Abstract
Methanol ingestion is neurotoxic in humans due to its metabolites, formaldehyde and formic acid. Here, we compared the cytotoxicity of methanol and its metabolites on different types of cells. While methanol and formic acid did not affect the viability of the cells, formaldehyde (200–800 μg/mL) was strongly cytotoxic in all cell types tested. We investigated the effects of formaldehyde on oxidative stress, mitochondrial respiratory functions, and apoptosis on the sensitive neuronal SK-N-SH cells. Oxidative stress was induced after 2 h of formaldehyde exposure. Formaldehyde at a concentration of 400 μg/mL for 12 h of treatment greatly reduced cellular adenosine triphosphate (ATP) levels. Confocal microscopy indicated that the mitochondrial membrane potential (MMP) was dose-dependently reduced by formaldehyde. A marked and dose-dependent inhibition of mitochondrial respiratory enzymes, viz., NADH dehydrogenase (complex I), cytochrome c oxidase (complex IV), and oxidative stress-sensitive aconitase was also detected following treatment with formaldehyde. Furthermore, formaldehyde caused a concentration-dependent increase in nuclear fragmentation and in the activities of the apoptosis-initiator caspase-9 and apoptosis-effector caspase-3/-7, indicating apoptosis progression. Our data suggests that formaldehyde exerts strong cytotoxicity, at least in part, by inducing oxidative stress, mitochondrial dysfunction, and eventually apoptosis. Changes in mitochondrial respiratory function and oxidative stress by formaldehyde may therefore be critical in methanol-induced toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exposure to methanol is common in the workplace and the household (Barceloux et al. 2002; Jammalamadaka and Raissi 2010). However, the incidence of methanol intoxication differs markedly across countries. In some Asian countries, thousands of cases of acute pesticide intoxication occur each year as a result of accidental or deliberate ingestion of undiluted pesticides. Many individuals then also suffer from acute methanol intoxication, because methanol is a solvent in many pesticide formulations (Gil et al. 2012, 2013).
Methanol toxicity is mainly seen in humans and monkeys. Upon ingestion, 10 mL of pure methanol can cause permanent blindness, whereas 30 mL is considered lethal, although the typical lethal dose is 100 mL (1–2 mL of pure methanol per kilogram body weight) (Desai et al. 2013). In humans, methanol toxicity has been considered to be mediated by the methanol metabolites rather than by the parent molecule. Through a series of oxidative steps, methanol is oxidized to methanal (formaldehyde), methanoic acid (formic acid), and is then finally detoxified to carbon dioxide (Kruse 1992). Even at a low concentration, formaldehyde can inhibit retinal respiration as well as anaerobic glycolysis (Kini and Cooper 1962). Due to its chemical reactivity, formaldehyde can damage most of the components in cells. Reaction of formaldehyde with proteins leads to modification of biologically important proteins, such as ribonuclease and proteolytic enzymes, thereby increasing their susceptibility to proteases. Moreover, formaldehyde methylates the lysyl groups of histones and ribonuclease and also reacts with the amino groups of nucleic acids that may affect cells genetically (Skrzydlewska 2003). The main cause of formaldehyde toxicity in replicating cells is stalling of replication complexes by formaldehyde-induced DNA-protein crosslinks, which causes activation of the transcriptional factor p53 and proapoptotic gene targets (Wong et al. 2012). Cells protect against these bulky genetic lesions by proteolytically removing crosslinked proteins, promoting replication recovery and cell survival (Quievryn and Zhitkovich 2000; Ortega-Atienza et al. 2015). Another report showed that formaldehyde at very low concentrations can induce tauopathies by increasing human tau proteins to form aggregates (Nie et al. 2007).
Several mechanisms of formic acid-mediated toxicity have also been described. Previous reports have suggested that formic acid causes retinal and optic nerve toxicity upon methanol intoxication (Barceloux et al. 2002; Jammalamadaka and Raissi 2010; Nicholls 1975). Histologically, the observation of oxidative phosphorylation-mediated disruption of axonal and glial cells suggests that formic acid is a mitochondrial poison (Nicholls 1976). In highly oxidative organs, including the retina and brain, cytochrome oxidase is an important energy-generating enzyme (Wong-Riley 1989). Formic acid inhibits both cytochrome c oxidase activity and succinate-cytochrome c reductase activity (Nicholls 1975).
Earlier studies have revealed that the damage to the various cell components is provoked not only by formic acid but also by other metabolites as formaldehyde and free radicals (Skrzydlewska 2003). Moreover, formaldehyde showed more cytotoxicity than methanol and formic acid in our study. One earlier study showed that under physiological pH, methanol and formic acid had no cytotoxicity, while formaldehyde at 1–3 mM/L initiated cell death in dissociated rat thymocytes (Oyama et al. 2002). Furthermore, formaldehyde induces intracellular reactive oxygen species (ROS) generation in rat PC12 pheochromocytoma cells (Tang et al. 2013). Such free radicals react with membrane phospholipids to form hydroperoxides and, ultimately, the small aldehyde molecules formed can diffuse from the site of formation to a distal site and can initiate damage (Esterbauer et al. 1992). Formaldehyde at low or higher concentrations has an aberrant effect on cell cycle (Miao et al. 2013). However, the endogenous formaldehyde production in human cells causes chronic impairments of the brain during aging (He et al. 2010). The mechanism responsible for cytotoxicity of methanol and its metabolites, and of formaldehyde specifically, is not completely elucidated.
During the last three decades, many biological roles have been defined for the mitochondria (Henze and Martin 2003). Mitochondrial enzyme complexes have key functions in the mitochondrial electron transport chain and production of a proton gradient across the inner membrane, and they synthesize adenosine triphosphate (ATP) by ATP synthase. In addition to the synthesis of ATP, the mitochondria are involved in various aspects of cell biology, including cell signaling (Zorov et al. 2014). cellular differentiation (St John 2014). and cell death (van Vliet et al. 2014). Any loss of the mitochondrial membrane potential can release cytochrome c, activating downstream caspases and eventually, apoptosis (Indran et al. 2011). However, the influence of formaldehyde on mitochondrial enzyme complexes and apoptosis has never been addressed.
Therefore, in this study, we proceed our work with formaldehyde to investigate its effects on mitochondrial respiratory function and apoptosis pathways in neuronal SK-N-SH cells.
Materials and methods
Chemicals
Methanol (100 %) was purchased from Merck (Darmstadt, Germany), formaldehyde from Wako (Osaka, Japan), and formic acid from Daejung (Seoul, South Korea). MTT [3-(4, 5-dimethylthiaxol-2-yl)-2, 5-diphenyl tetrazolium bromide], dimethylsulfoxide (DMSO), trypan blue, and Hoechst 33258 were obtained from Sigma-Aldrich (St. Louis, MO, USA). All primary antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA), except β-actin antibody which was purchased from Abcam (Cambridge, MA, USA). Horseradish peroxidase-conjugated anti-rabbit IgG and anti-mouse IgG were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell culture and treatment
Human brain neuroblastoma SK-N-SH and human hepatocellular liver carcinoma HepG2 cells were purchased from the Korean Cell Line Bank (KCLB; Seoul, South Korea). Human lung carcinoma A549 and human retinal pigment epithelial ARPE-19 cells were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). SK-N-SH, HepG2, A549, and ARPE-19 cells were maintained in RPMI-1640, EMEM, DMEM, and DMEM:F12 media, respectively, supplemented with 10 % FBS and 1 % penicillin-streptomycin antibiotic. Cells were expanded in a humidified CO2 incubator (5 %) at 37 °C using T-75 flasks, after trypsinization and subsequent washing with phosphate-buffered saline (PBS).
A stock concentration of each chemical used for treating cells was prepared in deionized water. Test chemicals were diluted in their specific medium at desired concentrations required.
Cell viability assay
To set up the range of experimental conditions, we performed cell viability assays with methanol, formaldehyde, and formic acid on SK-N-SH, A549, HepG2, and ARPE-19 cells. We adjusted the pH to 7.3–7.4 to simulate the physiological pH range. A chemosensitive and colorimetric MTT assay was performed, as previously described (Mickisch et al. 1990). Briefly, 100 μL aliquots of cell suspensions was seeded into 96-well plates overnight to reach 70–80 % confluence. Then, 100 μL of the test chemicals (freshly prepared in the respective media) was added into the wells, and the cells incubated for another 24 h at 37 °C. MTT reagent (20 μL of a 5 mg/mL solution) was added to each well and kept at 37 °C for 4 h. Live cells constitutively produce a dehydrogenase enzyme that reacts with tetrazolium salt, producing purple formazan crystals. The culture media were aspirated, and 100 μL DMSO was added to each well to dissolve these crystals. The color intensity was measured at 595 nm using a Victor™ X3 multilabel reader (Perkin Elmer, Waltham, MA, USA).
Intracellular ROS detection
To detect intracellular ROS generation, cells were seeded in a black 96-well plate and incubated overnight to reach 70–80 % confluence. Cells were washed with 1× Hanks balanced salt solution (HBSS) and incubated with 10 μM 2,7–dichlorofluorescein diacetate (H2DCFDA; Invitrogen, Carlsbad, CA, USA) in 1× HBSS containing 0.1 mg/mL glucose, for 30 min at 37 °C. Following washing with 1 × HBSS, cells were treated with hydrogen peroxide (H2O2) as positive control; H2O2 and ROS inhibitor; n-acetyl-l-cysteine (NAC) as negative control; and 50–200 μM methanol, formaldehyde, and formic acid. ROS generation was measured at time points from 10 min to 4 h. We treated cells with both H2O2 and NAC to observe the ROS-quenching effect of NAC in the negative control cells. Intracellular fluorescence was detected using a Victor™ X3 multilabel reader (Perkin Elmer, Waltham, MA, USA) with an excitation wavelength of 485 nm and emission wavelength of 530 nm. Fluorescence values of wells lacking cells were subtracted from those of the test and control samples.
Extraction and purification of mitochondrial fraction
Cellular mitochondrial fractions were extracted and purified using Mitochondria/Cytosol Fraction kits (BioVision, Milpitas Boulevard, Milpitas, CA, USA) according to the manufacturer’s protocol. Briefly, cells were obtained from treated and control (non-treated) cultures by centrifugation. Cells were first treated with the cytosol extraction buffer provided in the kit and homogenized. This homogenate was centrifuged at ×700g for 10 min. The pellet containing intact mitochondria were dissolved in PBS and kept in a −80 °C freezer until used.
Measurement of mitochondrial respiratory function and ATP content
The enzyme activities of complex I (NADH dehydrogenase) and IV (cytochrome c oxidase) were assessed in the cell lysates and freshly isolated mitochondria extracted from formaldehyde-treated SK-N-SH cells, respectively. Complex I and IV were assessed using a Complex I Enzyme Activity Microplate Assay kit (Abcam, Cambridge, MA, USA) and a Cytochrome Oxidase Activity Colorimetric Assay kit (BioVision, Milpitas, CA, USA), respectively, according to the manufacturers’ instructions (Lopes Costa et al. 2013; Favaro et al. 2010).
Mitochondrial aconitase is used as a biomarker for oxidative damage. This enzyme activity was measured by Aconitase Activity Assay Kit (Sigma-Aldrich, St. Louis, MO, USA). Data are presented as the percentage of activity compared to control.
The ATP level in formaldehyde-treated and untreated cells was determined using the recombinant firefly luciferase and its substrate D-luciferin in a bioluminescence assay, according to the manufacturer’s recommended protocol (Molecular Probes, Eugene, OR, USA) (Baron et al. 2005; Mishra et al. 2006). Briefly, the cells (1 × 106/mL) were harvested following desired treatment and suspended in a standard reaction solution containing reaction buffer, dithiothreitol, luciferin, and luciferase. After a gentle mix, ATP level was measured using a Victor™ X3 multilabel reader and expressed as the percentage of the control.
Detection of MMP
Collapse of mitochondrial membrane potential (MMP) in formaldehyde-treated cells was determined by confocal laser scanning microscopy with rhodamine123 staining. In brief, cells were seeded onto coverslips and treated with the indicated concentrations of formaldehyde for 24 h. Following treatment, 5 μg/mL rhodamine123 (stock concentration 1 mg/mL in DMSO) was added and incubated at 37 °C for 30 min. Cells were washed with PBS and fixed with 4 % paraformaldehyde. The fixed cells were then washed with PBS three times and mounted in the Fluorescence Mounting Medium (DAKO; Glostrup, Denmark). The cells were then observed using a model FV10i-W confocal microscope (Olympus; Tokyo, Japan), and images were analyzed using FV10i-ASW 3.0 Viewer software.
Nuclear staining
Nuclear fragmentation is a hallmark of apoptosis and can be detected by Hoechst dye staining. The cells were seeded onto coverslips and treated as required, then washed with PBS, and fixed with 4 % paraformaldehyde. Following fixation, cells were stained with 2 μg/mL Hoechst 33258 for 15 min at room temperature. The stained cells were washed with PBS three times, mounted on glass slides, and analyzed using a model FV10i-W confocal microscope (Olympus; Tokyo, Japan). Images were analyzed using FV10i-ASW 3.0 Viewer software.
Detection of caspase-9 and caspase-3/-7 activities
The activity of caspase-9 was determined by colorimetric assay using a commercially available kit (Invitrogen; Carlsbad, CA, USA). In brief, treated and untreated cells were harvested; proteins were extracted, quantified, and diluted to 1–4 mg/mL in cell lysis buffer. Then, 50 μL of 2× reaction buffer containing 10 mM DTT was added to each sample. LEHD–pNA substrate was added to a final concentration of 200 μM and incubated at 37 °C for 2 h in the dark. Samples were read at 405 nm in a Victor™ X3 multilabel reader.
The downstream substrates of caspase-9 include caspase-3/-7. The activity of caspase-3/-7 was detected by luminescent assay following the vendor’s protocol (Promega; WI, USA). Briefly, cells were seeded in white 96-well plates and treated as required. After treatment, an equal volume of caspase-Glo 3/7 reagent (mixture of caspase-Glo substrate and caspase-Glo buffer) was added to each well. The plate was incubated at room temperature in a vertical shaker for 2 h, and luminescence was recorded at indicated times by a Victor™ X3 multilabel reader.
Western blot analysis
Cells were harvested following desired treatment. Proteins were collected using RIPA lysis buffer containing a protease inhibitor cocktail (Santa Cruz Biotechnology) and quantified using BCA protein assay kit (Pierce; Rockford, IL, USA). Equal amount of proteins were electrophoresed by 4–20 % sodium dodecyl sulfate polyacrylamide electrophoresis gradient gel (Mini-PROTEAN® TGX™ Precast Gel; Bio-Rad, Hercules, CA, USA) at 100 V for 1.30 h and then transferred onto polyvinylidene fluoride membrane (Trans-Blot SD Semi-Dry Cell; Bio-Rad) at 15 V for 1 h. The membranes were blocked with 5 % skim milk in Tris-buffered saline containing 0.1 % (v/v) Tween-20 for 1 h and then incubated with primary antibodies overnight. Following a brief wash, membranes were again incubated with horseradish peroxidase-conjugated secondary antibodies for 1.5 h. After washing, protein band images were visualized using enhanced chemiluminescence Western blotting detection reagents (Bio-Rad) and images were captured using a ChemiDoc™ XRS+ System with Image Lab™ software (Bio-Rad).
Apoptosis by FACS analysis
Apoptosis assay was performed using Alexa Fluor® 488 Annexin V/Dead cell apoptosis kit (Invitrogen, USA). Exponentially growing cells were seeded in a 6-well plate overnight to reach 70–80 % confluence. Following treatment, cells were washed with PBS, trypsinized, and harvested by centrifugation. As mentioned, cells were stained with 5 μL fluorescein isothiocyanate (FITC)-conjugated annexin V and 1 μL of 100 μg/mL propidium iodide (PI) in each 100 μL cell suspension for 15 min at room temperature. Apoptosis was directly assessed by fluorescence-activated cell sorting (FACS) (MACS Miltenyi Biotec, Bergisch Gladbach, Germany), and the data were analyzed using MACSQuant analysis software version 2.4. FITC and PI emissions were detected with 515–545 and 564–606 nm fluorescence detectors, and a minimum of 10,000 cells was examined per data point. Cells that stained positive for annexin V and negative for PI were considered early apoptotic cells; those that were positive for both annexin V and PI were considered to be in the late stage of apoptosis, undergoing necrosis or already dead, and cells that stained negative for both were considered alive and not undergoing measurable apoptosis.
Statistical analyses
At least three separate tests were performed for each experiment with three to five replicates per individual test, and the mean ± SD of at least three culture dishes is presented. Satisfactory correlation was achieved between the results of individual experiments. Differences between groups were analyzed using one-way analysis of variance, followed by Student’s t test, with a p value of <0.05 considered as statistically significant.
Results
Effects of methanol and its metabolites on cell viability and ROS generation
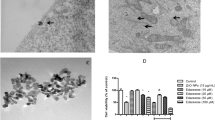
Cell viability was compared in these different cells after treatment with 12.5–800 μM methanol/formaldehyde/formic acid for 24 h. Methanol at the indicated concentrations did not show any cytotoxic effects on any of the cells, whereas formaldehyde at the indicated dosages caused a gradual cell death in all four cell lines, with the highest cytotoxicity noted in neuroblastoma SK-N-SH cells. The cytotoxicity was first noted at 100 μM formaldehyde and increased with increasing concentrations of formaldehyde in both SK-N-SH and ARPE-19 cells. However, in A549 and HepG2 cells, formaldehyde only started having cytotoxic effects at 400 μM. Conversely, formic acid did not show marked cytotoxicity in any cell type. Only in SK-N-SH cells, 800 μM formic acid resulted in reduction of cell viability to 80 % after 24 h of treatment (Fig. 1). As SK-N-SH cells were the most sensitive of the cell lines tested, we opted to use these cells in our further experiments.
Comparative cytotoxicity of methanol and its metabolites on various cells by MTT analysis. Methanol, formaldehyde, and formic acid were introduced to SK-N-SH (a), A549 (b), HepG2 (c), and ARPE-19 (d) cells at a concentration range of 12.5–800 μM for 24 h and MTT assay was performed. Untreated cells were used as controls. Data are presented as mean ± SD of three independent experiments. Asterisk (*) denotes significant differences relative to control (p < 0.05)
We then investigated whether methanol and its metabolites play a role in intracellular ROS generation in SK-N-SH cells. Only formaldehyde concentration- and time-dependently increased ROS generation. In contrast, methanol and formic acid did not show any release of ROS at any time point. This may be because the concentration ranges used for ROS generation are not cytotoxic. We used H2O2 as a positive control, which resulted in marked ROS generation, and we used NAC to quench the ROS generated by H2O2, as a negative control (Fig. 2).
Comparative ROS generation by methanol and its metabolites in SK-N-SH cells. Intracellular ROS generation was measured in SK-N-SH cells treated with 50–200 μM of methanol, formaldehyde, and formic acid, from 10 min to 4 h. Furthermore, 200 μM H2O2 (PC positive control) and 5 mM NAC (I, an inhibitor for ROS) were used as treatment controls. C stands for control without any treatment. Data are presented as mean ± SD of three independent experiments. Asterisk (*) and hashtag (#) denote significant differences relative to control and positive control (p < 0.05), respectively
From our results, we speculate that the toxic effect of methanol poisoning is mainly due to its metabolite, formaldehyde. Therefore, only formaldehyde was used for subsequent experiments, as it resulted in marked cell loss and intracellular ROS release.
Effects of formaldehyde on mitochondrial respiratory function and ATP content
Alterations in mitochondrial respiratory enzymes and bioenergetics were assessed in the samples extracted from SK-N-SH cells exposed to the various concentrations of formaldehyde. At 24 h, around 27 % cells survived after 200 μM formaldehyde treatment, making it difficult to extract mitochondria. Therefore, we treated cells with 200 μM formaldehyde for 12 h only, resulting in nearly 68 % cells surviving (data not shown). Although significant complex I activity inhibition was observed (Fig. 3a), the activity of terminal respiratory enzymes of complex IV was markedly inhibited by 200 μM formaldehyde (Fig. 3b). Reduction of the activity of mitochondrial oxidative damage marker, aconitase, was found in 100 and 200 μM formaldehyde-treated SK-N-SH cells (Fig. 3c). Similarly, ATP levels decreased with increasing concentrations of formaldehyde (Fig. 3d).
The effects of formaldehyde on mitochondrial enzyme activity and ATP generation in SK-N-SH cells. SK-N-SH cells were treated with 25–200 μM formaldehyde or were left untreated for 12 h to detect complex I (a), complex IV (b), and aconitase (c) activities. Cell lysates were used to study complex I activity, but freshly isolated mitochondria were used to assay complex IV and aconitase activities. We also measured ATP levels in cells treated with 12.5–400 μM formaldehyde and compared these to the levels in untreated cells. Percentage of ATP level was determined in 12-h treated cells (d). Data are presented as mean ± SD of three independent experiments. Asterisk (*) denotes significant differences relative to control (p < 0.05)
Effect of formaldehyde on MMP
The membrane permeant dye rhodamine123 was used to detect mitochondrial membrane potential. Treatment of SK-N-SH cells with 50–200 μM formaldehyde showed a clear reduction in the MMP following 24 h of treatment, which was noticeable from 50 μM and was prominent at 200 μM (Fig. 4).
The effect of formaldehyde on MMP in SK-N-SH cells. SK-N-SH cells were treated with 50–200 μM formaldehyde or left untreated for 24 h. Collapse of the MMP in cells was detected by confocal laser scanning microscopy using rhodamine123 staining. The lower panels show the phase contrast images of the respective fluorescent pictures. Magnification scale, 50 μm
Effect of formaldehyde on nuclear fragmentation
Nuclear fragmentation and chromatin condensation are the nuclear hallmarks of apoptosis. We evaluated formaldehyde-treated SK-N-SH cells for any changes in their nuclei by staining them with Hoechst 33258. Condensed and fragmented nuclei were visible with treatment with 100 μM or more formaldehyde, and increased with 200 μM formaldehyde, as seen in confocal images (Fig. 5).
The effect of formaldehyde on chromatin fragmentation in SK-N-SH cells. Apoptosis was detected using Hoechst 33258 dye staining of 50–200 μM formaldehyde-exposed SK-N-SH cells. Nuclear damage in 24-h formaldehyde-treated cells was detected by confocal laser scanning microscopy. Arrows indicated condensed or fragmented nuclei. Scale bar, 20 μm (a). Apoptotic rate was determined by scoring the percentage of cells containing fragmented nuclei with the total number of cells. Data are presented as mean ± SD of three independent experiments. Asterisk (*) denotes significant differences relative to control (p < 0.05) (b)
Effect of formaldehyde on caspase-9 and caspase-3/-7 activities
To determine whether formaldehyde exposure activates the caspase cascade in SK-N-SH cells, the functional activity of the apoptosis initiator caspase-9 and the apoptosis effector caspase-3/-7 were detected (Fig. 6). Activity of caspase-9 was found to increase with increasing concentrations (from 25 to 200 μM) of formaldehyde (Fig. 6a). Activation of caspase-9 is an indication of the release of mediators from the mitochondria, indicating the involvement of mitochondria in the apoptosis. All apoptosis pathways terminate in activation of the key enzyme, caspase-3. With 3.125 to 100 μM formaldehyde, SK-N-SH cells did not show marked differences in caspase-3/-7 activity (Fig. 6b), but the activity prominently increased at 200 μM formaldehyde. The activation of caspases further is verified by Western blot analysis. Both caspase-9 and caspase-3 showed significant cleavage at increasing dosages (Fig. 6c). Although the activity and protein expression analyses did not exactly follow similar plateau, both the experiments showed increased activity or cleavage with increased dosage.
The effect of formaldehyde on caspase-9 and caspase-3/-7 activities in SK-N-SH cells. SK-N-SH cells were treated with 12.5–200 μM of formaldehyde to detect caspase-9 activity (a) and with 3.125–200 μM of formaldehyde to detect caspase-3/-7 activity (b). In both cases, cells were treated for 24 h and a sample without any treatment was used as control. Data are presented as mean ± SD of three independent experiments. Asterisk (*) denotes significant differences relative to control (p < 0.05). Cells were treated with 50–200 μM of formaldehyde for 24 h to detect the cleavage of caspase-9 and caspase-3 by Western blot analysis. β-actin was used as endogenous control (c)
Effect of formaldehyde on apoptosis by FACS analysis
As formaldehyde increased nuclear fragmentation and caspase-9 and caspase-3/-7 activities, we further performed FACS analysis to detect apoptosis in formaldehyde-treated cells directly. A concentration-dependent increase of apoptotic cells was evident (Fig. 7). A gradual increase in the total number of apoptotic cells was apparent. Figure 7 shows that at 200 μM formaldehyde, early apoptotic cells accounted for 25.34 % of cells and late apoptotic cells accounted for 30.93 % of cells. When the concentration was increased to 400 μM, early apoptotic cells comprised 22.10 %, but late apoptotic cells increased to 46.50 % of cells.
The effect of formaldehyde on apoptosis in SK-N-SH cells. Representative flow cytometric analysis of cells stained with annexin V/PI following treatment with 100–400 μM formaldehyde for 24 h. The lower left panel shows the live cells, the lower right panel shows the early apoptotic cells, the upper right panel shows the late apoptotic cells or cells undergoing necrosis, and the upper left panel shows necrotic cells
Discussion
In general, the clinical presentation of acute methanol intoxication is characterized by neurological manifestations, consisting of optic neuropathy, coma, and seizure (Barceloux et al. 2002; Jammalamadaka and Raissi 2010). Upon ingestion, methanol is metabolized in the liver via several enzymatic and free radical pathways, in which oxidation of methanol results in the formation of formaldehyde, formic acid, carbon dioxide, and free radicals. Methanol alters the oxidant/antioxidant balance and causes oxidative stress in lymphoid organs of the rat in vivo (Parthasarathy et al. 2006). However, until now, the fate of various human cells upon direct exposure to methanol and its metabolites has never been compared with respect to cytotoxicity and intracellular ROS generation. In our study, formaldehyde showed the highest cytotoxicity to any of the cells tested, compared to methanol and formic acid. Previous studies showed that 120 μM formaldehyde caused around 30 % cytotoxicity in PC12 rat pheochromocytoma cells, whereas 30 mM formic acid resulted in around 45 and 30 % cytotoxicity in mouse photoreceptor 661W and human RPE ARPE-19 cells, respectively, after 24 h of treatment (Tang et al. 2013; Treichel et al. 2004). Our data showed somewhat similar results, where 800 μM formic acid showed little or no cytotoxicity in any of the four cell lines, while 200–400 μM formaldehyde showed robust cytotoxicity in most of the cells. Thus, in agreement with a previous report (Oyama et al. 2002; Yang et al. 2014). our comparative study showed that formaldehyde is more toxic than formic acid and methanol. Although formaldehyde is a metabolite of methanol but along with those previous reports, we did not find any toxicity with the indicated dosage of methanol treatment. We assume the methanol did not metabolize in the cell lines. This might be the lack of ADH enzyme that is highly present in the liver and the lining of the stomach (Farrés et al. 1994). The concentration of methanol and the free active sites of enzymes present in the surrounding environment also matter. It should be evaluated to in vivo studies to confirm the effect of methanol and its metabolites.
It is known that the mitochondria play a major role in energy metabolism, redox function, and osmotic regulation. Moreover, mitochondrial oxidative phosphorylation supplies bioenergy to each tissue; in particular, muscle and nerve tissues rely more on oxidative phosphorylation than on the glycolysis process. As a consequence of chemical-mediated cell toxicity, mitochondrial dysfunction may lead to cell injury and death (Wallace et al. 1997). Our results clearly show that formaldehyde inhibits the mitochondrial respiratory enzymes NADH dehydrogenase (complex I) and cytochrome c oxidase (complex IV). We could not explain the mechanism of formaldehyde-mediated inhibition on those enzyme complexes. However, we hypothesize that formaldehyde might induce an imbalance in the redox status or hamper the mitochondrial membrane potential of the mitochondria, which might lead to structural degradation of the relevant enzymes. The reduction of mitochondrial membrane potential is most likely a consequence of the inhibition of the activities of the mitochondrial enzyme complexes and of the ROS-sensitive aconitase enzyme, followed by inhibition of ATP synthesis. Further studies are necessary to determine whether formaldehyde induces structural degradation of these enzymes or merely alters an epitope on the enzyme, causing a change in the affinity and/or avidity of the antigen–antibody immune reaction.
The generation of oxidative stress by ROS production is an upstream event for mitochondrial dysfunction, leading to cytochrome c release and ultimately, activation of the caspase cascade and apoptosis. MMP has been shown to be affected in various oxidative stress models. When MMP is impeded, cytochrome c is released, leading to the activation of caspase-9 and triggering the executioner caspase-3/-7, which results in dismantling of cells through nuclear fragmentation (Indran et al. 2011; Jones et al. 2003; Xie et al. 2008). Our study depicted impairment of MMP may be the reason for formaldehyde-mediated apoptosis. Other mechanisms involving formaldehyde-mediated neuroblastoma cell death includes hyperphosphorylation and polymerization of tau protein lead to DNA impairment (Lu et al. 2013a, b). Formaldehyde was also found as the most detrimental component of methanol toxicity related to microtubule collapse, tau hyperphosphorylation, and subsequent impaired memory in mice (Yang et al. 2014). Inhibition of DNA methyltransferase function in aging-associated formaldehyde contributes to pathophysiology in Alzheimer’s disease (Tong et al. 2015).
Conclusion
In summary, formaldehyde is the most toxic metabolite of methanol. The mechanism of formaldehyde-mediated cellular toxicity probably involves the depletion of ATP in cells by the reduction in the activities of the mitochondrial respiratory enzymes. We also showed that the ROS-sensitive mitochondrial aconitase enzyme is also markedly inhibited by formaldehyde treatment, indicative of oxidative stress inside the cells. Thus, the alteration of mitochondrial energy metabolism and mitochondrial membrane potential and oxidative stress may be the major causes for the activation of the caspase cascade that results in nuclear fragmentation and apoptosis in response to methanol poisoning. However, further detailed studies are required to evaluate the effect of methanol and its metabolites to in vivo system.
Abbreviations
- ADH:
-
alcohol dehydrogenase
- ATP:
-
adenosine triphosphate
- FACS:
-
fluorescence-activated cell sorting
- FBS:
-
fetal bovine serum
- FITC:
-
fluorescein isothiocyanate
- HBSS:
-
Hanks balanced salt solution
- MMP:
-
mitochondrial membrane potential
- PBS:
-
phosphate-buffered saline
- ROS:
-
reactive oxygen species
References
Barceloux DG, Bond GR, Krenzelok EP, Cooper H, Vale JA. American Academy of Clinical Toxicology practice guidelines on the treatment of methanol poisoning. J Toxicol, Clin Toxicol. 2002;40:415–46.
Baron S, Caplanusi A, van de Ven M, Radu M, Despa S, Lambrichts I, et al. Role of mitochondrial Na + concentration, measured by CoroNa red, in the protection of metabolically inhibited MDCK cells. J Am Soc Nephrol. 2005;16:3490–7.
Desai T, Sudhalkar A, Vyas U, Khamar B. Methanol poisoning: predictors of visual outcomes. JAMA Ophthalmol. 2013;131:358–64.
Esterbauer H, Gebicki J, Puhl H, Jürgens G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radic Biol Med. 1992;13:341–90.
Farrés J, Moreno A, Crosas B, Peralba JM, Allali-Hassani A, Hjelmqvist L, et al. Alcohol dehydrogenase of class IV (sigma sigma-ADH) from human stomach. cDNA sequence and structure/function relationship. Eur J Biochemistry. 1994;224:549–57.
Favaro E, Ramachandran A, McCormick R, Gee H, Blancher C, Crosby M, et al. MicroRNA-210 regulates mitochondrial free radical response to hypoxia and Krebs cycle in cancer cells by targeting iron sulfur cluster protein ISCU. PLoS One. 2010;5, e10345.
Gil HW, Hong JR, Song HY, Hong SY. A case of methanol intoxication caused by methomyl pesticide ingestion. Hum Exp Toxicol. 2012;31:1299–302.
Gil HW, Jeong MH, Park JS, Choi HW, Kim SY, Hong SY. An outbreak of food borne illness due to methomyl pesticide intoxication in Korea. J Korean Med Sci. 2013;28:1677–81.
He RQ, Lu J, Miao JY. Formaldehyde stress. Sci China Life Sci. 2010;53:1399–404.
Henze K, Martin W. Evolutionary biology: essence of mitochondria. Nature. 2003;426:127–8.
Indran IR, Tufo G, Pervaiz S, Brenner C. Recent advances in apoptosis, mitochondria and drug resistance in cancer cells. Biochim Biophys Acta. 2011;1807:735–45.
Jammalamadaka D, Raissi S. Ethylene glycol, methanol and isopropyl alcohol intoxication. Am J Med Sci. 2010;339:276–81.
Jones DC, Prabhakaran K, Li L, Gunasekar PG, Shou Y, Borowitz JL, et al. Cyanide enhancement of dopamine-induced apoptosis in mesencephalic cells involves mitochondrial dysfunction and oxidative stress. Neurotoxicology. 2003;24:333–42.
Kini MM, Cooper JR. Biochemistry of methanol poisoning. 4. The effect of methanol and its metabolites on retinal metabolism. Biochem J. 1962;82:164–72.
Kruse JA. Methanol poisoning. Intensive Care Med. 1992;18:391–7.
Lopes Costa A, Le Bachelier C, Mathieu L, Rotig A, Boneh A, De Lonlay P, et al. Beneficial effects of resveratrol on respiratory chain defects in patients’ fibroblasts involve estrogen receptor and estrogen-related receptor alpha signaling. Hum Mol Genet. 2013;23:2106–19.
Lu J, Miao JY, Su T, Liu Y, He RQ. Formaldehyde induces hyperphosphorylation and aggregation of tau protein both in vitro and in vivo. Biochim Biophys Acta. 2013a;1830:4102–16.
Lu Y, He HJ, Zhou J, Miao JY, Lu J, He YG, et al. Hyperphosphorylation results in tau dysfunction in DNA folding and protection. J Alzheimers Dis. 2013b;37:551–63.
Miao JY, Lu J, Zhang ZJ, Tong ZQ, He RQ. The effect of formaldehyde on cell cycle is in a concentration-dependent manner. Prog Biochem Biophys. 2013;40:641–51.
Mickisch G, Fajta S, Keilhauer G, Schlick E, Tschada R, Alken P. Chemosensitivity testing of primary human renal cell carcinoma by a tetrazolium based microculture assay (MTT). Urol Res. 1990;18:131–6.
Mishra DP, Pal R, Shaha C. Changes in cytosolic Ca2+ levels regulate Bcl-xS and Bcl-xL expression in spermatogenic cells during apoptotic death. J Biol Chem. 2006;281:2133–43.
Nicholls P. Formate as an inhibitor of cytochrome c oxidase. Biochem Biophys Res Commun. 1975;67:610–6.
Nicholls P. The effect of formate on cytochrome aa3 and on electron transport in the intact respiratory chain. Biochim Biophys Acta. 1976;430:13–29.
Nie CL, Wang XS, Liu Y, Perrett S, He RQ. Amyloid-like aggregates of neuronal tau are induced by formaldehyde exposure and promote apoptosis of neuronal cells. BMC Neurosci. 2007;8:9.
Ortega-Atienza S, Wong VC, DeLoughery Z, Luczak MW, Zhitkovich A. ATM and KAT5 safeguard replicating chromatin against formaldehyde damage. Nucl Acids Res. 2015. doi:10.1093/nar/gkv957.
Oyama Y, Sakai H, Arata T, Okano Y, Akaike N, Sakai K, et al. Cytotoxic effects of methanol, formaldehyde, and formate on dissociated rat thymocytes: a possibility of aspartame toxicity. Cell Biol Toxicol. 2002;18:43–50.
Parthasarathy NJ, Kumar RS, Manikandan S, Devi RS. Methanol-induced oxidative stress in rat lymphoid organs. J Occup Health. 2006;48:20–7.
Quievryn G, Zhitkovich A. Loss of DNA-protein crosslinks from formaldehyde-exposed cells occurs through spontaneous hydrolysis and an active repair process linked to proteosome function. Carcinogenesis. 2000;21:1573–80.
Skrzydlewska E. Toxicological and metabolic consequences of methanol poisoning. Toxicol Mech Meth. 2003;13:277–93.
St John J. The control of mtDNA replication during differentiation and development. Biochim Biophys Acta. 2014;1840:1345–54.
Tang XQ, Fang HR, Zhou CF, Zhuang YY, Zhang P, Gu HF, et al. A novel mechanism of formaldehyde neurotoxicity: inhibition of hydrogen sulfide generation by promoting overproduction of nitric oxide. PLoS One. 2013;8, e54829.
Tong Z, Han C, Qiang M, Wang W, Lv J, Zhang S, et al. Age-related formaldehyde interferes with DNA methyltransferase function on causing memory loss in Alzheimer’s disease. Neurobiol Aging. 2015;36:100–10.
Treichel JL, Henry MM, Skumatz CM, Eells JT, Burke JM. Antioxidants and ocular cell type differences in cytoprotection from formic acid toxicity in vitro. Toxicol Sci. 2004;82:183–92.
van Vliet AR, Verfaillie T, Agostinis P. New functions of mitochondria associated membranes in cellular signaling. Biochim Biophys Acta. 2014;1843:2253–62.
Wallace KB, Eells JT, Madeira VM, Cortopassi G, Jones DP. Mitochondria-mediated cell injury. Symposium overview. Fundam Appl Toxicol. 1997;38:23–37.
Wong VC, Cash HL, Morse JL, Lu S, Zhitkovich A. S-phase sensing of DNA-protein crosslinks triggers TopBP1-independent ATR activation and p53-mediated cell death by formaldehyde. Cell Cycle. 2012;13:2526–37.
Wong-Riley MT. Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Trends Neurosci. 1989;12:94–101.
Xie L, Zhu X, Hu Y, Li T, Gao Y, Shi Y, et al. Mitochondrial DNA oxidative damage triggering mitochondrial dysfunction and apoptosis in high glucose-induced HRECs. Invest Ophthalmol Vis Sci. 2008;49:4203–9.
Yang M, Lu J, Miao J, Rizak J, Yang J, Zhai R, et al. Alzheimer’s disease and methanol toxicity (part 1): chronic methanol feeding led to memory impairments and tau hyperphosphorylation in mice. J Alzheimers dis. 2014;41:1117–29.
Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94:909–50.
Acknowledgements
This work was carried out with the support of “the Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ2015)”, Rural Development Administration, South Korea.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Zerin, T., Kim, JS., Gil, HW. et al. Effects of formaldehyde on mitochondrial dysfunction and apoptosis in SK-N-SH neuroblastoma cells. Cell Biol Toxicol 31, 261–272 (2015). https://doi.org/10.1007/s10565-015-9309-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-015-9309-6