Social Behavior, 54, 498–515.
McMakin, D. L., & Alfano, C. A. (2015). Sleep and anxiety in late childhood and early adolescence. Current Opinion in Psychiatry, 28, 483–489.
Meltzer, L. J., & Mindell, J. A. (2014). Systematic review and meta-analysis of behavioral interventions for pediatric insomnia. Journal of Pediatric Psychology, 39, 932–948.
Meltzer, L. J., Montgomery-Downs, H. E., Insana, S. P., & Walsh, C. M. (2012). Use of actigraphy for assessment in pediatric sleep research. Sleep Medicine Reviews, 16, 463–475.
Millman, R. (2005). Excessive sleepiness in adolescents and young adults: Causes, consequences, and treatment strategies. Pediatrics, 115(6), 1774–1786.
Moher, D., Hopewell, S., Schulz, K. F., Montori, V., Gøtzsche, P. C., Devereaux, P. J., et al. (2012). CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. International Journal of Surgery, 10, 28–55.
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews, 4, 1–9.
Moore, M., Kirchner, H. L., Drotar, D., Johnson, N., Rosen, C., Ancoli-Israel, S., et al. (2009). Relationships among sleepiness, sleep time, and psychological functioning in adolescents. Journal of Pediatric Psychology, 34, 1175–1183.
Morgenthaler, T., Kramer, M., Alessi, C., Friedman, L., Boehlecke, B., Brown, T., et al. (2006). Practice parameters for the psychological and behavioral treatment of insomnia: An update. An American Academy of Sleep Medicine Report. Sleep, 29, 1415–1419.
Morin, C. (2003). Measuring outcomes in randomized clinical trials of insomnia treatments. Sleep Medicine Reviews, 7, 263–279.
Morin, C., Beaulieu-Bonneau, S., Ivers, H., Vallières, A., Guay, B., Savard, J., et al. (2014). Speed and trajectory of changes of insomnia symptoms during acute treatment with cognitive–behavioral therapy, singly and combined with medication. Sleep Medicine, 15(6), 701–707.
Morin, C., Bootzin, R. R., Buysse, D. J., Edinger, J. D., Espie, C. A., & Lichstein, K. L. (2006). Psychological and behavioral treatment of insomnia: Update of the recent evidence (1998–2004). Sleep, 29, 1398–1414.
Morin, C. M., Culbert, J. P., & Schwartz, S. M. (1994). Nonpharmacological interventions for insomnia: A meta-analysis of treatment efficacy. American Journal of Psychiatry, 151(8), 1172–1180.
Morin, C., & Espie, C. (2003). Insomnia: A clinical guide to assessment and treatment. New York, NY: Kluwer.
Murtagh, D., & Greenwood, K. (1995). Identifying effective psychological treatments for insomnia. Journal of Consulting and Clinical Psychology, 63, 78–89.
National Sleep Foundation. (2011). 2011 sleep in America poll: Communications technology in the bedroom. Summary of findings. https://sleepfoundation.org/sites/default/files/sleepinamericapoll/SIAP_2011_Summary_of_Findings.pdf.
Nowell, P. D., Mazumdar, S., Buysse, D. J., Dew, M. A., Reynolds, C. F., & Kupfer, D. J. (1997). Benzodiazepines and zolpidem for chronic insomnia: A meta-analysis of treatment efficacy. JAMA, 278(24), 2170–2177.
Office of Disease Prevention and Health Promotion. (2011). Healthy people 2020 objective topic areas. Washington, DC: US Dept of Health and Human Services.
Ohayon, M. M., & Roberts, R. E. (2001). Comparability of sleep disorders diagnoses using DSM-IV and ICSD classifications with adolescents. Sleep, 24(8), 920–925.
Ohayon, M. M., Roberts, R. E., Zulley, J., Smirne, S., & Priest, R. G. (2000). Prevalence and patterns of problematic sleep among older adolescents. Journal of the American Academy of Child and Adolescent Psychiatry, 39, 1549–1556.
Ong, J. C., Manber, R., Segal, Z., Xia, Y., Shapiro, S., & Wyatt, J. K. (2014). A randomized controlled trial of mindfulness meditation for chronic insomnia. Sleep, 37, 1553–1563.
Owens, J. A., Dearth-Wesley, T., Lewin, D., Gioia, G., & Whitaker, R. C. (2016). Self-regulation and sleep duration, sleepiness, and chronotype in adolescents. Pediatrics, 138(6), e20161406.
Paruthi, S., Brooks, L. J., D’Ambrosio, C., Hall, W. A., Kotagal, S., Lloyd, R. M., et al. (2016). Recommended amount of sleep for pediatric populations: A consensus statement of the American Academy of Sleep Medicine. Journal of Clinical Sleep Medicine, 12(6), 785–786.
Pasch, K. E., Laska, M. N., Lytle, L. A., & Moe, S. G. (2010). Adolescent sleep, risk behaviors, and depressive symptoms: Are they linked? American Journal of Health Behavior, 34, 237–248.
Pelayo, R. P., Thorpy, M. J., & Glovinsky, P. (1988). Prevalence of delayed sleep phase syndrome among adolescents. Journal of Sleep Research, 17, 392.
Perez-Lloret, S., Videla, A. J., Richaudeau, A., Vigo, D., Rossi, M., Cardinali, D. P., et al. (2013). A multi-step pathway connecting short sleep duration to daytime somnolence, reduced attention, and poor academic performance: An exploratory cross-sectional study in teenagers. Journal of Clinical Sleep Medicine, 9(5), 469–473.
Qaseem, A., Kansagara, D., Forciea, M. A., Cooke, M., & Denberg, T. D. (2016). Management of chronic insomnia disorder in adults: A clinical practice guideline from the American College of Physicians. Annals of Internal Medicine, 165(2), 125–133.
Riemann, D., & Perlis, M. L. (2009). The treatments of chronic insomnia: A review of benzodiazepine receptor agonists and psychological and behavioral therapies. Sleep Medicine Reviews, 13, 205–214.
Roberts, R. E., Roberts, C. R., & Duong, H. T. (2009). Chronic insomnia and its negative consequences for health and functioning of adolescents: A 12-month prospective study. Journal of Adolescent Health, 42, 294–302.
Roeser, K., Schwerdtle, B., Kubler, A., & Schlarb, A. (2016). Further evidence for the just program as treatment for insomnia in adolescents: Results from a 1-year follow-up study. Journal of Clinical Sleep Medicine, 12, 257–262.
Rohde, P. (2015). Cognitive-behavioral prevention of depression in adolescents. Current Opinion in Psychology, 4, 136–141.
Sack, R., Auckley, D., Auger, R., Carskadon, M., Wright, K., Vitiello, M., et al. (2007). Circadian rhythm sleep disorders: Part II: Advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder and irregular sleep-wake rhythm. Sleep, 30(11), 1484–1501.
Saxvig, I. W., Pallesen, S., Wilhelmsen-Langeland, A., Molde, H., & Bjorvatn, B. (2012). Prevalence and correlates of delayed sleep phase in high school students. Sleep Medicine, 13(2), 193–199.
Schlarb, A. A., Liddle, C. C., & Hautzinger, M. (2011). JuSt—A multimodal program for treatment of insomnia in adolescents: A pilot study. Nature and Science of Sleep, 3, 13–20.
Scholle, S., Beyer, U., Bernhard, M., Eichholz, S., Erler, T., Graness, P., et al. (2011). Normative values of polysomnographic parameters in childhood and adolescence: Quantitative sleep parameters. Sleep Medicine, 12, 542–549.
Schwartz, D. R., & Carney, C. E. (2012). Mediators of cognitive-behavioral therapy for insomnia: A review of randomized controlled trials and secondary analysis studies. Clinical Psychology Review, 32, 664–675.
Short, M. A., Gradisar, M., Wright, H., Lack, L. C., Dohnt, H., & Carskadon, M. A. (2011). Time for bed: Parent-set bedtimes associated with improved sleep and daytime functioning in adolescents. Sleep, 34, 797–800.
Sivertsen, B., Omvik, S., Pallesen, S., Bjorvatn, B., Havik, O. E., Kvale, G., et al. (2006). Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults: A randomized controlled trial. JAMA, 295(24), 2851–2858.
Taylor, D. J., & Pruiksma, K. E. (2014). Cognitive and behavioural therapy for insomnia (CBT-I) in psychiatric populations: A systematic review. International Review of Psychiatry, 26, 205–213.
R Core Team. (2015). R: A language and environment for statistical computing. Vienna, Austria: Foundation for Statistical Computing. Retrieved from https://www.R-project.org/.
Telzer, E. H., Goldenberg, D., Fuligni, A. J., Lieberman, M. D., & Gálvan, A. (2015). Sleep variability in adolescence is associated with altered brain development. Developmental Cognitive Neuroscience, 14, 16–22.
Trauer, M., Qian, M. Y., Doyle, J. S., Rajaratnam, S. M., & Cunnington, D. (2015). Cognitive behavioral therapy for chronic insomnia: A systematic review and meta-analysis. Annals of Internal Medicine, 163, 191–204.
Van Straten, A., van der Zweerde, T., Kleiboer, A., Cuijpers, P., Morin, C. M., & Lancee, J. (2017). Cognitive and behavioral therapies in the treatment of insomnia: A meta-analysis. Sleep Medicine Reviews. doi:10.1016/j.smrv.2017.02.001.
Verbeek, I., Konings, G., Aldenkamp, A., Declerck, A., & Klip, E. (2006). Cognitive behavioral treatment in clinically referred chronic insomniacs: Group versus individual treatment. Behavioral Sleep Medicine, 4(3), 135–151.
Viechtbauer, W. (2010). Conducting meta-analyses in R with the metafor package. Journal of Statistical Software, 36(3), 1–48.
Vitiello, M. V., McCurry, S. M., & Rybarczyk, B. D. (2013). The future of cognitive behavioral therapy for insomnia: What important research remains to be done? Journal of Clinical Psychology, 69, 1013–1021.
Warner, S., Murray, G., & Meyer, D. (2008). Holiday and school-term sleep patterns of Australian adolescents. Journal of Adolescence, 31, 595–608.
Weisz, J. R., Ng, M. Y., & Bearman, S. K. (2014). Odd couple? Reenvisioning the relation between science and practice in the dissemination-implementation era. Clinical Psychological Science, 2(1), 58–74.
Wensing, M., Bosch, M., & Grol, R. (2010). Developing and selecting interventions for translating knowledge to action. CMAJ, 182(2), 85–88.
Wing, Y., Chan, N., Man Yu, M., Lam, S., Zhang, J., Li, S., et al. (2015). A school-based sleep education program for adolescents: A cluster randomized trial. Pediatrics, 135, e635–e643.
Wolfson, A., & Carskadon, M. (1998). Sleep schedules and daytime functioning in adolescents. Child Development, 69, 875–887.
World Health Organization. (2015). Health for the world’s adolescents. A second chance in the second decade, 2014. http://apps.who.int/adolescent/second-decade/files/1612_MNCAH_HWA_Executive_Summary.pdf.
Yamadera, W., Sato, M., Harada, D., Iwashita, M., & Aoki, R. (2013). Comparisons of short-term efficacy between individual and group cognitive behavioral therapy for primary insomnia. Sleep and Biological Rhythms, 11(3), 176–184.
Acknowledgements
The authors would like to thank Mr. Adam Pettitt for assisting with the study quality ratings and risk-of-bias assessments.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Blake, M.J., Sheeber, L.B., Youssef, G.J. et al. Systematic Review and Meta-analysis of Adolescent Cognitive–Behavioral Sleep Interventions. Clin Child Fam Psychol Rev 20, 227–249 (2017). https://doi.org/10.1007/s10567-017-0234-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10567-017-0234-5