Abstract
Serine proteases play an important role in inflammation via PARs. However, little is known of expression levels of PARs on monocytes of allergic patients, and influence of serine proteases and PARs on TNF-α secretion from monocytes. Using quantitative real-time PCR (qPCR) and flowcytometry techniques, we observed that the expression level of PAR-2 in monocytes of patients with allergic rhinitis and asthma was increased by 42.9 and 38.2 %. It was found that trypsin, thrombin, and tryptase induced up to 200, 320, and 310 % increase in TNF-α release from monocytes at 16 h, respectively. PAR-1 agonist peptide, SFLLR-NH2, and PAR-2 agonist peptide tc-LIGRLO-NH2 provoked up to 210 and 240 % increase in release of TNF-α. Since SCH 79797, a PAR-1 antagonist, and PD98059, an inhibitor of ERK inhibited thrombin- and SFLLR-NH2-induced TNF-α release, the action of thrombin is most likely through a PAR-1- and ERK-mediated signaling mechanism. Similarly, because FSLLRN-NH2, an inhibitor of PAR-2 diminished tryptase- and tc-LIGRLO-NH2-induced TNF-α release, the action of tryptase appears PAR-2 dependent. Moreover, in vivo study showed that both recombinant cockroach major allergens Per a 1 and Per a 7 provoked upregulation of PAR-2 and PAR-1 expression on CD14+ cells in OVA-sensitized mouse peritoneum. In conclusion, increased expression of PAR-2 in monocytes of AR and asthma implicates that PAR-2 likely play a role in allergy. PAR-2- and PAR-1-mediated TNF-α release from monocytes suggests that these unique protease receptors are involved in the pathogenesis of inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proteinase-activated receptors (PARs) belong to a novel family of G-protein-coupled receptors with seven transmembrane domains activated via proteolytic cleavage by serine proteinases. To date, four PARs have been identified and cloned. PAR-1, PAR-3, and PAR-4 are targets for thrombin and trypsin (Ossovskaya and Bunnett 2004). In contrast, PAR-2 is resistant to thrombin, but can be activated by trypsin, mast cell tryptase (Molino et al. 1997), neutrophil elastase (Uehara et al. 2003), and bacterial-derived proteinase (Sun et al. 2001). Proteinases activate PARs by proteolytic cleavage within the extracellular N terminus of their receptors, thereby exposing a novel-specific residue (about six amino acids) within the tethered ligand domain. Synthetic peptides correspond to the sequence of the “tethered ligand” are capable of activating the receptor independently of N-terminal proteolysis, providing a useful experimental tool for the specific activation of PARs (Ossovskaya and Bunnett 2004).
PARs are expressed by various cells involved in inflammatory and immune responses, such as epithelial cells (Asokananthan et al. 2002), mast cells (D’Andrea et al. 2000), T cells (Mari et al. 1996), neutrophils (Wang and He 2006a), and monocytes (Colognato et al. 2003). In these cells, activation of PARs affects their main functions such as proliferation, degranulation, and release of inflammatory mediators (Ossovskaya and Bunnett 2004). Through activation of PAR-1, thrombin is able to mediate induction of IL-1β and IL-6 production from PBMCs (Nieuwenhuizen et al. 2013) and IL-8 (Wang et al. 2006b) and matrix metalloprotease (MMP)-9 release (Wang et al. 2007) from human primary dermal fibroblasts. PAR-1 agonist peptide significantly stimulates vascular endothelial growth factor secretion from cultured human airway epithelial cells (Shimizu et al. 2011). Similarly, tryptase, trypsin, SLIGKV-NH2, and tc-LIGRLO-NH2 can induce IL-8 and lactoferrin secretion from peripheral blood neutrophils (Wang and He 2006a), and PAR-2 agonists upregulate granulocyte colony-stimulating factor, IL-8, and VCAM-1 expression in human bronchial fibroblasts (Ramachandran et al. 2006), implicating that the activation of PAR-2 is likely involved in the inflammatory process.
TNF-α is a major proinflammatory cytokine that is thought to be important in the pathogenesis of asthma (Busse et al. 2009), food allergy (Heyman and Desjeux 2000), ocular allergy (Leonardi 2002), and atopic dermatitis (Vestergaard et al. 2004). It has been reported that increased number of TNF-α-expressing cells and levels of TNF-α were observed in the bronchoalveolar lavage and in the airways of asthmatics (Waserman et al. 2000). Inhaled TNF-α increases airway responsiveness to methacholine in asthmatic subjects associated with a sputum neutrophilia (Thomas 2001). Since PARs, TNF-α, and monocytes all play roles in inflammation, we believe there must be some linkages between them. Because asthma and allergic rhinitis are two major allergic airway diseases, and they are very different, we investigated clinical relevance of PARs in these two allergic airway disorders, and influence of agonists of PARs on secretion of TNF-α from monocytes in the present study.
Materials and methods
Reagents
Human thrombin, trypsin, leupeptin, soybean trypsin inhibitor (SBTI), ovalbumin (OVA), and bovine serum albumin (BSA, fraction V) were purchased from Sigma (St Louis, MO, USA). Recombinant hirudin was obtained from Calbiochem (San Diego, CA, USA). SCH 79797 was from Tocris Cookson (Ellisville, MO, USA). The sequences of the active and reverse peptides were: PAR-1, SFLLR-NH2, and RLLFS-NH2; PAR-2, trans-cinnamoyl-Leu-Ile-Gly-Arg-Leu-Orn-amide (tc-LIGRLO-NH2), and trans-cinnamoyl-Orn-Leu-Arg-Gly-Ile-Leu-amide (tc-OLRGIL-NH2); PAR-2 antagonist peptide Phe-Ser-Leu-Leu-Arg-Asn-NH2 (FSLLRN-NH2) were synthesized in CL Bio-Scientific Inc (Xi An, China). RPMI 1640 and fetal calf serum (FCS) were obtained from HyClone (Logan, UT, USA). Monocyte Isolation Kit I was from Miltenyi Biotec (Bergisch Gladbach, Germany). UniCAP tryptase kit was from Pharmacia Diagnostics AB (Uppsala, Sweden). FITC-conjugated goat anti-mouse PAR-2 monoclonal antibody and rabbit anti-mouse PAR-1 polyclonal antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). TNF-α OptEIA ELISA kit, APC/CY7-anti-human CD14 antibody, PE-conjugated goat anti-rabbit polyclonal antibody and APC/CY7 rat anti-mouse CD14 antibody were purchased from BD Pharmingen (San Jose, CA, USA). PE-anti-human PAR-1 antibody and APC-anti-human PAR-2 antibody were purchased from R&D Systems, Inc., (Minneapolis, MN, USA). 2-(2-Diamino)-3-methoxyphenyl-4H-1-benzopyran-4-one (PD98059) was purchased from Cell Signaling Technology (Beverly, MA, USA). Recombinant human lung β-tryptase was obtained from Promega (Madison, WI, USA). N3-cyclopropyl-7- [[4-(1-methylethyl)phenyl]methyl]-7H-pyrrolo[3, 2-f]quinazoline-1,3-diamine (SCH 79797) was purchased from Tocris Cookson (Ellisville, MO, USA). TRIzol Reagent was obtained from Invitrogen (Carlsbad, CA, USA). ExScript™ RT reagent kit and SYBR® Premix Ex Taq™ (perfect real time) was obtained from TaKaRa Biotechnology Co. Ltd. (DaLian, China). Oligonucleotide primers for real-time PCR were synthesized by Invitrogen Biotechnology Co. (Shanghai, China). Recombinant Per a 1 (He et al. 2011) and Per a 7 (Zhang et al. 2008) and their blocking antibodies were prepared by our own laboratory. Cellular activation of signaling ELISA (CASE) kits for extracellular signal-regulated kinase (ERK) was purchased from SuperArray Bioscience Corporation (Frederick, MD, USA). Most of the general chemicals such as salts and buffer components were of analytical grade.
Patients and samples
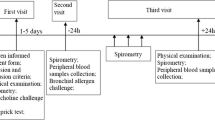
A total of 25 allergic rhinitis (AR), 22 asthma, and 13 healthy control subjects (HC) were recruited in the study from July 2014 to June 2015. The diagnosing criteria of asthma were conformed to the Global Initiative for Asthma (Von Mutius 2000); diagnosis for allergic rhinitis was based on Allergic Rhinitis and its Impact on Asthma (ARIA) (Demoly et al. 2003). All patients were asked to stop anti-allergy medication for at least 2 weeks prior to attending the study (those could not stop anti-allergy drugs were excluded). The recruited patients did not have any airway infection more than 1 month. The informed consent from each volunteer according to the declaration of Helsinki and agreement with the ethical committee of the First Affiliated Hospital of Liaoning Medical University (protocol number JY201405). The general characteristics of the patients and control subjects were summarized in Table 1. Peripheral venous blood sample was collected from each patient and HC, and were immediately processed to collect cells and plasma for analysis.
Animals
BALB/c mice (18–22 g) were obtained from Beijing Laboratory Animal Centre, China, Grade II, certificate No. 11400700056944. The animal experiment procedures were approved by the Animal Care Committee at Liaoning Medical University (protocol number DJ201407).
Isolation and culture of monocytes
Human monocytes were isolated from peripheral blood mononucleated cells (PBMC) by a magnet-activated cell sorting (MACS) system with Monocyte Isolation Kit I according to the manufacturer’s protocol. In brief, PBMC was isolated from fresh blood donated by the patients and HC, 50 ml from each individual per visit. After being separated from red blood cells by Ficoll-Paque density gradient, PBMC was collected, washed twice in PBS, and resuspended in MACS buffer containing 0.1 % EDTA and 1 % BSA in PBS. After incubation with microbead-linked anti-CD14 monocolonal antibody for 15 min at 8 °C, CD14+ monocytes were separated from other cells by passing through a magnetic cell separation system. The purity of the monocytes was consistently more than 95 %, as determined by flow cytometry and cell count. The cell viability was more than 98 %, as judged by trypan blue dye exclusion.
qPCR analysis of PAR mRNA expression in monocytes of allergic patients
The expression of PAR-1 and PAR-2 mRNAs in monocytes was determined by qPCR following the manufacture’s protocol. Briefly, after synthesizing cDNA from total RNA by using Superscript first strand synthesis system for RT-PCR and oligo-dT primers, real-time PCR was performed by using SYBR® Premix Ex Taq™ kit on the ABI Prism 7700 Sequence Detection System (Perkin Elmer Applied Systems, Foster City, CA, USA). Sequence-specific standard curves were generated using 10-fold serial dilutions of plasmid DNA, and the values for the initial concentrations of unknown samples were calculated by using the software (version 1.7) provided with the ABI 7700 system. PAR mRNA expression in each sample was finally determined after correction with β-actin expression. Each measurement of a sample was conducted in duplicate. The primers for PAR-1 and PAR-2 are summarized in Tables 2 and 3.
Flow cytometry examination of expression of PARs in monocytes
To detect PAR-1 and PAR-2 expression on human monocytes, PE-conjugated anti-human PAR-1, APC-conjugated anti-human PAR-2, and APC/CY7-conjugated anti-human CD14 antibodies were added to 100 μl whole blood at 4 °C for 30 min, respectively according to the manufacturer’s instructions. Red blood cells were removed with BD cell ligation buffer. Following washing with BD washing buffer, the cell pellets were resuspended in FACS-Flow and analyzed with FACS verse flow cytometer (BD Biosciences, San Jose, CA, USA). A total of 10,000 events were analyzed for each sample. For consistency with our previous work, expression of PARs was quantitated with mean fluorescence intensity. For detection of PAR-1 and PAR-2 expression on mouse monocytes, FITC-conjugated goat anti-mouse PAR-2 monoclonal antibody, rabbit anti-mouse PAR-1 polyclonal antibody, and APC/CY7-conjugated rat anti-mouse CD14 antibody were added to monocytes, followed by adding PE-conjugated goat anti-rabbit polyclonal antibody. Data were analyzed with CellQuest software (BD Immunocytometry systems), and represented as percentage of positive PAR expressing cells.
Monocyte challenge
Monocytes were cultured in 24-well culture plates at a density of 1.0 × 106 cells/well in RPMI 1640 medium at 37 °C for 1 h with 5 % CO2. The culture supernatants were then removed and cells were washed twice with fresh serum free RPMI 1640 medium at 300×g for 10 min. For challenge experiments, cells were exposed to various concentrations of thrombin (0.01–3.0 μg/ml, 1 U/ml = 6.7 nM, U = NIH unit), trypsin (0.01–0.3 μg/ml, 1 U/ml = 4.2 nM; specific activity, ∼10,000 BAEE U/mg protein), tryptase (0.25–2.0 μg/ml, 1 U/ml = 7.4 nM; specific activity, ~1,000 Nα CBZ-L-Lysine Thiobenzyl Ester U/mg protein) with or without their corresponding inhibitors or antagonist peptides, and to agonist peptides of PAR-1 and PAR-2 (all at 0.1–100 μM) and their reverse peptides, respectively for 16 h. Cells were then harvested for flowcytometry and qPCR analysis, and culture supernatants were collected and stored at −40 °C until use. For calculation of percentage inhibition of protease or agonists of PARs-induced TNF-α release by protease inhibitor or antagonists of PARs, the formula is (actual concentration of TNF-α induced by stimulus − concentration of TNF-α in medium alone) − (actual concentration of TNF-α induced by stimulus with inhibitor − concentration of TNF-α in medium alone)/(actual concentration of TNF-α induced by stimulus − concentration of TNF-α in medium alone) × 100 %.
For cell signaling experiments, cultured cells at a density of 1.0 × 106 cells/ml were treated with PD98059 (50 μM) for 30 min or 2 h before being challenged with trypsin (0.3 μg/ml) or thrombin (1.0 μg/ml) for 6 h.
Mouse sensitization and challenge
Mice were sensitized on days 0, 7, 14, and 21 with an subcutaneous multi-point injection of 50 μg of OVA and 1.5 mg of Al (OH)3 suspended in normal saline to a total volume of 0.5 ml. Nonsensitized control animals received only the equal volume (0.5 ml) of normal saline on the same days. On the day 25, sensitized mice were challenged with intraperitoneal injection of 0.5 ml of various concentrations of Per a 1 or Per a 7 in Al (OH)3 solution. At 3 h following challenge, animals were killed and their peritoneal lavages were taken for analysis. For certain experiments, specific anti-Per a 7 monoclonal antibody and anti-Per a 1 polyclonal antibody were preincubated with their corresponding allergens for 30 min before injection.
Analysis of phosphorylation of ERK
The phosphorylation of ERK was analyzed by using CASE kits. Briefly, a total of two sets of cells were prepared with one set of cells being incubated with the phospho-protein-specific antibody to measure phosphorylated protein, and the other set of cells being incubated with the pan-protein-specific antibody to measure total protein. After being fixed with cell fixing buffer, the quenching buffer and antigen retrieval buffer was added, respectively. Cells were blocked with blocking buffer for 1 h before addition of primary antibody to each appropriate well for 1 h, followed by adding secondary antibody for 1 h. Color was developed with developing solution before adding the stop solution. The plate was read at 450 nm on an ELISA Plate Reader (Max 340PC, Molecular Devices, CA, USA).
In order to determine relative cell number, the assay solution was removed from each well. The cell staining buffer was then placed into each well for 30 min, followed by adding sodium dodecyl sulfate for 1 h. The plate was read at 595 nm. To normalize the antibody reading to the relative cell number, the OD450 reading for each well was divided by its OD595 reading. To determine the relative extent of target protein phosphorylation, the OD450:OD595 ratio for phosphor-protein-specific antibody is normalized by the ratio for pan-protein-specific antibody under the same experimental condition.
Statistical analysis
Statistical analyses were performed by using SPSS software (Version 13.0). Data were expressed as mean ± SEM. Where analysis of variance indicated significant differences between groups with ANOVA, Student’s t test was applied. Data for allergic patients’ serum are presented as scatter plot. Where Kruskal–Wallis analysis indicated significant differences between groups, for the pre-planned comparisons of interest, the paired Mann–Whitney U test was employed. For all experiments, P < 0.05 was taken as significant.
Results
Upregulated expression of PAR-2 in monocytes of the patients with AR and asthma
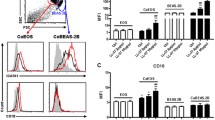
The findings that expression levels of PAR-2 mRNA and immunoreactivity of PAR-2 in the nasal mucosa of AR are significantly up-regulated as compared with normal nasal mucosa (Lee et al. 2007), and that PAR-2 staining in asthmatic epithelium is increased in comparison with normal epithelium (Knight et al. 2001) implicate that PAR-2 may be involved in the pathogenesis of allergic airway disorders. Since little is known of expression of PARs in monocytes of patients with AR and asthma, we investigated expression of PAR-1 and PAR-2 in monocytes in the present study. The results showed that expression of mRNAs for PAR-1 (Fig. 1a) and PAR-2 (Fig. 1b) was elevated by 34.7 and 37.9 %, and by 32.6 and 31.6 % in monocytes from patients with AR and asthma, respectively. At protein level, expression of PAR-2 (Fig. 1d, e) was increased by 42.9 and 38.2 %, whereas expression of PAR-1 (Fig. 1c, e) was decreased by 28.8 and 24.8 % in monocytes from AR and asthma, respectively. As showing in Fig. 1e, the percentage of PAR-1 expressing monocytes in HC subjects was 77.7 %, which was reduced to 56.3 and 45.3 % in AR and AS patients. On the other hand, the percentage of PAR-2 expressing monocytes in HC subjects was 12.4 %, which was enhanced to 29.1 and 20.4 % in AR and AS patients, respectively.
Scatter plots of expression of protease-activated receptor (PAR)s in peripheral blood monocytes. Expression of mRNA (a) and protein (c) of PAR-1, and mRNA (b) and protein (d) of PAR-2 in peripheral blood monocytes of patients with allergic rhinitis (AR), asthma (AS), and healthy control (HC) subjects was demonstrated. Each symbol represents the value from one individual subject. The median is indicated with a horizontal line. Expression of mRNA and protein was determined by using quantitative real-time PCR and flowcytometry analysis, respectively. e shows a representative graph of changes in mean fluorescent intensity, and f is a representative graph of changes in the number of positively stained cells detected by flowcytometry analysis. *P < 0.05 is taken as significant
PAR mediated release of TNF-α from monocytes
In order to understand potential mechanism by which PAR-2 is involved in AR and asthma, and because TNF-α is a key proinflammatory cytokine, we investigated PAR mediated TNF-α release from monocytes. The results showed that trypsin, thrombin, and tryptase provoked concentration-dependent increase in TNF-α release from monocytes at 16 h, respectively. Trypsin at the concentration of 0.3 μg/ml (Fig. 2a), thrombin at 3.0 μg/ml (Fig. 2b), and tryptase at 2.0 μg/ml (Fig. 2c) induced approximately 200, 320, and 310 % increase in TNF-α release from monocytes, respectively. As little as 0.03 μg/ml of trypsin, 0.1 μg/ml of thrombin, and 0.25 μg/ml of tryptase were able to provoke marked TNF-α release. PAR-1 agonist peptide SFLLR-NH2 at the concentration of 100 μM (Fig. 2d) and PAR-2 agonist peptide tc-LIGRLO-NH2 at the concentration of 100 μM (Fig. 2e) induced approximately up to 210 and 240 % increase in release of TNF-α at 16 h following incubation. In the same experiments, reverse peptide RLLFS-NH2 and tc-OLRGIL-NH2 had little effect on release of TNF-α from monocytes. Since SCH 79797, a PAR-1 antagonist at the concentration of 1 μM, inhibited 78.6 % thrombin-induced and 87.7 % SFLLR-NH2-induced TNF-α release from monocytes, this indicates that thrombin-induced release of TNF-α is most likely through PAR-1. Similarly, FSLLRN-NH2 (400 μM), an inhibitor of PAR-2, diminished 82.9 % tryptase-induced and 87 % tc-LIGRLO-NH2-induced TNF-α release from monocytes, indicating that tryptase-induced release of TNF-α is most likely through PAR-2.
Induction of TNF-α secretion from human monocytes by agonists of protease-activated receptor (PAR)s. Agonists of PARs were added with or without their inhibitors or antagonist peptides. Cells were incubated with a various concentrations of trypsin (in microgram per milliliter) or trypsin with soybean trypsin inhibitor (SBTI, in microgram per milliliter), leupeptin (Leup, in microgram per milliliter), PD 98059 (PD, in micromolar), FSLLRY-NH2 (FSLLRY, in micromolar), or SCH 79797 (SCH, in micromolar); b various concentrations of thrombin (in microgram per milliliter) or thrombin with hirudin (in microgram per milliliter), PD or SCH; c various concentrations of tryptase (in microgram per milliliter) or tryptase with Leup or FSLLRY; d various concentrations of an agonist peptide of PAR-1 SFLLR-NH2 (SFLLR, in micromolar), a reverse peptide of SFLLR RLLFS-NH2 (RLLFS) or SFLLR with SCH; and e various concentrations of an agonist peptide of PAR-2 tc-LIGRLO-NH2 (tc-LIGRLO, in micromolar) , a reverse peptide of tc-LIGRLO tc-OLRGIL-NH2 (tc-OLRGIL), tc-LIGRLO with FSLLRY for 16 h at 37 °C. Values shown are mean ± SEM for four to six independent experiments from different donors. *P < 0.05 compared with the response to medium alone control. † P < 0.05 compared with the response to the corresponding stimulus alone
It was noticed that hirudin, a specific thrombin inhibitor inhibited up to 80.6 % thrombin-induced secretion of TNF-α from monocytes; leupeptin reduced up to 88.8 % tryptase-induced secretion of TNF-α from monocytes; SBTI and leupeptin diminished approximately up to 77.6 and 87.1 % trypsin-induced secretion of TNF-α from monocytes; whereas hirudin, leupeptin, and SBTI alone at the concentrations examined had little effect on TNF-α release from monocytes (Fig. 2). These indicated that the actions of trypsin, thrombin, and tryptase are dependent on their enzymatic activity.
PAR-mediated upregulation of expression TNF-α mRNA in monocytes
Following the above findings, we anticipated that PARs are likely to cause alteration of the expression of TNF-α mRNA in monocytes. To confirm the anticipation, we examined the effect of trypsin and thrombin on expression of TNF-α mRNA in monocytes. It was found that trypsin (Fig. 3a) and thrombin (Fig. 3b) induced up to 200 and 290 % increase in expression of TNF-α mRNA over baseline control, respectively. Since FSLLRN-NH2, SBTI, and leupeptin were able to inhibit trypsin-induced up-regulation of expression of TNF-α mRNA, the action of trypsin was likely dependent on PAR-2 and required enzymatic activity of trypsin. Similarly, thrombin-induced up-regulation of expression of TNF-α mRNA was diminished by SCH 79797 and hirudin, indicating that the action of thrombin was PAR-1 and enzymatic activity dependent.
Quantitative real-time PCR analysis of expression of TNF-α mRNA in monocytes. Expression of TNF-α mRNA was induced by agonists of protease-activated receptor (PAR)s with or without their inhibitors or antagonist peptides. In a, cells were treated with various concentrations of trypsin (in microgram per milliliter) or trypsin with soybean trypsin inhibitor (SBTI, in microgram per milliliter), FSLLRY-NH2 (FSLLRY, in micromolar), or SCH 79797 (SCH, in micromolar) at 37 °C for 30 min and 2 h. In b, cells were treated with various concentrations of thrombin (in microgram per milliliter) or thrombin with hirudin (in microgram per milliliter) or SCH at 37 °C for 30 min and 2 h, respectively. The data were expressed as mean ± SE for four separate experiments performed in duplicate. *P < 0.05 compared with the response to corresponding medium alone control. † P < 0.05 compared with the response to the corresponding stimulus alone
Inhibition of trypsin- and thrombin-induced phosphorylation of ERK in monocytes
In order to further understand the mechanism of trypsin and thrombin-induced release of TNF-α, we examined the phosphorylation of ERK in monocytes. The result showed that an inhibitor of ERK PD98059 completely abolished trypsin- and thrombin-induced phosphorylation of ERK in monocytes following 30 min and 2 h preincubation periods (Fig. 4).
Effect of PD98059 (PD) on trypsin- and thrombin-induced phosphorylation of ERK in monocytes. Cells were preincubated with PD an inhibitor of ERK (50 μM) for 30 min and 2 h, respectively, before trypsin (in microgram per milliliter) or thrombin (in microgram per milliliter) being added for 6 h at 37 °C. The values shown are mean ± SEM for four separate experiments performed in duplicate. *P < 0.05 compared with the response to corresponding medium alone control. † P < 0.05 compared with the response to the corresponding stimulus alone
Cockroach allergen induced upregulation of expression of PARs in CD14+ cells from the peritoneal lavage of sensitized mice
In order to further understand the increased expression of PARs in monocytes of allergic airway disorders, we examined the expression of PARs in CD14+ cells from the peritoneal lavage of sensitized mice. The results showed that both recombinant Per a 1 and Per a 7 provoked upregulation of PAR-1 expression on CD14+ cells, but only Per a 7 induced an enhanced expression of PAR-2. The specific anti-human Per a 1 antibody inhibited approximately 72.1 % Per a 1-induced PAR-1 expression, and specific anti-human Per a 7 antibody abolished 82.2 and 51.4 % Per a 7-provoked PAR-1 and PAR-2 expression, respectively, when these antibodies were preincubated with their corresponding allergens for 30 min before injection (Fig. 5).
Induction of expression of protease-activated receptor (PAR)s in CD14+ cells of the peritoneal lavage fluid. Expression of PAR-1 (a) and PAR-2 (b) in CD14+ cells of the peritoneal lavage fluid of ovalbumin sensitized mouse was enhanced by cockroach allergens. Various concentrations of recombinant Per a 1 and recombinant Per a 7 were injected into the peritoneum of mice for 3 h before peritoneal lavage fluid being collected. Data were expressed as Mean ± SE for six to seven mice. *P < 0.05 compared with the response to medium alone. † P < 0.05 compared with the response to the corresponding stimulus alone. Ab = antibody
Discussion
In the current study, it is found for the first time that the expression of PAR-2 was selectively increased in monocytes from AR and asthma, and serine proteases was able to induce TNF-α release from monocytes via PAR-2 and PAR-1 in a enzymatic dependent manner. Although asthma and allergic rhinitis are very different diseases, they seem to express similar levels of PAR-1 and PAR-2 in their monocytes, which provides an evidence to support they share some commonalities. Since TNF-α is an important proinflammatory factor and upregulated expression of PAR-2 has been observed in the nasal mucosa of AR and asthmatic airway epithelium we believe our current findings may contribute to the understanding of the pathogenesis of allergic airway disorders.
In recent years, it has been discovered that PAR-2 has potential to play a role in allergy. Thus, suppression of connexin 26 in house dust mite-sensitized AR patients is related to a PAR-2 mediated pathway and may be involved in the initiation and maintenance of AR (Zheng et al. 2012). Airway hyperreactivity to inhaled methacholine is diminished 38 % in mice lacking PAR-2 and increased by 52 % in mice overexpressing PAR-2 (Schmidlin et al. 2002). Our finding that the expression of PAR-2 was increased in monocytes from the patients with AR and asthma implicates further that PAR-2 plays a role in allergy. To our surprise, the expression of PAR-1 protein was decreased in monocytes from the patients with AR and asthma, which may be not supportive to the previous report that the expression of PAR-1 was upregulated by thrombin that induces the expression of TGF-β1 to promote airway remodeling in OVA-allergic rats (Zhu et al. 2013). The explanation for the discrimination could be the expression of PAR-1 may be downregulated on monocytes, but upregulated on some other cells in allergy, which obviously requires further work to clarify. It appeared that expression of PAR-1 mRNA was upregulated in AR and AS patients, which is contrary to the decrease in PAR-1 protein. The unmatched expressions of mRNA and protein may be caused by a rapid mRNA decay as mRNA stability (Frasca et al. 2007) and protein synthesis (Kuwahara et al. 2006) involve several physiological regulators. However, altered function of posttranscriptional regulatory pathways (Esnault and Malter 2003) may also be involved. We have previously demonstrated that monocytes from healthy subjects express PAR-1, PAR-3, PAR-4, but not PAR-2 (Li et al. 2006). Our current experiments also show low levels of PAR-2 mRNA and protein expression on monocytes from HC subjects, but markedly increased in AR and AS. These observations emphasize the specificity of PAR-2 in allergy.
Our findings that trypsin, thrombin, tryptase, SFLLR-NH2, and tc-LIGRLO-NH2 enhanced TNF-α release from highly purified monocytes suggest a novel potential of protease-provoked and PAR-mediated inflammation. It has long been recognized that tryptase displays proinflammatory actions including induction of inflammatory cell accumulation and microvascular leakage in laboratory animals, and stimulation of activation of mast cells, endothelial cells, and epithelial cells (He et al. 2012). The finding that tryptase provoked TNF-α release from monocytes confirms further that tryptase possesses proinflammatory actions. Similarly, it was reported that thrombin stimulated the release of IL-1β from monocytes (Nieuwenhuizen et al. 2013) and elicited monocyte chemotaxis (Popovic et al. 2008), which implicated that thrombin may exert a proinflammatory effect by regulating monocyte functions. Our previous work which demonstrated that serine proteinases thrombin, trypsin, and tryptase are potent stimuli of IL-6 secretion from human monocytes (Li et al. 2006), and a report that tryptase activates peripheral blood mononuclear cells causing the synthesis and release of TNF-α, IL-6, and IL-1 beta (Malamud et al. 2003) may reinforce the current observation that thrombin, trypsin, and tryptase are potent stimuli of TNF-α secretion from human monocytes.
As little as 100 ng/ml of thrombin and 250 ng/ml tryptase were able to induce significant TNF-α release from monocytes suggests that these proteinases are potent secretagogues of TNF-α. These concentrations of thrombin should be easily achieved in blood, particularly when the processes of platelet aggregation and coagulation are initiated (Rand et al. 1996). However, little reliable information on serum level of tryptase available, we presume that 250 ng/ml tryptase should be reached under allergic conditions. It appears that the actions of trypsin and thrombin in induction of TNF-α secretion dependent on phosphorylation of ERK. Since the signaling pathway MAPK/ERK has been found to be involved in thrombin - and trypsin-induced TNF-α release from T cells (Yang et al. 2013), and that PD98059 blocked thrombin-induced phosphorylation of ERK and IL-8 production in human dermal fibroblasts (Wang et al. 2006b), and ERK-associated cell signaling pathway is a common pathway for mediator generation, growth, and migration of inflammatory cells, our current observation may suggest a novel mechanism of protease involved in inflammation.
Using OVA-sensitized mouse allergy model, it was observed in the present study that American cockroach major allergen Per a 1 enhanced PAR-1 expression on CD14+ cells, whereas another major allergen Per a 7 increased both PAR-1 and PAR-2 expression on CD14+ cells, suggesting that these major allergens can induce upregulated expression of PARs on CD14+ cells under allergic conditions. The previous reports that German cockroach fecal remnants augmented TNF-α-induced MMP-9 expression by a mechanism involving PAR-2 and ERK (Page et al. 2006) and that German cockroach extract activated PAR-2 and thereby produces TNF-α from alveolar macrophages (Kim et al. 2012) may support our current observation. Through the upregulation and activation of PAR-2, allergen-derived proteases are sufficient to induce cytokine and inflammatory mediator production in the airways, leads to the initiation of allergic airway responses (Day et al. 2012).
In conclusion, we have demonstrated that the expression of PAR-2 is selectively increased in monocytes from patients with AR and asthma, and through which tryptase and trypsin induce TNF-α release from monocytes possibly by an ERK mediated mechanism (summarized in Fig. 6). Since expression of PAR-2 on macrophages can be upregulated by major allergen, and GB88 an antagonist of PAR-2 (Lohman et al. 2012) has recently been proved to possess therapeutic potential as anti-inflammatory drugs, our current findings may provide further evidence for understanding the role of PARs in allergy and for the development of novel anti-allergic drugs.
Abbreviations
- PARs:
-
Proteinase-activated receptors
- MMP:
-
Matrix metalloprotease
- SBTI:
-
Soybean trypsin inhibitor
- OVA:
-
Ovalbumin
- BSA:
-
Bovine serum albumin
- FCS:
-
Fetal calf serum
- AR:
-
Allergic rhinitis
- HC:
-
Healthy control subjects
- ARIA:
-
Allergic Rhinitis and its Impact on Asthma
- PBMC:
-
Peripheral blood mononucleated cells
- MACS:
-
Magnet-activated cell sorting
- CASE:
-
Cellular activation of signaling ELISA
- ERK:
-
Extracellular signal-regulated kinase
- TGF:
-
Transforming growth factor
- TNF:
-
Tumor necrosis factor
- VCAM:
-
Vascular cell adhesion molecule
References
Asokananthan N, Graham PT, et al. Activation of protease-activated receptor (PAR)-1, PAR-2, and PAR-4 stimulates IL-6, IL-8, and prostaglandin E2 release from human respiratory epithelial cells. J Immunol. 2002;168:3577–85.
Busse PJ, Zhang TF, et al. Decrease in airway mucous gene expression caused by treatment with anti-tumor necrosis factor alpha in a murine model of allergic asthma. Ann Allergy Asthma Immunol : Off Publ Am College Allergy Asthma Immunol. 2009;103:295–303.
Colognato R, Slupsky JR, et al. Differential expression and regulation of protease-activated receptors in human peripheral monocytes and monocyte-derived antigen-presenting cells. Blood. 2003;102:2645–52.
D’Andrea MR, Rogahn CJ, et al. Localization of protease-activated receptors-1 and -2 in human mast cells: indications for an amplified mast cell degranulation cascade. Biotech Histochem : Off Publ Biol Stain Commission. 2000;75:85–90.
Day SB, Ledford JR, et al. German cockroach proteases and protease-activated receptor-2 regulate chemokine production and dendritic cell recruitment. J Innate Immun. 2012;4:100–10.
Demoly P, Allaert FA, et al. Validation of the classification of ARIA (allergic rhinitis and its impact on asthma). Allergy. 2003;58:672–5.
Esnault S, Malter JS. Hyaluronic acid or TNF-alpha plus fibronectin triggers granulocyte macrophage-colony-stimulating factor mRNA stabilization in eosinophils yet engages differential intracellular pathways and mRNA binding proteins. J Immunol. 2003;171:6780–7.
Frasca D, Landin AM, et al. Tristetraprolin, a negative regulator of mRNA stability, is increased in old B cells and is involved in the degradation of E47 mRNA. J Immunol. 2007;179:918–27.
He S, Zhang H, et al. Self-amplification mechanisms of mast cell activation: a new look in allergy. Curr Mol Med. 2012;12:1329–39.
He S, Zhang Z, et al. Analysis of properties and proinflammatory functions of cockroach allergens per a 1.01 s. Scand J Immunol. 2011;74:288–95.
Heyman M, Desjeux JF. Cytokine-induced alteration of the epithelial barrier to food antigens in disease. Ann N Y Acad Sci. 2000;915:304–11.
Kim JY, Sohn JH, et al. Alveolar macrophages play a key role in cockroach-induced allergic inflammation via TNF-alpha pathway. PLoS One. 2012;7:e47971.
Knight DA, Lim S, et al. Protease-activated receptors in human airways: upregulation of PAR-2 in respiratory epithelium from patients with asthma. J Allergy Clin Immunol. 2001;108:797–803.
Kuwahara I, Lillehoj EP, et al. Neutrophil elastase induces IL-8 gene transcription and protein release through p38/NF-{kappa}B activation via EGFR transactivation in a lung epithelial cell line. Am J Physiol Lung Cell Mol Physiol. 2006;291:L407–16.
Lee HM, Kim HY, et al. Up-regulation of protease-activated receptor 2 in allergic rhinitis. Ann Otol Rhinol Laryngol. 2007;116:554–8.
Leonardi A. The central role of conjunctival mast cells in the pathogenesis of ocular allergy. Curr Allergy Asthma Rep. 2002;2:325–31.
Li T, Wang H, et al. Induction of interleukin-6 release from monocytes by serine proteinases and its potential mechanisms. Scand J Immunol. 2006;64:10–6.
Lohman RJ, Cotterell AJ, et al. An antagonist of human protease activated receptor-2 attenuates PAR2 signaling, macrophage activation, mast cell degranulation, and collagen-induced arthritis in rats. FASEB J : Off Publ Fed Am Soc Exp Biol. 2012;26:2877–87.
Malamud V, Vaaknin A, et al. Tryptase activates peripheral blood mononuclear cells causing the synthesis and release of TNF-alpha, IL-6 and IL-1 beta: possible relevance to multiple sclerosis. J Neuroimmunol. 2003;138:115–22.
Mari B, Guerin S, et al. Thrombin and trypsin-induced Ca(2+) mobilization in human T cell lines through interaction with different protease-activated receptors. FASEB J : Off Publ Fed Am Soc Exp Biol. 1996;10:309–16.
Molino M, Barnathan ES, et al. Interactions of mast cell tryptase with thrombin receptors and PAR-2. J Biol Chem. 1997;272:4043–9.
Nieuwenhuizen L, Falkenburg WJ, et al. Stimulation of naive monocytes and PBMCs with coagulation proteases results in thrombin-mediated and PAR-1-dependent cytokine release and cell proliferation in PBMCs only. Scand J Immunol. 2013;77:339–49.
Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev. 2004;84:579–621.
Page K, Hughes VS, et al. German cockroach proteases regulate matrix metalloproteinase-9 in human bronchial epithelial cells. Allergy. 2006;61:988–95.
Popovic M, Laumonnier Y, et al. Thrombin-induced expression of endothelial CX3CL1 potentiates monocyte CCL2 production and transendothelial migration. J Leukoc Biol. 2008;84:215–23.
Ramachandran R, Morice AH, et al. Proteinase-activated receptor2 agonists upregulate granulocyte colony-stimulating factor, IL-8, and VCAM-1 expression in human bronchial fibroblasts. Am J Respir Cell Mol Biol. 2006;35:133–41.
Rand MD, Lock JB, et al. Blood clotting in minimally altered whole blood. Blood. 1996;88:3432–45.
Schmidlin F, Amadesi S, et al. Protease-activated receptor 2 mediates eosinophil infiltration and hyperreactivity in allergic inflammation of the airway. J Immunol. 2002;169:5315–21.
Shimizu S, Gabazza EC, et al. Role of thrombin in chronic rhinosinusitis-associated tissue remodeling. Am J Rhinol Allergy. 2011;25:7–11.
Sun G, Stacey MA, et al. Interaction of mite allergens der p3 and der p9 with protease-activated receptor-2 expressed by lung epithelial cells. J Immunol. 2001;167:1014–21.
Thomas PS. Tumour necrosis factor-alpha: the role of this multifunctional cytokine in asthma. Immunol Cell Biol. 2001;79:132–40.
Uehara A, Muramoto K, et al. Neutrophil serine proteinases activate human nonepithelial cells to produce inflammatory cytokines through protease-activated receptor 2. J Immunol. 2003;170:5690–6.
Vestergaard C, Johansen C, et al. TARC augments TNF-alpha-induced CTACK production in keratinocytes. Exp Dermatol. 2004;13:551–7.
Von Mutius E. Presentation of new GINA guidelines for paediatrics. The Global Initiative on Asthma. Clin Exp Allergy : J Br Soc Allergy Clin Immunol. 2000;30(Suppl 1):6–10.
Wang H, He S. Induction of lactoferrin and IL-8 release from human neutrophils by tryptic enzymes via proteinase activated receptor-2. Cell Biol Int. 2006a;30:688–97.
Wang L, Luo J, et al. Induction of interleukin-8 secretion and activation of ERK1/2, p38 MAPK signaling pathways by thrombin in dermal fibroblasts. Int J Biochem Cell Biol. 2006b;38:1571–83.
Wang L, Luo J, et al. Induction of MMP-9 release from human dermal fibroblasts by thrombin: involvement of JAK/STAT3 signaling pathway in MMP-9 release. BMC Cell Biol. 2007;8:14.
Waserman S, Dolovich J, et al. TNF-alpha dysregulation in asthma: relationship to ongoing corticosteroidtherapy. Can Respir J : J Can Thoracic Soc. 2000;7:229–37.
Yang H, Li T, et al. Induction of tumor necrosis factor (TNF) release from subtypes of T cells by agonists of proteinase activated receptors. Mediat Inflamm. 2013;2013:165453.
Zhang Z, Zhang H, et al. Induction of T-helper type 2 cytokine release and up-regulated expression of protease-activated receptors on mast cells by recombinant American cockroach allergen Per a 7. Clinical and Experimental Allergy : Journal of the British Society for Allergy and Clinical Immunology. 2008;38:1160–7.
Zheng J, Liu W, et al. Suppression of connexin 26 is related to protease-activated receptor 2-mediated pathway in patients with allergic rhinitis. Am J Rhinol Allergy. 2012;26:e5–9.
Zhu W, Bi M, et al. Thrombin promotes airway remodeling via protease-activated receptor-1 and transforming growth factor-beta1 in ovalbumin-allergic rats. Inhal Toxicol. 2013;25:577–86.
Acknowledgments
This project was sponsored by the grants from the “12th Five-Year ” National Science and Technology Support Plan (2014BAI07B02), the National Natural Science Foundation of China (nos. 81172836, 81471592, 81472016 ); Major Science and Technology Platform for Institution of Higher Education in Liaoning Province (2014168); “Twelfth five-year” Public Welfare Industry Special Scientific Research Project (2015SQ00136); the National Natural Science Foundation of Liaoning Province (2014022027, 2014022019); Program for Liaoning Innovation Research Team in University (LNIRT, LT2013017); Climbing Scholar Project for Institution of Higher Education in Liaoning province (2013222); Allergic Disease Translational Medicine Research Centre of Liaoning Province (201341); Liaoning Provincial Engineering Research Centre for Diagnosing & Treating Inflammatory Disease (20141093); Clinical Capability Construction Project for Liaoning Provincial Hospitals ( LNCCC-A06-2014); and Science and Technology Planning Project of.
Suzhou (SYS201272).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no competing interest regarding the publication of this article.
Additional information
Shuqing Ge and Tao Li contributed equally to the study
Rights and permissions
About this article
Cite this article
Ge, S., Li, T., Yao, Q. et al. Expression of proteinase-activated receptor (PAR)-2 in monocytes from allergic patients and potential molecular mechanism. Cell Biol Toxicol 32, 529–542 (2016). https://doi.org/10.1007/s10565-016-9353-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-016-9353-x