Abstract
Nausea and vomiting are the most common symptoms in different diseases. Medicinal plants are considered as a reliable source of new drugs to control these symptoms. In this study, we evaluated the antiemetic and neuroprotective effects of the methanolic extract of Sambucus ebulus L. fruit and relationship between emesis (retching) and oxidative stress biomarkers in the mitochondria brain of young chickens. Emesis was induced by ipecac and copper sulphate (60 and 600 mg/kg, orally), respectively, and the methanolic extracts (50, 100, 200 mg/kg) were injected intraperitoneally (i.p.). The extract showed a significant antiemetic activity against ipecac and copper sulphate-induced emesis at all doses (p < 0.001; percentages of retching inhibition 46, 96.5 and 83 % against ipecac and 73, 79.5 and 69.2 % against copper sulphate, respectively). Lipid peroxidation (LPO) was significantly decreased (p < 0.001) at all doses of extract in retching induced by copper sulphate, and catalase (CAT) activity significantly increased (p < 0.05) in the extract (50 mg/kg) and metoclopromide groups in retching induced by ipecac in the chickens’ brain mitochondria. Protein carbonyl (PC) contents significantly (p < 0.05) decreased only in extract (100 mg/kg) group in retching induced by ipecac. Mitochondria function (MTT assay) significantly increased by extract (100 mg/kg) as compared to control group in retching induced by ipecac. The results of this study suggests that the extract has protective effects, possibly by central and peripheral mechanisms, and neuroprotective effect by increasing plasma antioxidants or scavenging of free radicals induced by retching. It seems that extract could prevent protein modification and improve oxidative stress in the early stages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nausea and vomiting are the important problems and the most common symptoms encountered in medicine which occurs in different diseases such as surgery and cancer patients receiving chemotherapy and drug treatments. Medications by different synthetic drugs to control these symptoms are available but in recent decades, medicinal plants are considered as a reliable source of new drugs because of numerous side effects of synthetic drugs. Traditional medicines have been used for thousands of years in Iran and other Middle East countries. In this regard, use of medicinal plants is common in Iran. Four species of the genus Sambucus plant are grown in Iran. Among these, Sambucus ebulus L. (Caprifoliaceae) is one of the most common species that commonly grows in the northern regions of Iran (Ebrahimzadeh et al. 2007). S. ebulus contains different compounds such as ribosome-inactivating proteins (ebulin) (Citores et al. 1998), which has been used for the construction of anticancer immunotoxins (Jiménez et al. 2015), ebulitins (de Benito et al. 1995), steroid substances, flavonoids, cardiac glycosides, tannins, caffeic acid derivatives and other isolated substances (Ahmadiani et al. 1998; Ghannadi and Ghassemi-Dehkordi 1997; de Benito et al. 1995; Saeedi Saravi and Shokrzadeh 2009). Other Sambucus species have been shown to contain mainly anthocyanins like “sambucina” or “sambucia-nina”, flavonoids as quercetin, organic acids, esters of fatty acids, triterpenes, polyphenols, sterols, ursolic acid and iridoids (Abdala et al. 2014). These species have demonstrated interesting anti-inflammatory and anti-nociceptive (Ahmadiani et al. 1998; Wang et al. 2013), anti-giardial (Rahimi-Esboei et al. 2013), hypolipidemic (Dubey et al. 2012), anti-microbial activities (Chashoo et al. 2012), analgesic and antipyretic effects (Abdala et al. 2014). In addition, the flower infusion is used to treat coughs and the juice of the fruit as gargle or mouthwash and laxative (Darias et al. 1986; Abdala et al. 2014). Iranian people living on the coast of the Caspian Sea use this plant as analgesics, anti-Helicobacter pylori, anti-hemorrhoid and anti-rheumatic drug. The investigations showed that, traditionally, these people use rhizomes, roots and leaves of S. ebulus for sore throat and arthritis (Tuzlaci and Tolon 2000; Yeşilada et al. 1999; Ebrahimzadeh et al. 2007). Also, it has been reported to be an insect repellent, treatment of burns and infectious wounds, eczema, edema, urticarial and rheumatism (Ebrahimzadeh et al. 2009b). Recently, the toxicity of ethyl acetate fraction (2nd fraction) and safety of methanolic fraction (3rd fraction) of S. ebulus have been reported (Ebrahimzadeh et al. 2007). However, in spite of many reports of antioxidant activity and other benefit attribute abut S. ebulus, no formal study has been done previously on the antiemetic effect of S. ebulus. Also intensive search of the literatures did not show any report about relation between retching and brain oxidative damage. So, the current study represents the first research into the relation between retching and oxidative stress biomarkers and antiemetic effect of methanolic extract fruit of S. ebulus employing in chickens animal.
The goals of this research are to (1) evaluate the antiemetic effect of methanolic extract fruit of S. ebulus and (2) to identify the relationship between emesis (retching) and oxidative stress biomarkers in the mitochondria brain of the chickens.
Materials and methods
Chemicals
All the chemicals used in this study were of analytical grade and purchased from Merck (Darmstadt, Germany) and Sigma Chemical Co. (St. Louis, MO, USA) unless otherwise stated. Metoclopramide from Hakim Pharmaceutical Co. Iran, and Ipecac from Lab. Chim. Farm. A. SELLA S. R.L were obtained.
Plant materials and preparation of methanolic extract
S. ebulus fruit was collected from Geleh Kola Sofla_Kordkheyl, Sari, Iran in September 2014 and authenticated by Dr B. Eslami. A voucher (No. 87) has been deposited in Sari School of Pharmacy Herbarium. Fruit was dried at room temperature and coarsely ground before extraction. Three hundred grams of sample was fractionated by successive solvent extraction at room temperature by maceration with hexane (700 ml × 3) then ethyl acetate (700 ml × 3) and finally methanol (700 ml × 3), successively. The resulting methanol extract (3rd fraction) was concentrated over a rotary vacuum evaporator (35 °C) until a solid extract sample was obtained. The resulting crude extracts were freeze-dried for complete water removal (yield 17 %).
Determination of total phenolic and flavonoid contents
Total phenolic compound contents were determined by the Folin-Ciocalteau method (Ebrahimzadeh et al. 2009a). The extract sample (0.5 ml) was mixed with 2.5 ml of a 0.2 N Folin-Ciocalteau reagent for 5 min, and then 2.0 ml of 75 g/l sodium carbonate was added. The absorbance of the reaction was recorded at 760 nm after 2 h of incubation at room temperature. The standard curve was prepared solutions (0–250 mg/ml) of gallic acid in methanol/water (50:50, v/v). Total phenol values are expressed in terms of gallic acid equivalent (mg/g of dry mass) (GAE), which is a common reference compound. AlCl3 method was used for determination of total flavonoid content of extract (Ebrahimzadeh et al. 2009a). Briefly, 0.5 ml solution of plant extract in methanol was separately mixed with 1.5 ml of methanol, 100 μl of 10 % aluminium chloride, 100 μl of 1 M potassium acetate, and 2.8 ml of distilled water and left at room temperature for 30 min. The absorbance of the reaction mixture was measured at 415 nm with a double beam spectrophotometer. The calibration curve was obtained by preparing quercetin solutions at concentrations 12.5 to 100 mg/ml in methanol. The total flavonoid contents were calculated as quercetin equivalent (mg/g of dry mass) (QE) from calibration curve.
Animals
Young chickens (male and female, 32–38 g, 3–5 days old) were obtained from a poultry local market. Young chickens were divided into 10 groups of 5 each. The antiemetic activity was assessed after 24 h fasting of the chickens. All animal experiments were achieved in accordance with the acts of the Ethical Committee of Mazandaran University of Medical Sciences.
The acute toxicity
The different doses of methanolic extract of S. ebulus fruit were injected to the separated groups of four young chickens, and mortalities were recorded after 24 h.
Antiemetic activity
The antiemetic activity was evaluated using the method explained by Yang et al., with different emetic agents (Yang et al. 1999). The emesis was induced with copper sulphate, 60 mg/kg (0.5 ml, orally) or ipecac, 600 mg/kg (1 ml, orally) in young chickens. Three doses of extracts (50,100, 200 mg/kg) and negative control (normal saline/Tween 80, 90:10, v/v) and positive control (metoclopramide 2 mg/kg) were administered to the chickens, i.p. at a volume of 0.2 ml, 1 h prior to treatment with the emetic agents. Frequency of retching was recorded for copper sulphate and ipecac 60 and 20 min after their treatments, respectively. The percent inhibition was calculated by the following formula:
where A = frequency of retching in control group
B = frequency of retching in test group
Mitochondrial preparation
Mitochondria were prepared from chickens’ brains using differential centrifugation. Briefly, tissues were minced and then homogenized. The nuclei and broken cell debris were sedimented with centrifuging (1500×g for 10 min at 4 °C), and the pellet was discarded. The supernatant was centrifuged at 11,000×g for 10 min, and the superior layer was carefully discarded. The mitochondrial pellet was washed gently then suspended in the isolation medium and centrifuged again at 10,000×g for 10 min. Final mitochondrial pellets were suspended in Tris buffer containing (0.25 M sucrose, 0.05 M Tris–HCl, 2.0 mM MgCl2, 20 Mm KCl, and 1.0 mM Na2HPO4, pH of 7.4) at 4 °C (Hosseini et al. 2013). Protein concentrations were measured using the Coomassie blue protein-binding method as explained by Bradford (Bradford 1976). Also, succinate dehydrogenase was determined in the isolated of mitochondria. Mitochondria were prepared fresh for each experiment and used within 4 h of isolation, and all process were carried out on ice to reduce degradation and hence to assure the isolation of high-quality mitochondrial preparation.
Measurement of lipid peroxidation
The content of malonedialdehyde (MDA) was measured using the method of Zhang et al. (2008). Briefly, 0.25 ml sulfuric acid (0.05 M) was added to 0.2 ml mitochondrial fractions (0.5 mg protein/ml) with the addition of 0.3 ml 0.2 % thiobarbituric acid (TBA). All the micro tubes were placed in a boiling water bath for 30 min. At the end, the tubes were shifted to an ice bath and 0.4 ml n-butanol was added to each tube. Then, they were centrifuged at 3500×g for 10 min. The amount of MDA formed in each of the samples was assessed through measuring the absorbance of the supernatant at 532 nm with ELIZA reader (Tecan, Rainbow Thermo, Austria). The method was calibrated with tetraethoxypropane standard solutions.
Measurement of catalase activity
Catalase enzyme activity was evaluated based on the disappearance of H2O2 and measured by the absorbance decrease at 240 nm in a reaction medium containing sodium phosphate buffer (50 mM, pH 7.0) and H2O2 (10 mM). One unit of the enzyme is defined as 1 mol H2O2 as substrate consumed/min, and the activity was reported as units per milligram of protein (Abei 1984).
Measurement of protein carbonyl
Protein carbonyl content was measured by spectrophotometric method. Briefly, samples were extracted in 500 μl of 20 % (w/v) trichloroacetic acid (TCA). Then, samples were placed at 4 C for 15 min. The precipitates were treated with 500 μl of 0.2 % DNPH (2,4-dinitrophenylhydrazine) and 500 Μl of 2 M HCl for control group, and samples were incubated at room temperature for 1 h with vortexing at 5-min intervals. Proteins were precipitated by adding 55 μl of 100 % TCA. The micro tubes were centrifuged and washed three times with 1000 μl of the ethanol-ethyl acetate (1:1, v/v) mixture. Then the micro tube contents were dissolved in 200 μl of 6 M guanidine hydrochloride. The carbonyl content was determined by reading the absorbance at 365 nm wavelength (Levine et al. 1990).
Assessment of mitochondrial toxicity
Mitochondrial toxicity was evaluated by measuring the reduction of MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide using the method explained by Ghazi-Khansari et al. (Ghazi-Khansari et al. 2006.
Statistical analysis
The data were expressed as the mean values ± S.E.M. Analysis of variance followed by the multiple comparison test of Tukey-Kramer was used to compare data. The minimal level of significance chosen was p < 0.05.
Results
Acute intoxication
For evaluation of the acute toxicity and the maximum non-lethal doses of extract, increasing doses of the four extracts were administered to young chickens beginning from 500 mg/kg. Up to 2000 mg/kg, no mortality was observed among chickens treated with any of the extracts. Doses higher than 2000 mg/kg were not administered because the mortality cannot be solely attributed to the toxicity of extracts at these doses and might be due to the imbalance of the electrolytes and osmotic pressure of biological fluids.
Antiemetic activity of methanolic extract
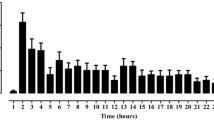
All doses of extract of S. ebelus fruit (50, 100, 200 mg/kg), significantly reduced the emesis induced by ipecac and copper sulphate in the chickens (Figs. 1 and 2). The percentages of retching inhibition induced by ipecac were 46, 96.5 and 83 % for doses 50, 100 and 200 mg/kg, respectively, and induced by copper sulphate were 73, 79.5 and 69.2 %, respectively. Also, induced emesis by ipecac and copper sulphate was significantly inhibited by metoclopramide (2 mg/kg) (Figs. 1 and 2).
The antiemetic effect of the methanolic extract of S. ebelus L. and metoclopramide on ipecac (600 mg/kg, orally)-induced emesis in young chickens. Data are mean ± SEM of five animals in each group. Significantly different from control group at p < 0.001 (aaa); Significantly different from Ext. 50 mg/kg at p < 0.001 (bbb); Significantly different from metoclopromide group at p < 0.05 (c)
Oxidative stress biomarkers
The effect of extract on lipid peroxidation (LPO) was investigated by assessing TBARS level in homogenates of the chickens’ brain mitochondria. TBARS level was significantly increased in the control group (p < 0.05) when compared to metoclopromide and extract (100 mg/kg) in retching induced by ipecac. Three doses of extract (50, 100, 200 mg/kg) and metoclopromide groups significantly decreased the TBARS levels (p < 0.001) when compared to the control group in retching induced by copper sulphate (Table 1).
As shown in Table 1, there was a significant decrease in catalase (CAT) activity (p < 0.05) in the extract (50 mg/kg) and metoclopromide groups when compared to the control group in retching induced by ipecac in the chickens’ brain mitochondria. There were no significant alterations observed on CAT activity in the brain mitochondria between control and other groups in retching induced by copper sulphate. Protein carbonyl (PC) contents significantly (p < 0.05) decreased only in extract (100 mg/kg) group when compared with the control and extract (50, 200 mg/kg) groups in retching induced by ipecac, but no significant changes observed on PC contents in retching induced by copper sulphate in the chickens’ brain mitochondria groups.
We used MTT test for assessment of mitochondria function after retching induced by ipecac or copper sulphate, and the effect of different concentrations of methanolic extracts of S. ebulus. As shown in Figs. 3 and 4, mitochondria function significantly increased (p < 0.05, 52 %) by extract (100 mg/kg) as compared to control group in retching induced by ipecac, but no significant changes observed on mitochondria function in retching induced by copper sulphate in different groups.
Effect of the methanolic extract of S. ebelus L. on mitochondrial function on ipecac (600 mg/kg, orally)-induced emesis in the young chickens’ mitochondria brain cells. Data are presented as percentage of changes to metoclopromide group. Data are mean ± SEM of five animals in each group. Significantly different from control group at p < 0.05 (a)
Discussion
For the first time, this study indicates that the retching can elevate oxidative stress biomarkers in the brain mitochondria and the fruit methanolic extract of S. ebulus has not only antiemetic but also neuroprotective effects in the brain of young chickens.
Nausea and vomiting are the common adverse events of different diseases, and extensive researches have led to use of safe drugs with low or lack of side effects. Nowadays, use of medicinal plants is still very important considering more and more ways of treatment in this area (Kültür 2007; Ertuğ 2000). S. ebulus is one of the medicinal plants that is commonly use in various disease because of its effectiveness. This plant contains different compounds such as anticancer substances (ebulin), flavonoids, tannins, steroid substances, cardiac glycosides, caffeic acid derivatives (Ahmadiani et al. 1998; Ghannadi and Ghassemi-Dehkordi 1997; de Benito et al. 1995; Saeedi Saravi and Shokrzadeh 2009), anthocyanins, organic acids, esters of fatty acids, triterpenes, polyphenols, sterols, ursolic acid and iridoids (Abdala et al. 2014).
In this study, the fruit extract of S. ebulus showed antiemetic effects against ipecac and copper sulphate-induced emesis in young chickens. Ipecac has an irritant effect in the stomach and produces emesis by the stimulation of the chemoreceptor trigger zone (CTZ) in the area postrema (central action) and by the direct action on the gastric mucosa (peripheral action) (Hosseinzadeh et al. 2008). According to our results, the extract prevented emesis induced by ipecac effectively; hence, it is possible that the extract acts centrally as well as peripherally to exert its antiemetic activity. Copper sulphate induces emesis only by peripheral action (Hosseinzadeh et al. 2008). The extract was able to prevent emesis induced by copper sulphate effectively. It seems that the extract has a peripheral antiemetic activity. Moreover, antiemetic effect of extract at dose of 100 mg/kg had equal or in some cases even more effective than metoclopramide effect (Figs. 1 and 2). It may be that extract stimulates the motility of the upper gastrointestinal tract by accelerating the gastric emptying rate, a similar effect to metoclopramide. According to the retching mechanism, emesis can be occurred by the stimulation of the dopaminergic system at the CTZ. Also mucosal damage releases the 5-hydroxytryptamine (5-HT) from the enterochromaffin cells. Released 5-HT possibly stimulates the vagal afferent nerves by interaction with 5-HT3 receptors. Likewise, liberated substance P from the gastric mucosa can affect on tachykinin NK1 receptors and may act cooperatively with 5-HT3 receptors in the upper gastrointestinal tract to induce vomiting. Therefore, the first thing that comes to mind is that extract, with various compounds, may have local or central effect via interaction with released mediated compounds related to emesis pathways and it was able to attenuate the vagal afferent nerve stimulation.
In extensive searches in the literatures, relationship between retching and oxidative stress markers was not found. Hence, the central focus of this study was to identify the relationship between retching and oxidative stress biomarkers in the chickens’ brain mitochondria. Usually, most markers of oxidative damage have revealed free radical attack on polyunsaturated fatty acids and proteins which results in lipid peroxidation, protein and DNA oxidation (Kadiiska et al. 2005). There are high levels of polyunsaturated fatty acids in the membrane brain cell lipids and, for that reason, particularly are sensitive to oxidation. The most widely investigated processes, induced by free radicals, are the PC and LPO and are therefore considered as excellent indexes of oxidative stress. Net ROS production is the sequel of imbalance between prooxidants and antioxidants ratio. Cellular macromolecule damaging occurs by LPO and induces cell function disturbances (Pederzolli et al. 2010). We first observed that level of LPO significantly elevated by retching. Significant increase in the level of LPO in control group may be due to the antioxidant inactivation of enzymes related to oxidative stress or poor antioxidant defence. Our results showed that enhancement in mitochondria ROS formation was prevented by all different extract doses of S. ebulus. LPO can markedly increase following ROS formation (Shahraki et al. 2014). We observed significant decline of LPO in different extract treatment groups. It can be understood that extract prevented cytotoxicity and sub-cellular organelle damage (mitochondria/lysosomes) as we observed in mitochondria MTT assay test (Fig. 3).
This impelled us to consider possible retching which provoked protein oxidation as determined by a noticeable increase of PC formation in control group. Oxidation of sulfhydryl groups of proteins to disulphide form leads to their inactivation. Reactive free radicals can oxidize the sulfhydryl groups of proteins and rise the carbonyl levels. Therefore, protein oxidative damage is presumed by attack of reactive species which are induced by this short branched chain fatty acid. Our results confirm this observation as PC content increased by retching in brain mitochondria.
Our results showed that despite lipid peroxidation, protein oxidation is also induced by retching in brain mitochondria through formation of ROS. Recent publications have revealed interesting anti-inflammatory and anti-nociceptive (Ahmadiani et al. 1998; Wang et al. 2013) effects of S. ebulus. It was demonstrated that polyphenols have higher antioxidant capacity compared with vitamins and carotenoids (Richelle et al. 2001). Different flavonoids (flavanols, flavonols and flavanones) and phenolic acids were identified in S. ebulus extract. Some studies have shown that the antioxidant activity and activity of hydrophilic extract is much higher against lipid peroxidation compared as the lipophilic extract (Gentile et al. 2007). These reports support and are consistent with our observation of protection effects by different S. ebulus extract doses against ROS formation and lipid peroxidation of chickens’ brain neurones. Intracellular organelles such as lysosomes and mitochondria can be the target of ROS, and mitochondria damage could be due to lipid peroxidative consequence of ROS formation. Hence, it seems that methanolic extract could abate ROS formation in our study (Table 1). Continuous stimulation of the neuron cells (dopaminergic and serotonergic neurons) and vagal afferent nerves can be regarded as an important mechanism underlying the sub-organelle (mitochondria/lysosomes) membrane damages. It can be confirmed in our results that showed induced oxidative damage in brain mitochondria by retching.
CAT is one of the most important defence mechanisms against toxic effects of oxygen metabolism which alleviate the ROS toxic effects. Highly reactive hydroxyl radical (OH−) produced by H2O2 induces oxidation of biological molecules. Our data showed that induced ROS formation by the intracellular H2O2 generation system can be modulated by extracts of S. ebulus (Table 1). Interestingly, no significant improvement in CAT was observed in our study. It may return to act as a direct scavenger of produced free radicals, by extract in this case, rather than an indirect activation of antioxidant enzymes resulting in no need of more enzyme activation. It was suggested that antioxidants and radical scavenging components of extract may attack the produced free radicals and inhibit them and that surely needs to be elucidated by further works.
Our study showed the best neuroprotective effect of methanolic extract against hydroperoxide-induced lipid peroxidation and PC formation. It could be ought to the ability of the extract on H2O2, superoxide or hydroxyl radical source disappearance. It was demonstrated that the phenolic compounds are effective in reduction of protein carbonylation (Shahraki et al. 2014). It seems that S. ebulus extract traps dicarbonyls, in this manner, reducing toxicity and reversing early stage of carbonyl formation. Additionally, the flavonoids found in S. ebulus fruit could effectively prevent protein carbonylation under physiological conditions in early stage.
Due to the diversity of compounds found in both non-polar and polar extracts, it is expected that several cytoprotective mechanisms are acting against carbonyl and oxidative induced neurotoxicity. Possibility, synergistic effects between several antioxidants could be present in which antioxidants recharge neighbouring antioxidants. In summary, our study has shown that S. ebulus extract prevented neuron oxidative stress (lipid peroxidation), carbonyl stress (protein carbonylation) and cytotoxicity induced by ROS that has been implicated in retching. This is confirmed by the results that the better effect of S. ebulus methanolic extract dose is 100 mg/kg on modulation oxidative stress biomarkers. Hence, practically, dose of 100 mg/kg of extract can be considered as an effective dose for its antioxidative potential.
Conclusion
To our information, we are the first to report the brain mitochondria disruption provoked by retching and neuroprotective activity of S. ebulus methanolic extract in this particular study. The results of this study suggest that the fruit extract of S. ebulus has both protective effects against ipecac and copper sulphate-induced retching in young chickens, possibly by central and peripheral mechanisms. Neuroprotective effect of extract may be due to scavenging of free radicals induced by retching. Overall, S. ebulus may present a valuable approach to counter elevated oxidative and carbonyl stress observed in retching, possible by increasing plasma antioxidants, bioactive compounds or oxygen scavenger which could prevent protein modification and improve oxidative stress in the early stages.
References
Abdala S, Dévora S, Martín-Herrera D, Pérez-Paz P. Antinociceptive and anti-inflammatory activity of Sambucus palmensis link, an endemic Canary Island species. J Ethnopharmacol. 2014;155:626–32.
Abei H. Catalase in vitro. Methods Enzymol. 1984;105:121–6.
Ahmadiani A, Fereidoni M, Semnanian S, Kamalinejad M, Saremi S. Antinociceptive and anti-inflammatory effects of Sambucus ebulus rhizome extract in rats. J Ethnopharmacol. 1998;61:229–35.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54.
Chashoo, I., Kumar, D., Bhat, Z., Khan, N., Kumar, V. & Nowshehri, J. A. 2012. Antimicrobial studies of Sambucus wightiana Wall. ex. Wight & Arn. J Pharm Res, 5.
Citores L, DE Benito FM, Iglesias R, Ferreras JM, Argüeso P, Jiménez P, et al. Presence of polymerized and free forms of the non-toxic type 2 ribosome-inactivating protein ebulin and a structurally related new homodimeric lectin in fruit of Sambucus ebulus L. Planta. 1998;204:310–7.
Darias V, Bravo L, Barquin E, Herrera DM, Fraile C. Contribution to the ethnopharmacological study of the Canary islands. J Ethnopharmacol. 1986;15:169–93.
de Benito FM, Citores L, Iglesias R, Ferreras JM, Soriano F, Arias J, et al. Ebulitins: a new family of type 1 ribosome-inactivating proteins (rRNA N-glycosidases) from leaves of Sambucus ebulus L. that coexist with the type 2 ribosome-inactivating protein ebulin 1. FEBS Lett. 1995;360:299–302.
Dubey P, Jayasooriya AP, Cheema SK. Fish oil induced hyperlipidemia and oxidative stress in BioF1B hamsters is attenuated by elderberry extract. Appl Physiol Nutr Metab. 2012;37:472–9.
Ebrahimzadeh MA, Mahmoudi M, Karami M, Saeedi S, Ahmadi AH, Salimi E. Separation of active and toxic portions in Sambucus ebulus. Pak J Biol Sci. 2007;10:4171–3.
Ebrahimzadeh M, Nabavi S, Nabavi S. Antioxidant activities of methanol extract of Sambucus ebulus L. flower. Pak J Biol Sci. 2009a;12:447.
Ebrahimzadeh MA, Ehsanifar S, Eslami B. Sambucus ebulus elburensis fruit: a good source for antioxidants. Pharmacogn Mag. 2009b;4:213–8.
Ertuğ F. An ethnobotanical study in central Anatolia (Turkey). Econ Bot. 2000;54:155–82.
Gentile C, Tesoriere L, Butera D, Fazzari M, Monastero M, Allegra M, et al. Antioxidant activity of Sicilian pistachio (Pistacia vera L. var. Bronte) nut extract and its bioactive components. J Agric Food Chem. 2007;55:643–8.
Ghannadi A, Ghassemi-Dehkordi N. Pharmacognostical investigations on Sambucus ebulus L. and Sambucus nigra L. DARU J Pharm Sci. 1997;7:55–65.
Ghazi-Khansari M, Mohammadi-Bardbori A, Hosseini MJ. Using Janus green B to study paraquat toxicity in rat liver mitochondria. Ann N Y Acad Sci. 2006;1090:98–107.
Hosseini M-J, Shaki F, Ghazi-Khansari M, Pourahmad J. Toxicity of vanadium on isolated rat liver mitochondria: a new mechanistic approach. Metallomics. 2013;5:152–66.
Hosseinzadeh H, Mirshojaeian M, Razavi BM. Antiemetic effect of Pistacia vera L. (pistachio) leaves and nuts aqueous extracts in young chicken. Pharmacol Online. 2008;2:568–71.
Jiménez P, Tejero J, Cordoba-Diaz D, Quinto EJ, Garrosa M, Gayoso MJ, et al. Ebulin from dwarf elder (Sambucus ebulus L.): a mini review. Toxins. 2015;7:648–58.
Kadiiska M, Gladen B, Baird D, Germolec D, Graham L, Parker C, et al. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med. 2005;38:698–710.
Kültür Ş. Medicinal plants used in Kırklareli Province (Turkey). J Ethnopharmacol. 2007;111:341–64.
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz A-G, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–78.
Pederzolli CD, Mescka CP, Zandoná BR, De Moura Coelho D, Sgaravatti ÂM, Sgarbi MB, et al. Acute administration of 5-oxoproline induces oxidative damage to lipids and proteins and impairs antioxidant defenses in cerebral cortex and cerebellum of young rats. Metab Brain Dis. 2010;25:145–54.
Rahimi-Esboei B, Ebrahimzadeh M, Gholami S, Falah-Omrani V. Anti-giardial activity of Sambucus ebulus. Eur Rev Med Pharmacol Sci. 2013;17:2047–50.
Richelle M, Tavazzi I, Offord E. Comparison of the antioxidant activity of commonly consumed polyphenolic beverages (coffee, cocoa, and tea) prepared per cup serving. J Agric Food Chem. 2001;49:3438–42.
Saeedi Saravi SS, Shokrzadeh M. Histopathological and biochemical disorders following administration of Sambucus ebulus extract on mice and rats and preventive effects of vitamins C and E on renal and hepatic disorders. Pharmacogn Mag. 2009;5:131–5.
Shahraki J, Zareh M, Kamalinejad M, Pourahmad J. Cytoprotective effects of hydrophilic and lipophilic extracts of Pistacia vera against oxidative versus carbonyl stress in rat hepatocytes. Iran J Pharm Res. 2014;13:1263.
Tuzlaci E, Tolon E. Turkish folk medicinal plants, part III: Sile (Istanbul). Fitoterapia. 2000;71:673–85.
Wang Q, Kuang H, SU Y, Sun Y, Feng J, Guo R, et al. Naturally derived anti-inflammatory compounds from Chinese medicinal plants. J Ethnopharmacol. 2013;146:9–39.
Yang Y, Kinoshita K, Koyama K, Takahashi K, Tai T, Nunoura Y, et al. Novel experimental model using free radical-induced emesis for surveying anti-emetic compounds from natural sources. Planta Med. 1999;65:574–6.
Yeşilada E, Gürbüz IL, Shibata H. Screening of Turkish anti-ulcerogenic folk remedies for anti-Helicobacter pylori activity. J Ethnopharmacol. 1999;66:289–93.
Zhang F, Xu Z, Gao J, Xu B, Deng Y. In vitro effect of manganese chloride exposure on energy metabolism and oxidative damage of mitochondria isolated from rat brain. Environ Toxicol Pharmacol. 2008;26:232–6.
Acknowledgments
Financial support for this work was provided by Mazandaran University of Medical Sciences, Sari, Iran with reference number 1155.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Fathi, H., Ebrahimzadeh, M.A., Ziar, A. et al. Oxidative damage induced by retching; antiemetic and neuroprotective role of Sambucus ebulus L.. Cell Biol Toxicol 31, 231–239 (2015). https://doi.org/10.1007/s10565-015-9307-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-015-9307-8