Abstract
Aging, injuries, and diseases can be considered as the result of malfunctioning or damaged cells. Regenerative medicine aims to restore tissue homeostasis by repairing or replacing cells, tissues, or damaged organs, by linking and combining different disciplines including engineering, technology, biology, and medicine. To pursue these goals, the discipline is taking advantage of pluripotent stem cells (PSCs), a peculiar type of cell possessing the ability to differentiate into every cell type of the body. Human PSCs can be isolated from the blastocysts and maintained in culture indefinitely, giving rise to the so-called embryonic stem cells (ESCs). However, since 2006, it is possible to restore in an adult cell a pluripotent ESC-like condition by forcing the expression of four transcription factors with the rejuvenating reprogramming technology invented by Yamanaka. Then the two types of PSC can be differentiated, using standardized protocols, towards the cell type necessary for the regeneration. Although the use of these derivatives for therapeutic transplantation is still in the preliminary phase of safety and efficacy studies, a lot of efforts are presently taking place to discover the biological mechanisms underlying genetic pathologies, by differentiating induced PSCs derived from patients, and new therapies by challenging PSC-derived cells in drug screening.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastrulation is the central phase of embryogenesis that transforms the blastocyst into a structure comprising the primary germ layers of the embryo. Before gastrulation, the inner cell mass of the blastocyst consists of a single homogeneous population, while, with the onset of gastrulation, clonal groups of cells restrict their developing fate. Indeed, starting from the pluripotent epiblast, three germ layers are specified, rearranged, and shaped into the body plan (Solnica-Krezel and Sepich 2012).
The determination of cell fate is a stepwise process that involves a combination of transcription factors (TFs) that, by binding to specific DNA sequences, control the transcription of the genetic information from DNA to messenger RNA. In 1987, the possibility to convert cellular lineage by forcing the expression of a single transfected cDNA was shown for the first time (Davis et al. 1987). Indeed, when the TF MyoD1 was expressed in fibroblasts or adipoblast cell lines, myotube formation as for skeletal muscle cells was observed, thus demonstrating the TF-mediated conversion of cell type (Tapscott et al. 1988). Since then, several other experiments suggest the instructive role of TFs in determining the cell type. Indeed, GATA1 expression converts monocytic precursors to erythroid-megakaryocytic cells or eosinophils (Kulessa et al. 1995) while Pax5 ablation or C/EBPa transduction convert B cells into macrophages (Nutt et al. 1999; Xie et al. 2004).
In 1981, the inner cell mass of the developing murine blastocyst was firstly isolated, dissociated, and cultured in vitro to obtain an embryonic stem cell (ESC) line with unlimited, undifferentiated proliferative potential (Evans and Kaufman 1981; Martin 1981). In 1998, human embryos, produced for clinical purposes by in vitro fertilization techniques, were cultured to the blastocyst stage; their inner cell masses were isolated by immunosurgery, plated on irradiated mouse embryonic fibroblasts, and cultured indefinitely as human ESC (Thomson et al. 1998). This procedure, which implies the destruction of a human embryo, has been the subject of profound, warm, and living discussions in ethics and morality (Annas et al. 1996; Doerflinger 1999; Robertson 2001).
Using murine- and human-derived ESC, several studies identified key genes, mainly TFs, directly linked to the maintenance of cell renewal (e.g., Oct 3/4; Niwa 2001) and pluripotency (e.g., Nanog; Mitsui et al. 2003), the two properties that define a cell as a stem cell.
In 2006, Shinya Yamanaka demonstrated the possibility to use TF not only to change cell fate but also to encompass the gastrulation process. Yamanaka undertook the challenge of finding the proper combination of transcription factors that converts mouse embryonic fibroblasts into induced pluripotent stem cells (iPSCs), an artificial state strictly related to ESC (Takahashi and Yamanaka 2006). The reprogramming procedure appeared to be very efficient when the combination of four TFs Oct4, Sox2, Klf4, and c-Myc (OSKM factors, also called Yamanaka’s factors) was applied. One year later, Yamanaka’s group successfully derived iPSCs also from human fibroblasts (Takahashi et al. 2007), thus opening the way to the therapeutic use in regenerative medicine of cells directly differentiated from iPSCs.
The outstanding innovation of Yamanaka’s procedure is the possibility to derive iPSCs from an individual patient and then transplant autologous iPSC derivatives for therapeutic/regenerative medicine. In the last decade, several reprogramming techniques that generate human PSCs from differentiated somatic cells have been successfully developed (Takahashi and Yamanaka 2006; Takahashi et al. 2007; Yu et al. 2007; Chung et al. 2014; Tachibana et al. 2013), and a number of protocols have been established to improve the induced reprogramming technique (Maherali and Hochedlinger 2008; González et al. 2011; Brouwer et al. 2016). The purpose of this extensive research resides in obtaining reprogrammed cells devoid of any genetic changes in chromosomal DNA induced by the reprogramming process.
Cell reprogramming
The reprogramming process serves to reawaken the endogenous TFs that characterize a pluripotent cell. Once the pluripotency-associated TFs are switched on by the transient ectopic expression of OSKM factors, the cell will indeed remain in the embryonic stem-like state (Stadtfeld and Hochedlinger 2010). A summary of the different reprogramming methods, together with the pros and cons of the specific technology, is reported in Table 1.
The delivery of the OSKM transcription factors into a mouse or human fibroblasts was originally achieved using modified retroviruses that provide a relatively easy and efficient way of introducing exogenous genes into somatic cells. However, since retroviruses do not pass the nuclear membrane, the gene transduction mediated by retroviral vectors is effective only in actively dividing cells (Matreyek and Engelman 2013).
Lentiviruses, unlike retroviruses, are able to transduce both non-dividing (slowly dividing or quiescent but metabolically active cells) and dividing cells. Then, lentiviral-based vectors were introduced to enhance the reprogramming process. In particular, lentiviral-derived polycistronic vectors have allowed the contemporary introduction of OSKM genes in a cell, a feature that considerably reduces vector copy number integration per cell. This procedure significantly decreased the risk for insertional mutagenesis and greatly enhanced the efficiency of the process.
Nevertheless, even though lentiviral-based vectors have been used in clinics to sustain the expression of arylsulfatase A (Biffi et al. 2013) or WASP (Aiuti et al. 2013), the exogenous viral DNA sequences inserted in the patient’s genome will last forever, thus limiting the use of the transduced cells to compassionate medicine patients.
Based on their behavior as episomal non-integrating DNA, adenoviral vectors have been considered as agents for cell reprogramming and indeed replication-defective adenoviruses expressing OSKM factors have been proven to be useful for the derivation of iPSCs from liver cells and fibroblasts (Stadtfeld et al. 2008).
As an alternative to viral delivery, non-integrating reprogramming approaches based on the use of episomal plasmid DNA vectors, EBNA-based vectors, or minicircle DNA have been developed, although, as well as in the adenoviral system, the efficiency of human-induced PSC (hiPSC) generation remains low (for a review, see Park et al. 2014).
In 2011, the efficient generation of transgene-free human iPSCs by temperature-sensitive Sendai virus (SeV) vectors has been reported (Ban et al. 2011). SeV efficiently introduces into the cells single-stranded RNA which remains in the cytoplasm and involves neither DNA intermediates nor the integration into the host genome (Bitzer et al. 2003). In addition, the SeV vector containing the reprogrammed genes can be cleared from the host cell using the functional temperature sensitivity mutations introduced into the key viral proteins (Ban et al. 2011). Indeed, recombinant F gene-deleted, non-transmissible SeV has already been used in the gene therapy approach, demonstrating no serious adverse event related to its administration (ClinicalTrials.gov identifier: NCT02276937).
PSCs and tumor formation
By definition, a cell is pluripotent if it can differentiate into cells derived from all three of the embryonic germ layers: ectoderm, mesoderm, and endoderm. There are several types of in vitro and in vivo assays to be performed in order to demonstrate that the procedure invented by Yamanaka indeed generates pluripotent cells. Among them, the teratoma formation assay serves as a robust and relatively simple way to demonstrate pluripotency. Briefly, the assay consists in injecting the cells, which are supposed to be pluripotent, in immunodeficient mice and waiting for the formation of a tumor. In the case of pluripotent cells, a teratoma, the classical tumor of germ cells, will arise, containing derivatives from all three of the germ layers. Indeed, the identification within the tumor of structures belonging to the three germ layers confirms the pluripotency of the cell line (Bulic-Jakus et al. 2016).
The concept that iPSCs, as well as ESCs, will form a teratoma if xeno- or allo-grafted strongly hampers their use in the clinical practice even if regenerative medicine procedures do not intend to use the iPSCs themselves but cells obtained from their differentiation. Although xenograft models may not accurately predict the fate of grafted cells in humans, for the most application it may be necessary to treat patients with billions of cells, for which the security process leading to the complete absence of undifferentiated cells must be greatly increased.
Nevertheless, the identification of surface markers specific for tumor-forming PSCs (Tang et al. 2011) or drugs that selectively suppress undifferentiated iPSC (Lee et al. 2013) will surely give rise to safe transplantable cells with no or minimal risk for tumor formation.
PSC-differentiated derivatives
iPSCs, like ESCs, have the potential to differentiate into every cell type of the body. Although several labs reported the transdifferentiation of adult mesenchymal stem cells in hepatocytes (e.g., Wu and Tao 2012), pancreatic islet cells (Bhonde et al. 2014), neurons (for a review, see Scuteri et al. 2011), or cardiomyocytes (e.g., Choi et al. 2010), the utilized protocols are poorly reproducible. At variance, several commercial kits to derive these and other types of terminally differentiated cells directly from PSCs are already available (see the catalog of the following companies: Thermo Fisher Scientific, Stem Cell Technologies, Pluriomics, R&D Systems, Clontech, Axol). Indeed, the fact that the companies sell commercial products guarantees a wide standardization leading to greater reproducibility of the methods that will be reflected in the quality of the obtained cells.
An updated list of cells that have been derived from both human ESCs or iPSCs can be found at the following website:https://research.cchmc.org/stemcell/differentiation#Directed_hPSC_diff_in_the_PSCF
Since the two cell types of PSC are very close (Mallon et al. 2014), the various differentiation protocols were applied indifferently or with some minor modifications. As shown, labile, stable, or perennial cells belonging to all of the three germ layers are reported, thus confirming the enormous differentiation capability of PSCs. Moreover, methods to obtain complex stem cell-derived autoassembling functional tissues, the so-called organoids, have been described. By definition, an organoid contains several cell types that self-organize similarly to the in vivo organogenesis process (for a review, see Lancaster and Knoblich 2014). During these years, the amelioration in the differentiation protocols of human PSCs has greatly improved the generation of these structures that have, even more than of single PSC derivatives, the potential to model the developmental process and the disease, thus representing a tool for drug testing as well as for therapeutic approach. Indeed, pulmonary (Dye et al. 2015), intestinal (Spence et al. 2011), retinal (Zhong et al. 2014), hepatic (Takebe et al. 2014), cerebral (Lancaster et al. 2013), or optic cup organoids (Nakano et al. 2012) have been successfully derived.
A major problem for the study of PSC or PSC-derived organoid resides in their functional immaturity. In the reprogramming process, cells undergo a “rejuvenation” that will produce terminally differentiated cells exhibiting features similar to the fetal or embryonic counterpart. Although in some experiments the age of the cells is a negligible detail, it would be appropriate to achieve a certain degree of maturation when modeling age-related diseases, e.g., neurodegenerative disorders. Indeed, long-term culture of dopaminergic neurons, supported by a monolayer of mouse postnatal cortical astrocytes, showed the expected features of maturation, including complex dendritic arborizations (Sánchez-Danés et al. 2012).
PSC-related clinical trials
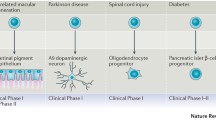
Nowadays, the search for PSC-based clinical trials in the “clinicaltrials.gov” website returns 51 results. Most of these studies are recruiting patients for disease modeling, some of which (less than 10%) are generating terminally differentiated cells for drug screening, while only one study, not yet opened, will use PSC derivatives in transplants (ClinicalTrials.gov identifier: NCT02464956).
PSC in disease modeling
In the past, disease modeling studies have been carried out using cellular or animal models. In the first case, several attempts have been made on immortalized cells, whose DNA has been modified according to the mutation which is thought to cause the disease; a similar procedure has been used also to genetically modify animals, usually mice, which in most cases do not precisely exhibit all of the symptoms of the human disease. At variance, the iPSC model has several advantages including the direct source from the patient. This means that all the differentiated cells will carry not only the mutation but also the entire genetic background of the patient which, in some cases, is crucial to correctly see and evaluate the pathology. Furthermore, when studying neurodegenerative disorders or cardiac pathologies, the possibility to differentiate postmitotic cells as neurons or cardiomyocytes carrying the mutation in the correct biological environment eliminates the doubtful results due to the absence, in the heterologous system, of cell-specific accessory proteins.
One example out of many, congenital long-QT syndromes (LQTSs) are a group of heritable, usually autosomal dominant disorders with an estimated prevalence of 1:2500, characterized by an abnormally delayed or prolonged ventricular repolarization phase (Crotti et al. 2008). To date, LQTSs have been associated with over 500 different mutations in at least 13 genes encoding cardiac ion channel proteins, but the most prevalent forms LQT1 and LQT2 are caused by potassium channel mutations with a percentage of genotyped cases of >50 and 30–40%, respectively (Crotti et al. 2008). LQT1 was the first cardiac disease modeled using hiPSC, and since then, several hiPSC-derived cardiomyocytes (CMs) from patients carrying mutations in LQTS-associated channels have been considered (Dell'Era et al. 2015).
Disease-specific abnormalities were observed in LQT1-derived CMs including a variation in the duration and a rate adaptation of the action potential, a 70 to 80% reduction in slow delayed rectifier potassium current IKs, and vulnerability to catecholaminergic stress (Moretti et al. 2010). Moreover, electrophysiological studies confirmed the protective effect of beta-blockers in the abnormal response to catecholamine stimulation, thus supporting the current therapeutic approach for LQT1 patients (Moretti et al. 2010).
Unlike classical genetic studies, the iPSC model is able to give a functional response also in the case of the sporadic form of a genetic disease, where no correlation with specific mutations has been found yet. Indeed, neurons generated from Parkinson’s disease (PD) patients carrying leucin-rich repeat kinase 2 mutation, as well as neurons generated from sporadic PD patients, developed evident signs of neurodegeneration, including fewer and shorter neurites and a significant increase in apoptotic cells, probably due to a deficient autophagic machinery (Sánchez-Danés et al. 2012).
PSC and pharmaceutics
Reliable data on potential toxic effects of novel therapeutic drugs are urgently needed. Cardiotoxicity, neurotoxicity, immunotoxicity, and hepatotoxicity are serious complications of clinical therapy and one of the main causes for failure of promising drug candidates.
Animal studies, such as mice, dogs, and pigs, have been traditionally used in toxicological research to provide preclinical security evaluation of various therapeutic agents under development, but differences in drug metabolism and related toxicity contribute to the failure of drug trials from animal models to humans (Wobus and Löser 2011; Giri and Bader 2015).
PSC-derived cells offer a humanized platform for preclinical efficacy and toxicity studies of innovative therapeutic drugs in development. Indeed, the unlimited supply of terminally differentiated human cardiomyocytes, neurons, and hepatocytes derived from PSC can be used in assays for drug screening and/or toxicity assessment (Ko and Gelb 2014). However, the clonal origin of an iPSC line as well as the young age of differentiated cells must be considered before reaching hurried conclusions. The use of this technology will allow producing high-quality drugs with a relatively low-cost drug discovery process and will fulfill the guiding principles of replacement, refinement, and reduction for an ethical use of animals in research.
Moreover, starting from iPSC-derived diseased cells, potential or new drug candidates can be easily screened in vitro and then tested in a clinical trial allowing to demonstrate their safety and dose, identify side effects (phase I), and further predict their efficacy in terms of toxicity, dosage, and human susceptibility (phase II).
Using patient-specific iPSCs, a clinical ready drug library was screened and multiple validated hits for novel treatment of alpha1-antitrypsin deficiency patients were identified (Choi et al. 2013). Furthermore, using iPSCs derived from amyotrophic lateral sclerosis (ALS) patients, a histone acetyltransferase inhibitor called anacardic acid was identified as a drug that rescued the abnormal ALS motor neuron phenotype (Egawa et al. 2012). In the last example, a surrogate of diabetic cardiomyopathy was chemically induced in iPSC-derived cardiomyocytes (CMs) and the cells were used in a screening assay for protective drugs (Drawnel et al. 2014). In parallel, iPSCs were derived from type 2 diabetes mellitus (T2DM) patients, and the resulting CMs showed a similar cardiomyopathic phenotype (Drawnel et al. 2014). Several protective drugs identified in the first screening were really effective also with T2DM CMs, thus revealing that the iPSC platform has the intrinsic potential to discover new disease mechanisms and valid treatments (Drawnel et al. 2014).
PSC-derived cells and transplantation
In January 2009, Geron, a company based in Menlo Park, California, received FDA clearance to begin the world’s first human long-awaited clinical trial of a human ESC (hESC)-based oligodendrocyte progenitor cell therapy (GRNOPC1). GRNOPC1 cells have demonstrated remyelinating, nerve growth stimulating, and angiogenic properties leading to restoration of function in rodent models of acute spinal cord injury (Lebkowski 2011). The phase I clinical trial enrolled its first patient in October 2010, and totally, five young patients, four males, and one female were treated. Although they received billions of cells within 2 weeks of injury, none of them developed an immune response to GRNOPC1 even though some had complete HLA mismatch (Ilic et al. 2015). At present, a similar study, conducted by Asterias Biotherapeutics, is recruiting participants with the aim to evaluate the safety of escalating doses of oligodendrocyte progenitor cells in subjects with subacute cervical spinal cord injuries (ClinicalTrials.gov identifier: NCT02302157).
In September 2013, the first heart-related clinical trial, made by the Assistance Publique—Hôpitaux de Paris, enrolled the first patient (ClinicalTrials.gov identifier: NCT02057900). Patients with ischemic heart failure receive a fibrin gel embedded with human ESC-derived cardiac-committed CD15+ Isl-1+ progenitors during coronary artery bypass grafting and/or a mitral valve procedure. The objective of this study is to assess both the feasibility and safety issues.
In September 2014, ViaCyte initiated clinical research on its VC-01™ product candidate for type 1 diabetes (http://viacyte.com/clinical/clinical-trials/). VC-01™ has two components: pancreatic progenitor cells (PEC-01) encapsulated in a device to deliver the cells to the patient and protect them from the attack by the patient’s immune system. PEC-01 cells are manufactured from a line of hESCs using a carefully controlled directed differentiation process, designed to yield a cell population, devoid of pluripotency, that can mature into glucose-responsive, insulin-producing cells. Indeed, in animal studies, implanted PEC-01 cells have been shown to further differentiate and mature into pancreatic endocrine cells, including beta cells that secrete human insulin in response to increases in blood glucose (Agulnick et al. 2015).
In parallel to these sporadic studies that still will give us essential information on PSC-derivative safety in clinical use, a massive intervention is taking place to produce and test PSC-derived retinal pigment epithelial (RPE) cells to treat macular degeneration and related diseases. Most of these clinical trials utilize hESC-derived RPE cells (MA09-hRPE), while just one is using hiPSC-derived RPE cells.
MA09-hRPE were derived from single-blastomere ESCs and differentiated into RPE cells at 99% purity (Klimanskaya et al. 2004). After the introduction to the subretinal space of rats, MA09-hRPE survived for more than 8 months without evidence of pathological consequences, but, more importantly, the cells rescued visual functions measured with both visual acuity and luminance threshold response (Lu et al. 2009).
Results from completed trials demonstrate no evidence of adverse proliferation, rejection, or serious ocular or systemic safety issues related to the transplanted tissue, and change from baseline in best-corrected visual acuity was observed in patients with age-related macular degeneration (ClinicalTrials.gov identifier: NCT01344993) and Stargardt’s macular dystrophy (NCT01354006) (Schwartz et al. 2015). Recently, Cell Cure Neuroscience Ltd. started a phase I/II trial to test hESC-derived RPE cells in age-related macular degeneration (ClinicalTrials.gov identifier: NCT02286089).
The first clinical trial involving transplantation of iPSC derivatives was carried out in Japan, at Rikagaku Kenkyūsho (RIKEN) Institute. On September 12, 2014, the first participant in the “Clinical study of autologous induced pluripotent stem cell-derived retinal pigment epithelium (RPE) cell sheets for exudative age-related macular degeneration (AMD),” a 70-year-old woman, underwent transplantation of a cell sheet graft. Skin sample was harvested approximately 10 months before and used to generate autologous iPSC-derived RPE cell sheets. A single RPE cell sheet (1.3 mm × 3 mm) was engrafted into the subretinal space of one eye without serious hemorrhaging or complications.
In March 2015, the enrollment of patients was suspended, in order to review the trial according to the Act on the Safety of Regenerative Medicine for which processed cells must be manufactured in a government-licensed and government-inspected cell processing facility. Nevertheless, the patient was examined at the end of the first year and no signs of tumorigenesis or other major abnormalities were observed as a result of the transplantation. However, the press release by RIKEN states that “the efficacy of the iPSC-derived RPE sheet was difficult to assess” (http://www.rikenibri.jp/AMD/img/20151009en.pdf).
Conclusions
The process of replacing, engineering, or regenerating human cells, tissues, or organs to restore or establish normal function, summarized with the words “regenerative medicine,” is a very complex field of research that has attracted a huge expectation from all mankind. Regenerated products can be easily derived from stem cells, particularly from those that hold a pluripotent differentiation capacity like ESC or iPSC. However, in common with past discoveries, the translation of these products in the clinical setting needs a lot of time to predict and evaluate every aspect that may interfere with the therapeutical healing process.
The long-term effects of a PSC-derived therapeutic approach are still unknown. The genetic and epigenetic alterations associated with reprogramming, the risk of teratocarcinoma formation, and the lack of robust and highly reproducible differentiation protocols all pose significant challenges to the use of iPSCs as cell-based therapies for human patients.
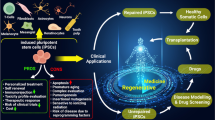
The preliminary studies demonstrated that if therapeutic terminally differentiated cells can be isolated and sorted from the original pluripotent population, a safe product is obtained, devoid of any undesirable side effect, especially the tumor forming capacity. The possibility to obtain iPSCs directly from the patients has focused even more attention in this research field, opening the possibility to personalize the pharmacological treatment, to eventually correct the pathology-linked DNA mutation, and to use patient-derived rejuvenated cells for an autologous transplant (see Fig. 1).
Overview of the regenerative medicine process that involves the derivation and use of iPSCs. iPSCs can be derived from healthy (blue) or genetically diseased patient (pink) and subjected to the desired differentiation protocol. Nowadays (solid-line arrows), terminally differentiated cells derived from the two conditions can be used for drug screening, while patient-derived cells are increasing the knowledge of disease modeling or gene therapy; soon (dashed-line arrows), PSC-derived cells will strengthen the field of personalized medicine and will be finally challenged in autologous transplant (color figure online)
The great expectation on regenerative medicine therapeutic protocols will certainly be answered but with the time needed and the systematic methods of a serious and rigorous science.
References
Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26(11):1276–84.
Agulnick AD, Ambruzs DM, Moorman MA, Bhoumik A, Cesario RM, et al. Insulin-producing endocrine cells differentiated in vitro from human embryonic stem cells function in macroencapsulation devices in vivo. Stem Cells Transl Med. 2015;4(10):1214–22.
Aiuti A, Biasco L, Scaramuzza S, Ferrua F, Cicalese MP, Baricordi C, et al. Lentiviral hematopoietic stem cell gene therapy in patients with wiskott-aldrich syndrome. Science. 2013;341(6148):1233151-1233151.
Annas GJ, Caplan A, Elias S. The politics of human-embryo research—avoiding ethical gridlock. N Engl J Med. 1996;334(20):1329–32.
Ban H, Nishishita N, Fusaki N, Tabata T, Saeki K, et al. Efficient generation of transgene-free human induced pluripotent stem cells (iPSCs) by temperature-sensitive Sendai virus vectors. Proc Natl Acad Sci U S A. 2011;108(34):14234–9.
Bhonde RR, Sheshadri P, Sharma S, Kumar A. Making surrogate β-cells from mesenchymal stromal cells: perspectives and future endeavors. Int J Biochem Cell Biol. 2014;46:90–102.
Biffi A, Montini E, Lorioli L, Cesani M, Fumagalli F, et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341(6148):1233158.
Bitzer M, Armeanu S, Lauer UM, Neubert WJ. Sendai virus vectors as an emerging negative-strand RNA viral vector system. J Gene Med. 2003;5(7):543–53.
Brouwer M, Zhou H, Nadif KN. Choices for induction of pluripotency: recent developments in human induced pluripotent stem cell reprogramming strategies. Stem Cell Rev. 2016;12(1):54–72.
Bulic-Jakus F, Katusic Bojanac A, Juric-Lekic G, Vlahovic M, Sincic N. Teratoma: from spontaneous tumors to the pluripotency/malignancy assay. Wiley Interdiscip Rev Dev Biol. 2016;5(2):186–209.
Chang CW, Lai YS, Pawlik KM, Liu K, Sun CW, et al. Polycistronic lentiviral vector for “hit and run” reprogramming of adult skin fibroblasts to induced pluripotent stem cells. Stem Cells. 2009;27(5):1042–9.
Choi YS, Dusting GJ, Stubbs S, Arunothayaraj S, Han XL, et al. Differentiation of human adipose-derived stem cells into beating cardiomyocytes. J Cell Mol Med. 2010;14(4):878–89.
Choi SM, Kim Y, Shim JS, Park JT, Wang RH, et al. Efficient drug screening and gene correction for treating liver disease using patient-specific stem cells. Hepatology. 2013;57(6):2458–68.
Chung YG, Eum JH, Lee JE, Shim SH, Sepilian V, et al. Human somatic cell nuclear transfer using adult cells. Cell Stem Cell. 2014;14(6):777–80.
Crotti L, Celano G, Dagradi F, Schwartz PJ. Congenital long QT syndrome. Orphanet J Rare Dis. 2008;3:18.
Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51(6):987–1000.
Dell'Era P, Benzoni P, Crescini E, Valle M, Xia E, et al. Cardiac disease modeling using induced pluripotent stem cell-derived human cardiomyocytes. World J Stem Cells. 2015;7(2):329–42.
Doerflinger RM. The ethics of funding embryonic stem cell research: a Catholic viewpoint. Kennedy Inst Ethics J. 1999;9(2):137–50.
Drawnel FM, Boccardo S, Prummer M, Delobel F, Graff A, et al. Disease modeling and phenotypic drug screening for diabetic cardiomyopathy using human induced pluripotent stem cells. Cell Rep. 2014;9(3):810–21.
Dye BR, Hill DR, Ferguson MA, Tsai YH, Nagy MS, et al. In vitro generation of human pluripotent stem cell derived lung organoids. Elife. 2015;4.
Egawa N, Kitaoka S, Tsukita K, Naitoh M, Takahashi K, et al. Drug screening for ALS using patient-specific induced pluripotent stem cells. Sci Transl Med. 2012;4(145):145ra104.
Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154–6.
Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85(8):348–62.
Giri S, Bader A. A low-cost, high-quality new drug discovery process using patient-derived induced pluripotent stem cells. Drug Discov Today. 2015;20(1):37–49.
González F, Boué S, Izpisúa Belmonte JC. Methods for making induced pluripotent stem cells: reprogramming à la carte. Nat Rev Genet. 2011;12(4):231–42.
Grabundzija I, Wang J, Sebe A, Erdei Z, Kajdi R, et al. Sleeping beauty transposon-based system for cellular reprogramming and targeted gene insertion in induced pluripotent stem cells. Nucleic Acids Res. 2013;41(3):1829–47.
Hockemeyer D, Soldner F, Cook EG, Gao Q, Mitalipova M, Jaenisch R. A drug-inducible system for direct reprogramming of human somatic cells to pluripotency. Cell Stem Cell. 2008;3(3):346-353.
Ilic D, Devito L, Miere C, Codognotto S. Human embryonic and induced pluripotent stem cells in clinical trials. Br Med Bull. 2015;116:19–27.
Jia F, Wilson KD, Sun N, Gupta DM, Huang M, et al. A nonviral minicircle vector for deriving human iPS cells. Nat Methods. 2010;7(3):197–9.
Kim D, Kim CH, Moon JI, Chung YG, Chang MY, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4(6):472–6.
Klimanskaya I, Hipp J, Rezai KA, West M, Atala A, et al. Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells. 2004;6(3):217–45.
Ko HC, Gelb BD. Concise review: drug discovery in the age of the induced pluripotent stem cell. Stem Cells Transl Med. 2014;3(4):500–9.
Kulessa H, Frampton J, Graf T. GATA-1 reprograms avian myelomonocytic cell lines into eosinophils, thromboblasts, and erythroblasts. Genes Dev. 1995;9(10):1250–62.
Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345(6194):1247125.
Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501(7467):373–9.
Lebkowski J. GRNOPC1: the world’s first embryonic stem cell-derived therapy interview with Jane Lebkowski. Regen Med. 2011;6(6 Suppl):11–3.
Lee MO, Moon SH, Jeong HC, Yi JY, Lee TH, et al. Inhibition of pluripotent stem cell-derived teratoma formation by small molecules. Proc Natl Acad Sci U S A. 2013;110(35):E3281–90.
Lu B, Malcuit C, Wang S, Girman S, Francis P, et al. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells. 2009;27(9):2126–35.
Maherali N, Hochedlinger K. Guidelines and techniques for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;3(6):595–605.
Maherali N, Ahfeldt T, Rigamonti A, Utikal J, Cowan C, et al. A high-efficiency system for the generation and study of human induced pluripotent stem cells. Cell Stem Cell. 2008;3(3):340–5.
Mallon BS, Hamilton RS, Kozhich OA, Johnson KR, Fann YC, et al. Comparison of the molecular profiles of human embryonic and induced pluripotent stem cells of isogenic origin. Stem Cell Res. 2014;12(2):376–86.
Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78(12):7634–8.
Matreyek KA, Engelman A. Viral and cellular requirements for the nuclear entry of retroviral preintegration nucleoprotein complexes. Viruses. 2013;5(10):2483–511.
Mitani K, Kubo S. Adenovirus as an integrating vector. Curr Gene Ther. 2002;2(2):135–44. Review
Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113(5):631–42.
Moretti A, Bellin M, Welling A, Jung CB, Lam JT, et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med. 2010;363(15):1397–409.
Nakanishi M, Otsu M. Development of Sendai virus vectors and their potential applications in gene therapy and regenerative medicine. Curr Gene Ther. 2012;12(5):410–6.
Nakano T, Ando S, Takata N, Kawada M, Muguruma K, et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10(6):771–85.
Narsinh KH, Jia F, Robbins RC, Kay MA, Longaker MT, et al. Generation of adult human induced pluripotent stem cells using nonviral minicircle DNA vectors. Nat Protoc. 2011;6(1):78–88.
Niwa H. Molecular mechanism to maintain stem cell renewal of ES cells. Cell Struct Funct. 2001;26(3):137–48.
Nutt SL, Heavey B, Rolink AG, Busslinger M. Pillars article: commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature. 1999;401: 556–562. J Immunol. 2015;195(3):766–72.
Park HJ, Shin J, Kim J, Cho SW. Nonviral delivery for reprogramming to pluripotency and differentiation. Arch Pharm Res. 2014;37(1):107–19.
Ramalingam S, London V, Kandavelou K, Cebotaru L, Guggino W, et al. Generation and genetic engineering of human induced pluripotent stem cells using designed zinc finger nucleases. Stem Cells Dev. 2013;22(4):595–610.
Robertson JA. Human embryonic stem cell research: ethical and legal issues. Nat Rev Genet. 2001;2(1):74–8. Review
Sánchez-Danés A, Richaud-Patin Y, Carballo-Carbajal I, Jiménez-Delgado S, Caig C, et al. Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson’s disease. EMBO Mol Med. 2012;4(5):380–95.
Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385(9967):509–16.
Scuteri A, Miloso M, Foudah D, Orciani M, Cavaletti G, et al. Mesenchymal stem cells neuronal differentiation ability: a real perspective for nervous system repair? Curr Stem Cell Res Ther. 2011;6(2):82–92.
Solnica-Krezel L, Sepich DS. Gastrulation: making and shaping germ layers. Annu Rev Cell Dev Biol. 2012;28:687–717.
Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470(7332):105–9.
Stadtfeld M, Hochedlinger K. Induced pluripotency: history, mechanisms, and applications. Genes Dev. 2010;24(20):2239–63.
Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322(5903):945–9.
Struhl G. A homoeotic mutation transforming leg to antenna in Drosophila. Nature. 1981;292(5824):635–8.
Tachibana M, Amato P, Sparman M, Gutierrez NM, Tippner-Hedges R, et al. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell. 2013;153(6):1228–38.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72.
Takebe T, Zhang RR, Koike H, Kimura M, Yoshizawa E, et al. Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. Nat Protoc. 2014;9(2):396–409.
Tang C, Lee AS, Volkmer JP, Sahoo D, Nag D, et al. An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat Biotechnol. 2011;29(9):829–34.
Tapscott SJ, Davis RL, Thayer MJ, Cheng PF, Weintraub H, et al. MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science. 1988;242(4877):405–11.
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–7.
Wobus AM, Löser P. Present state and future perspectives of using pluripotent stem cells in toxicology research. Arch Toxicol. 2011;85(2):79–117.
Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458(7239):766–70.
Wu XB, Tao R. Hepatocyte differentiation of mesenchymal stem cells. Hepatobiliary Pancreat Dis Int. 2012;11(4):360–71.
Xie H, Ye M, Feng R, Graf T. Stepwise reprogramming of B cells into macrophages. Cell. 2004;117(5):663–76.
Yakubov E, Rechavi G, Rozenblatt S, Givol D. Reprogramming of human fibroblasts to pluripotent stem cells using mRNA of four transcription factors. Biochem Biophys Res Commun. 2010;394(1):189–93.
Yoshioka N, Gros E, Li H-R, Kumar S, Deacon DC, Maron C, et al. Efficient generation of human iPSCs by a synthetic self-replicative RNA. Cell Stem Cell. 2013;13(2):246-254.
Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324(5928):797-801.
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–20.
Zhong X, Gutierrez C, Xue T, Hampton C, Vergara MN, et al. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat Commun. 2014;5:4047.
Zhou W, Freed CR. Adenoviral gene delivery can reprogram human fibroblasts to induced pluripotent stem cells. Stem Cells. 2009;27(11):2667–74.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by a grant by Fondazione Cariplo to P.D.E. (ref. no. 2014-0822) and by BFU2013-49157-P and RETICTerCel grants from MINECO and the European Research Council (ERC) 2012-StG (311736- PD-HUMMODEL) to A.C.
Rights and permissions
About this article
Cite this article
Mora, C., Serzanti, M., Consiglio, A. et al. Clinical potentials of human pluripotent stem cells. Cell Biol Toxicol 33, 351–360 (2017). https://doi.org/10.1007/s10565-017-9384-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-017-9384-y